Increasing Performance of Spiral-Wound Modules (SWMs) by Improving Stability against Axial Pressure Drop and Utilising Pulsed Flow
Abstract
:1. Introduction
2. Materials and Methods
2.1. Filtration Plant and Experimental Design
2.1.1. Plant Modification and Experimental Design to Utilise Pulsed Flow
Filtration Experiments
Cleaning Experiments
2.1.2. Membrane Modification and Experimental Design to Investigate Increased Axial Pressure Drops
2.2. Chemical and Statistical Analyses
3. Results and Discussion
3.1. Optimisation of SWM’s Process Efficiency via the Utilisation of Pulsed Flow
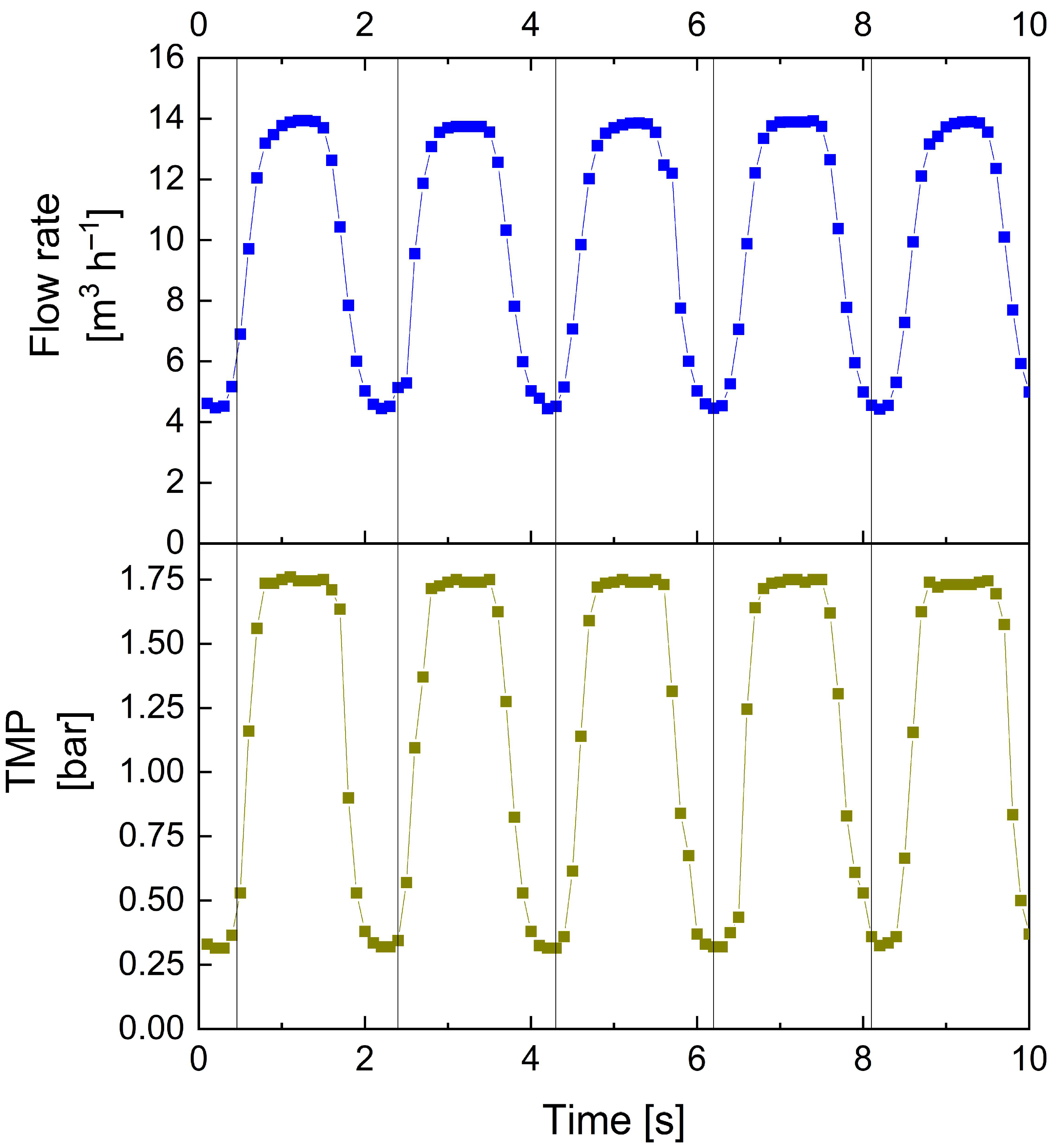
3.1.1. Validation of Plant Modifications
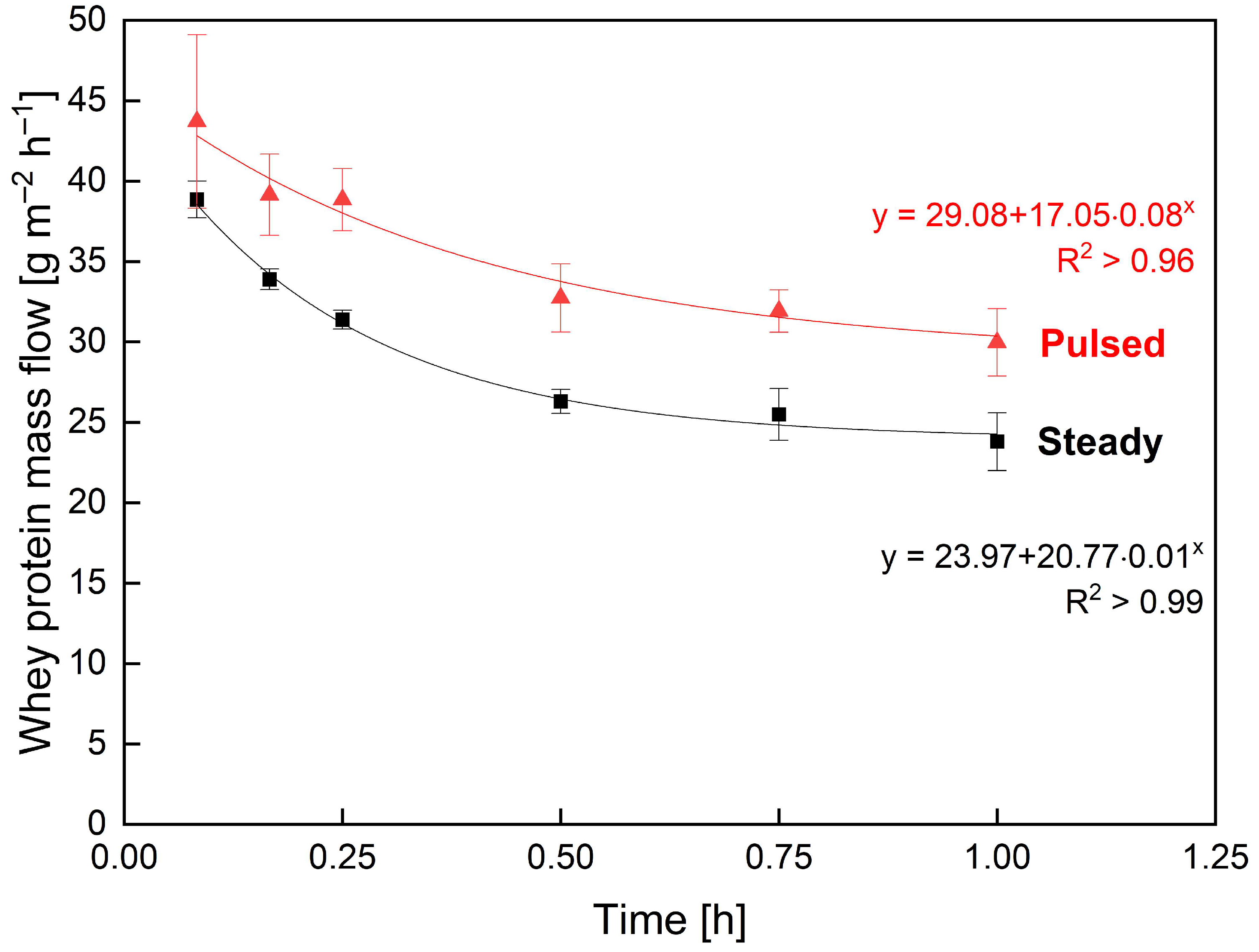
3.1.2. Influence of Pulsed Flow on Filtration Performance in Industrial-Scale SWMs
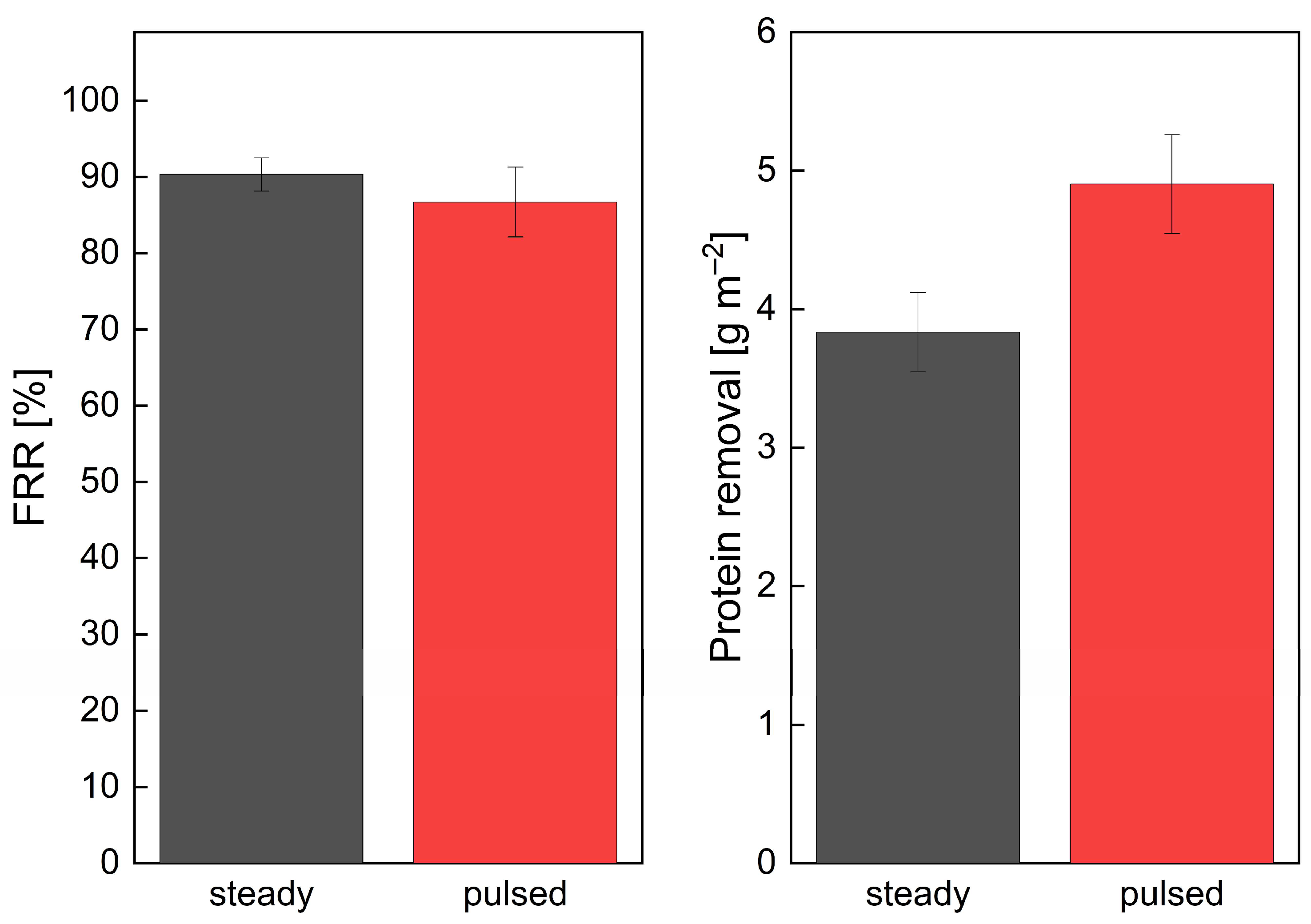
3.1.3. Influence of Pulsed Flow on Cleaning Efficiency in Industrial-Scale SWMs
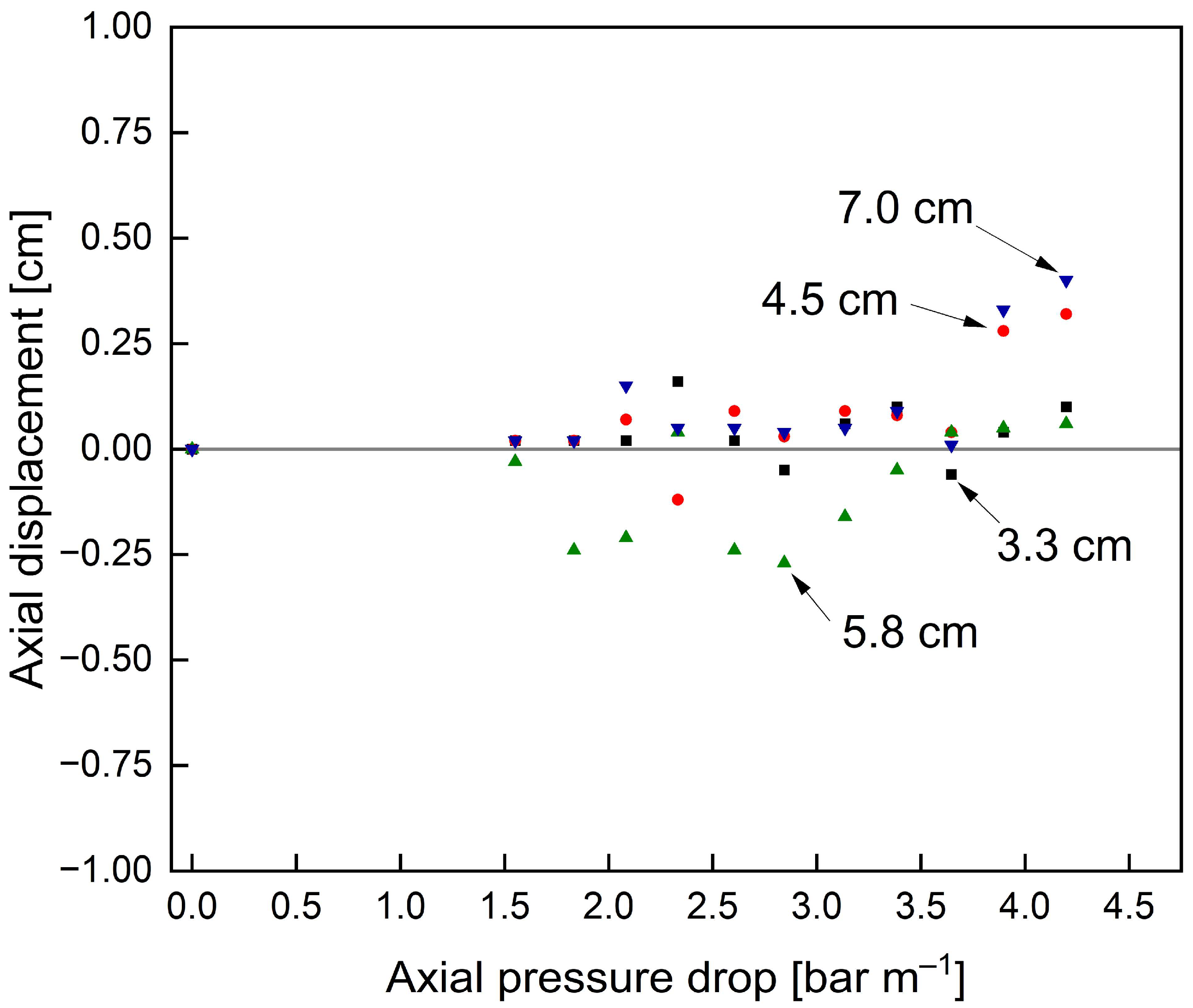
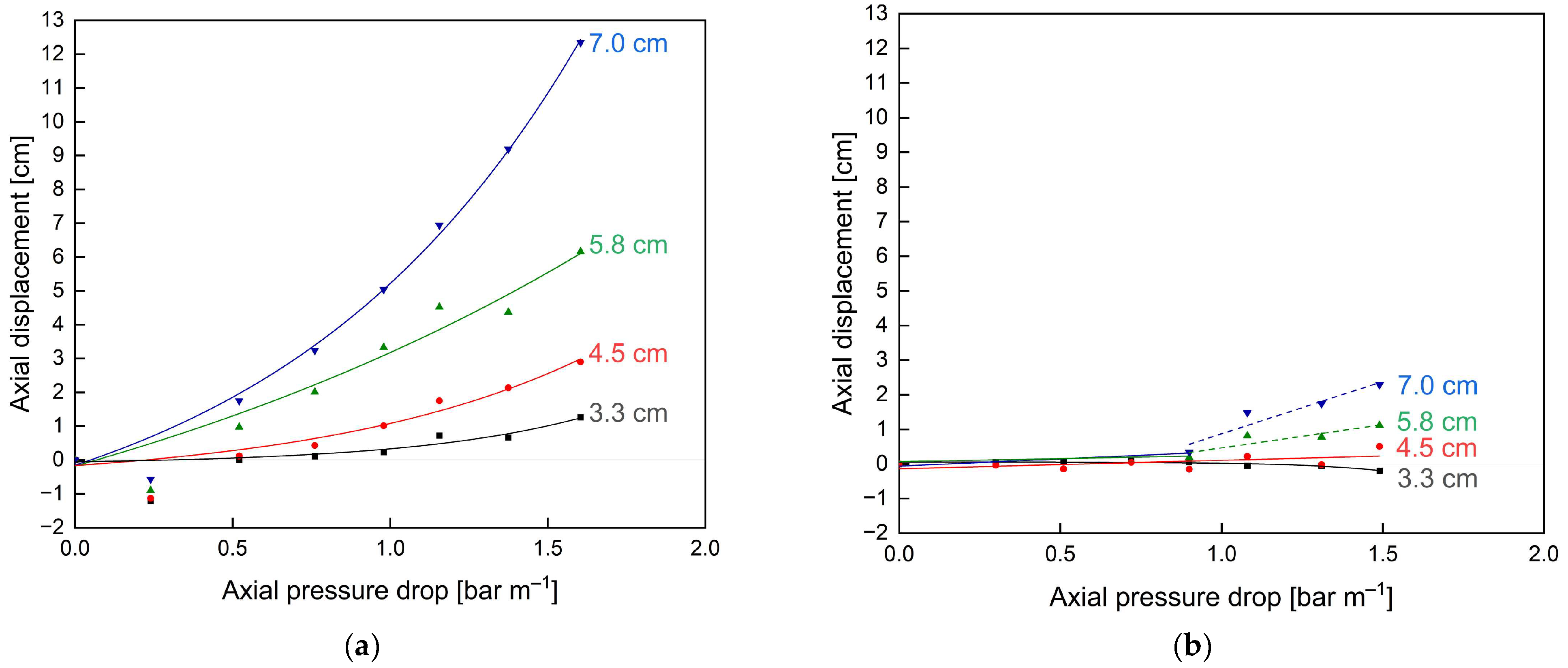
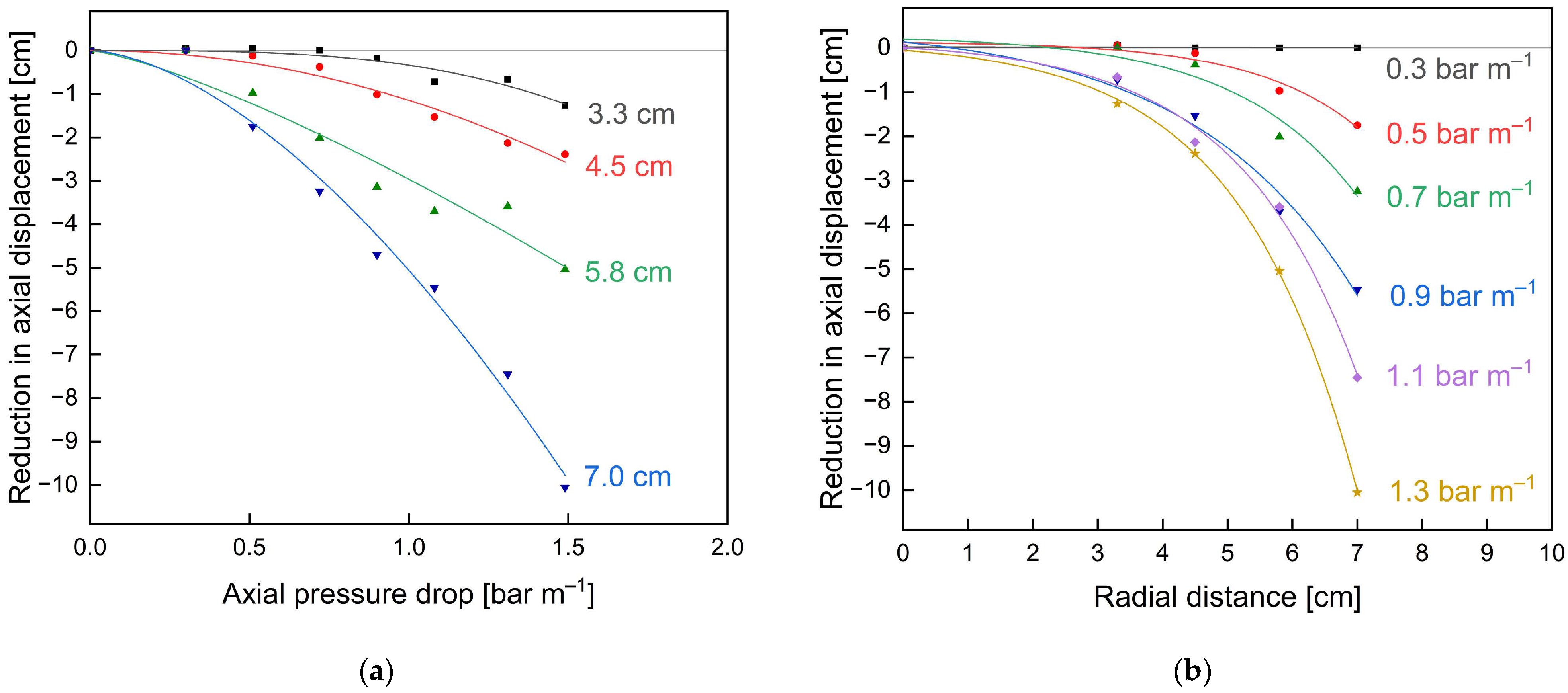
3.2. Optimisation of SWMs’ Mechanical Stability by Feed-Side Glue Connections
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartlett, M.; Bird, M.R.; Howell, J.A. An experimental study for the development of a qualitative membrane cleaning model. J. Membr. Sci. 1995, 105, 147–157. [Google Scholar] [CrossRef]
- Bird, M.R.; Bartlett, M. Measuring and modelling flux recovery during the chemical cleaning of MF membranes for the processing of whey protein concentrate. J. Food Eng. 2002, 53, 143–152. [Google Scholar] [CrossRef]
- Cui, Z.F.; Jiang, Y.; Field, R.W. Fundamentals of pressure-driven membrane separation processes. In Membrane Technology; Elsevier: Oxford, UK, 2010; pp. 1–18. ISBN 9781856176323. [Google Scholar]
