Purification of Liquid Fraction of Digestates from Different Origins—Comparison of Polymeric and Ceramic Ultrafiltration Membranes Used for This Purpose
Abstract
1. Introduction
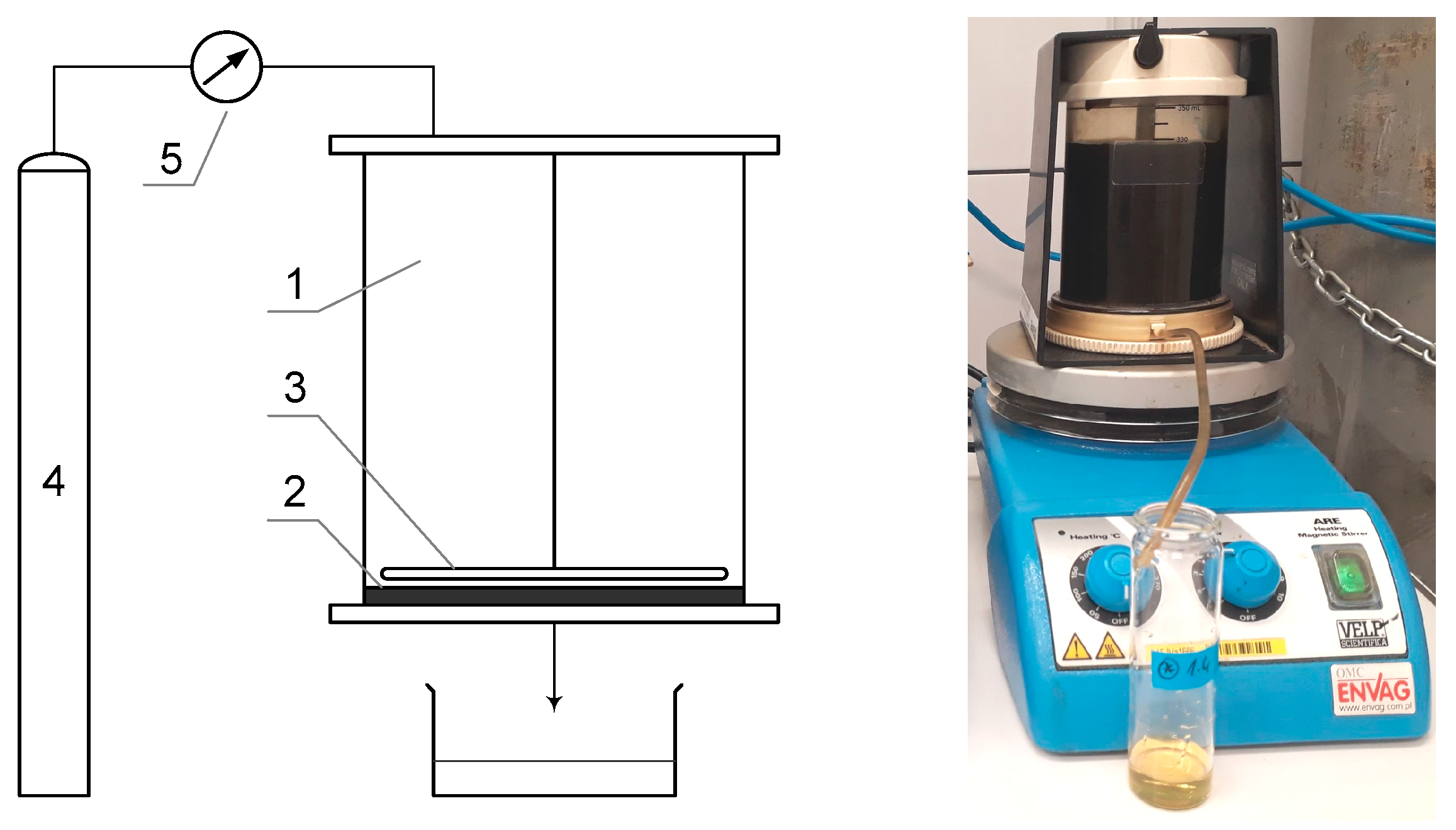
2. Materials and Methods
- V—volume of permeate, m3;
- A—membrane surface area, m2;
- t—filtration time, d.