- Saxena, A.; Tripathi, B.P.; Kumar, M.; Shahi, V.K. Membrane-based techniques for the separation and purification of proteins: An overview. Adv. Colloid. Interface Sci. 2009, 145, 1–22. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2012; ISBN 9781118359686. [Google Scholar]
- Ng, K.S.; Haribabu, M.; Harvie, D.J.; Dunstan, D.E.; Martin, G.J. Mechanisms of flux decline in skim milk ultrafiltration: A review. J. Membr. Sci. 2017, 523, 144–162. [Google Scholar] [CrossRef]
- Ripperger, S.; Altmann, J. Crossflow microfiltration—state of the art. Sep. Purif. Technol. 2002, 26, 19–31. [Google Scholar] [CrossRef]
- Ripperger, S.; Grein, T. Filtrationsverfahren mit Membranen und ihre Modellierung. Chem. Ing. Tech. 2007, 79, 1765–1776. [Google Scholar] [CrossRef]
- Schiffer, S.; Kulozik, U. Effect of temperature-dependent bacterial growth during milk protein fractionation by means of 0.1 µM microfiltration on the length of possible production cycle times. Membranes 2020, 10, 326. [Google Scholar] [CrossRef] [PubMed]
- Chamberland, J.; Messier, T.; Dugat-Bony, E.; Lessard, M.-H.; Labrie, S.; Doyen, A.; Pouliot, Y. Influence of feed temperature to biofouling of ultrafiltration membrane during skim milk processing. Int. Dairy J. 2019, 93, 99–105. [Google Scholar] [CrossRef]
- Burgess, S.A.; Lindsay, D.; Flint, S.H. Thermophilic bacilli and their importance in dairy processing. Int. J. Food Microbiol. 2010, 144, 215–225. [Google Scholar] [CrossRef]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry—A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef]
- Ng, K.S.; Dunstan, D.E.; Martin, G.J. Influence of processing temperature on flux decline during skim milk ultrafiltration. Sep. Purif. Technol. 2018, 195, 322–331. [Google Scholar] [CrossRef]
- Brans, G.; Schroën, C.G.; van der Sman, R.G.; Boom, R.M. Membrane fractionation of milk: State of the art and challenges. J. Membr. Sci. 2004, 243, 263–272. [Google Scholar] [CrossRef]
- Qu, P.; Gésan-Guiziou, G.; Bouchoux, A. Dead-end filtration of sponge-like colloids: The case of casein micelle. J. Membr. Sci. 2012, 417–418, 10–19. [Google Scholar] [CrossRef]
- Qu, P.; Bouchoux, A.; Gésan-Guiziou, G. On the cohesive properties of casein micelles in dense systems. Food Hydrocoll. 2015, 43, 753–762. [Google Scholar] [CrossRef]
- Bouchoux, A.; Gésan-Guiziou, G.; Pérez, J.; Cabane, B. How to squeeze a sponge: Casein micelles under osmotic stress, a SAXS study. Biophys. J. 2010, 99, 3754–3762. [Google Scholar] [CrossRef]
- Bouchoux, A.; Cayemitte, P.-E.; Jardin, J.; Gésan-Guiziou, G.; Cabane, B. Casein micelle dispersions under osmotic stress. Biophys. J. 2009, 96, 693–706. [Google Scholar] [CrossRef]
- Horne, D.S. Casein micelle structure and stability. In Milk Proteins; Elsevier: Cambridge, UK, 2020; pp. 213–250. ISBN 9780128152515. [Google Scholar]
- Hartinger, M.; Heidebrecht, H.-J.; Schiffer, S.; Dumpler, J.; Kulozik, U. Technical concepts for the investigation of spatial effects in spiral-wound microfiltration membranes. Membranes 2019, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Blanpain-Avet, P.; Migdal, J.F.; Bénézech, T. Chemical cleaning of a tubular ceramic microfiltration membrane fouled with a whey protein concentrate suspension—Characterization of hydraulic and chemical cleanliness. J. Membr. Sci. 2009, 337, 153–174. [Google Scholar] [CrossRef]
- Samuelsson, G.; Dejmek, P.; Trägårdh, G.; Paulsson, M. Minimizing whey protein retention in cross-flow microfiltration of skim milk. Int. Dairy J. 1997, 7, 237–242. [Google Scholar] [CrossRef]
- Defrance, L.; Jaffrin, M.Y. Comparison between filtrations at fixed transmembrane pressure and fixed permeate flux: Application to a membrane bioreactor used for wastewater treatment. J. Membr. Sci. 1999, 152, 203–210. [Google Scholar] [CrossRef]
- Qiu, T.Y.; Davies, P.A. Concentration polarization model of spiral-wound membrane modules with application to batch-mode RO desalination of brackish water. Desalination 2015, 368, 36–47. [Google Scholar] [CrossRef]
- Farhat, N.M.; Staal, M.; Bucs, S.; van Loosdrecht, M.; Vrouwenvelder, J.S. Spatial heterogeneity of biofouling under different cross-flow velocities in reverse osmosis membrane systems. J. Membr. Sci. 2016, 520, 964–971. [Google Scholar] [CrossRef]
- Altmann, J.; Ripperger, S. Particle deposition and layer formation at the crossflow microfiltration. J. Membr. Sci. 1997, 124, 119–128. [Google Scholar] [CrossRef]
- Hartinger, M.; Schiffer, S.; Heidebrecht, H.-J.; Dumpler, J.; Kulozik, U. Milk protein fractionation by custom-made prototypes of spiral-wound microfiltration membranes operated at extreme crossflow velocities. J. Membr. Sci. 2020, 605, 118110. [Google Scholar] [CrossRef]
- Schopf, R.; Schmidt, F.; Linner, J.; Kulozik, U. Comparative assessment of tubular ceramic, spiral wound, and hollow fiber membrane microfiltration Module Systems for Milk Protein Fractionation. Foods 2021, 10, 692. [Google Scholar] [CrossRef]
- Kürzl, C.; Kulozik, U. Comparison of the efficiency of pulsed flow membrane cleaning in hollow fibre (HFM) and spiral-wound microfiltration membranes (SWM). Food Bioprod. Process. 2023, 139, 166–177. [Google Scholar] [CrossRef]
- Fischer, N.; Masoudian, M.; Germann, N. Impact of non-Newtonian fluid behavior on hydrodynamics and mass transfer in spacer-filled channels. Fluid. Dyn. Res. 2020, 52, 65502. [Google Scholar] [CrossRef]
- Kürzl, C.; Kulozik, U. Influence of Pulsed and Alternating Flow on the Filtration Performance during Skim Milk Microfiltration with Flat-Sheet Membranes. Sep. Purif. Technol. 2023, 321, 124234. [Google Scholar] [CrossRef]
- Geraldes, V. Flow management in nanofiltration spiral wound modules with ladder-type spacers. J. Membr. Sci. 2002, 203, 87–102. [Google Scholar] [CrossRef]
- Han, Z.; Terashima, M.; Liu, B.; Yasui, H. CFD investigation of the effect of the feed spacer on hydrodynamics in spiral wound membrane modules. MCA 2018, 23, 80. [Google Scholar] [CrossRef]
- Kavianipour, O.; Ingram, G.D.; Vuthaluru, H.B. Investigation into the effectiveness of feed spacer configurations for reverse osmosis membrane modules using Computational Fluid Dynamics. J. Membr. Sci. 2017, 526, 156–171. [Google Scholar] [CrossRef]
- Schwinge, J.; Wiley, D.E.; Fletcher, D.F. A CFD study of unsteady flow in narrow spacer-filled channels for spiral-wound membrane modules. Desalination 2002, 146, 195–201. [Google Scholar] [CrossRef]
- Koutsou, C.P.; Yiantsios, S.G.; Karabelas, A.J. Direct numerical simulation of flow in spacer-filled channels: Effect of spacer geometrical characteristics. J. Membr. Sci. 2007, 291, 53–69. [Google Scholar] [CrossRef]
- Hartinger, M.; Napiwotzki, J.; Schmid, E.-M.; Hoffmann, D.; Kurz, F.; Kulozik, U. Influence of spacer design and module geometry on the filtration performance during skim milk microfiltration with flat sheet and spiral-wound membranes. Membrane 2020, 10, 57. [Google Scholar] [CrossRef]
- Habenicht, G. Kleben: Grundlagen, Technologien, Anwendungen, 6. Aufl. 2009; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-85266-7. [Google Scholar]
- Dilger, K. Selecting the right joint design and fabrication techniques. In Advances in Structural Adhesive Bonding; Elsevier: Cambridge, UK, 2010; pp. 295–315. ISBN 9781845694357. [Google Scholar]
- Grote, K.-H.; Bender, B.; Göhlich, D. (Eds.) Dubbel–Taschenbuch für den Maschinenbau; 25. Auflage; Springer Vieweg: Berlin/Heidelberg, Germany, 2018; ISBN 9783662548042. [Google Scholar]
- Althof, W. Verformungs-Und Festigkeitseigenschaften von Klebstoffen bei Kurz-Und Langzeitbeanspruchung. N39 1984, 141–162. [Google Scholar]
- Karabelas, A.J.; Koutsou, C.P.; Sioutopoulos, D.C. Comprehensive performance assessment of spacers in spiral-wound membrane modules accounting for compressibility effects. J. Membr. Sci. 2018, 549, 602–615. [Google Scholar] [CrossRef]
- Weinberger, M.E.; Kulozik, U. Pulsatile crossflow improves microfiltration fractionation of cells and proteins. J. Membr. Sci. 2021, 629, 119295. [Google Scholar] [CrossRef]
- Kürzl, C.; Tran, T.; Kulozik, U. Application of a pulsed crossflow to improve chemical cleaning efficiency in hollow fibre membranes following skim milk microfiltration. Sep. Purif. Technol. 2022, 302, 122123. [Google Scholar] [CrossRef]
- Gillham, C.R.; Fryer, P.J.; Hasting, A.P.; Wilson, D.I. Enhanced cleaning of whey protein soils using pulsed flows. J. Food Eng. 2000, 46, 199–209. [Google Scholar] [CrossRef]
- Blel, W.; Le Gentil-Lelièvre, C.; Bénézech, T.; Legrand, J.; Legentilhomme, P. Application of turbulent pulsating flows to the bacterial removal during a cleaning in place procedure. Part 1: Experimental analysis of wall shear stress in a cylindrical pipe. J. Food Eng. 2009, 90, 422–432. [Google Scholar] [CrossRef]
- Blel, W.; Legentilhomme, P.; Bénézech, T.; Legrand, J.; Le Gentil-Lelièvre, C. Application of turbulent pulsating flows to the bacterial removal during a cleaning in place procedure. Part 2: Effects on cleaning efficiency. J. Food Eng. 2009, 90, 433–440. [Google Scholar] [CrossRef]
- Yang, J.; Kjellberg, K.; Jensen, B.B.B.; Nordkvist, M.; Gernaey, K.V.; Krühne, U. Investigation of the cleaning of egg yolk deposits from tank surfaces using continuous and pulsed flows. Food Bioprod. Process. 2019, 113, 154–167. [Google Scholar] [CrossRef]
- Weidemann, C.; Vogt, S.; Nirschl, H. Cleaning of filter media by pulsed flow—Establishment of dimensionless operation numbers describing the cleaning result. J. Food Eng. 2014, 132, 29–38. [Google Scholar] [CrossRef]
- Bode, K.; Hooper, R.J.; Paterson, W.R.; Wilson, D.I.; Augustin, W.; Scholl, S. Pulsed flow cleaning of whey protein fouling layers. Heat Transf. Eng. 2007, 28, 202–209. [Google Scholar] [CrossRef]
- Augustin, W.; Fuchs, T.; Föste, H.; Schöler, M.; Majschak, J.-P.; Scholl, S. Pulsed flow for enhanced cleaning in food processing. Food Bioprod. Process. 2010, 88, 384–391. [Google Scholar] [CrossRef]
- Dumpler, J.; Wohlschläger, H.; Kulozik, U. Dissociation and coagulation of caseins and whey proteins in concentrated skim milk heated by direct steam injection. Dairy Sci. Technol. 2017, 96, 807–826. [Google Scholar] [CrossRef]
- Kürzl, C.; Kulozik, U. Alternating flow direction improves chemical cleaning efficiency in hollow fibre membranes following skim milk microfiltration. J. Food Eng. 2023, 356, 111587. [Google Scholar] [CrossRef]
- Augustin, W.; Bohnet, M. Influence of pulsating flow on fouling behaviour. In Proceedings of the International Conference on Mitigation of Heat Exchanger Fouling and Its Economic and Environmental Implications, Banff, Canada, 18–23 July 1999; ISBN 1-56700-172-6. [Google Scholar]
- Föste, H.; Schöler, M.; Majschak, J.-P.; Augustin, W.; Scholl, S. Modeling and validation of the mechanism of pulsed flow cleaning. Heat Transf. Eng. 2013, 34, 753–760. [Google Scholar] [CrossRef]
- Gu, B.; Adjiman, C.S.; Xu, X.Y. The effect of feed spacer geometry on membrane performance and concentration polarisation based on 3D CFD simulations. J. Membr. Sci. 2017, 527, 78–91. [Google Scholar] [CrossRef]
- Schwinge, J.; Wiley, D.E.; Fletcher, D.F. Simulation of unsteady flow and vortex shedding for narrow spacer-filled channels. Ind. Eng. Chem. Res. 2003, 42, 4962–4977. [Google Scholar] [CrossRef]
- Schwinge, J.; Neal, P.R.; Wiley, D.E.; Fletcher, D.F.; Fane, A.G. Spiral wound modules and spacers. J. Membr. Sci. 2004, 242, 129–153. [Google Scholar] [CrossRef]
- Koutsou, C.P.; Yiantsios, S.G.; Karabelas, A.J. A numerical and experimental study of mass transfer in spacer-filled channels: Effects of spacer geometrical characteristics and Schmidt number. J. Membr. Sci. 2009, 326, 234–251. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kürzl, C.; Hartinger, M.; Ong, P.; Schopf, R.; Schiffer, S.; Kulozik, U. Increasing Performance of Spiral-Wound Modules (SWMs) by Improving Stability against Axial Pressure Drop and Utilising Pulsed Flow. Membranes 2023, 13, 791. https://doi.org/10.3390/membranes13090791
Kürzl C, Hartinger M, Ong P, Schopf R, Schiffer S, Kulozik U. Increasing Performance of Spiral-Wound Modules (SWMs) by Improving Stability against Axial Pressure Drop and Utilising Pulsed Flow. Membranes. 2023; 13(9):791. https://doi.org/10.3390/membranes13090791
Chicago/Turabian StyleKürzl, Christian, Martin Hartinger, Patrick Ong, Roland Schopf, Simon Schiffer, and Ulrich Kulozik. 2023. "Increasing Performance of Spiral-Wound Modules (SWMs) by Improving Stability against Axial Pressure Drop and Utilising Pulsed Flow" Membranes 13, no. 9: 791. https://doi.org/10.3390/membranes13090791
APA StyleKürzl, C., Hartinger, M., Ong, P., Schopf, R., Schiffer, S., & Kulozik, U. (2023). Increasing Performance of Spiral-Wound Modules (SWMs) by Improving Stability against Axial Pressure Drop and Utilising Pulsed Flow. Membranes, 13(9), 791. https://doi.org/10.3390/membranes13090791