- cp—concentration of impurities in the treated solution, g/m3;
- cn—initial concentration of impurities in the solution to be purified, g/m3.
3. Results
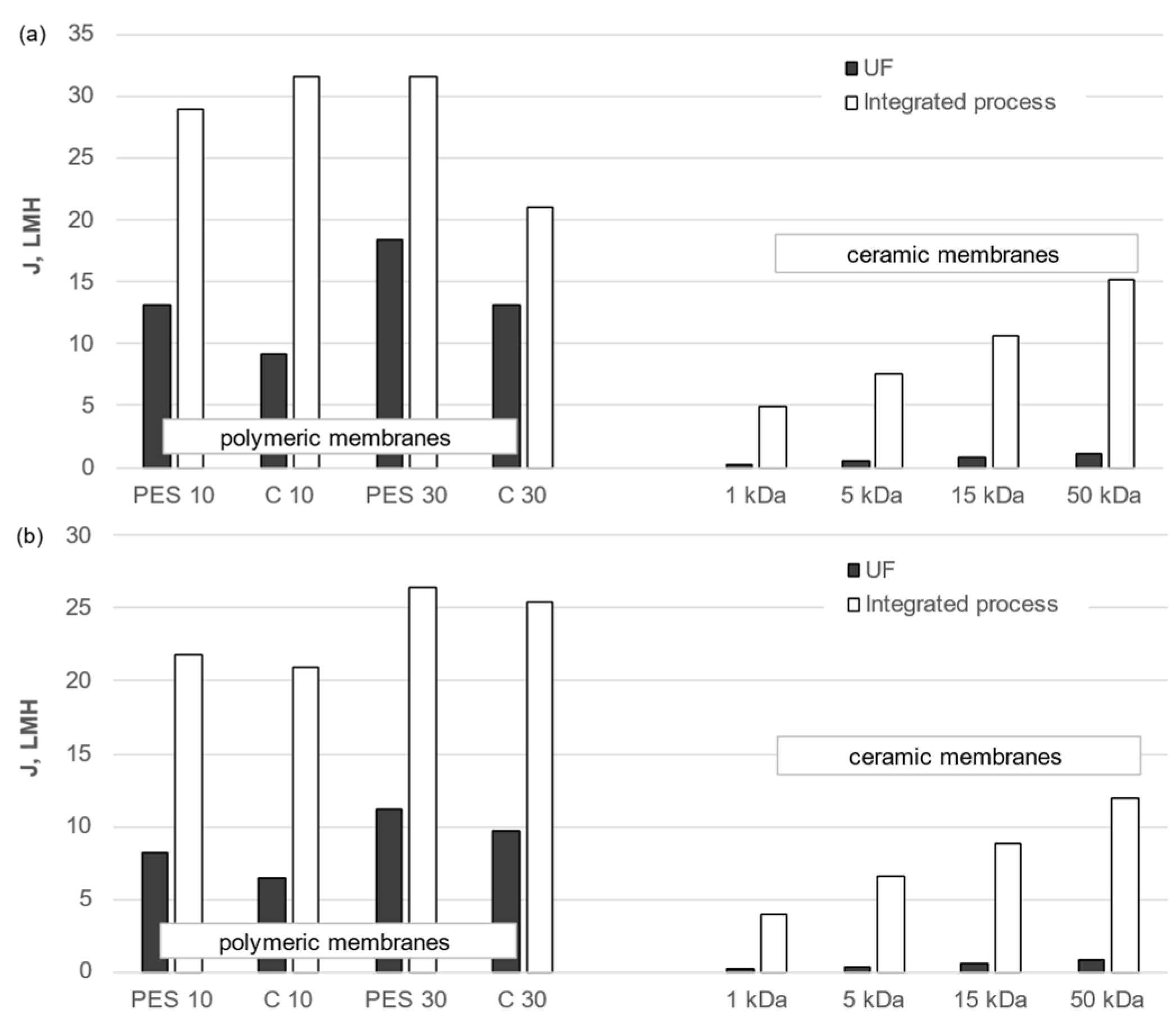
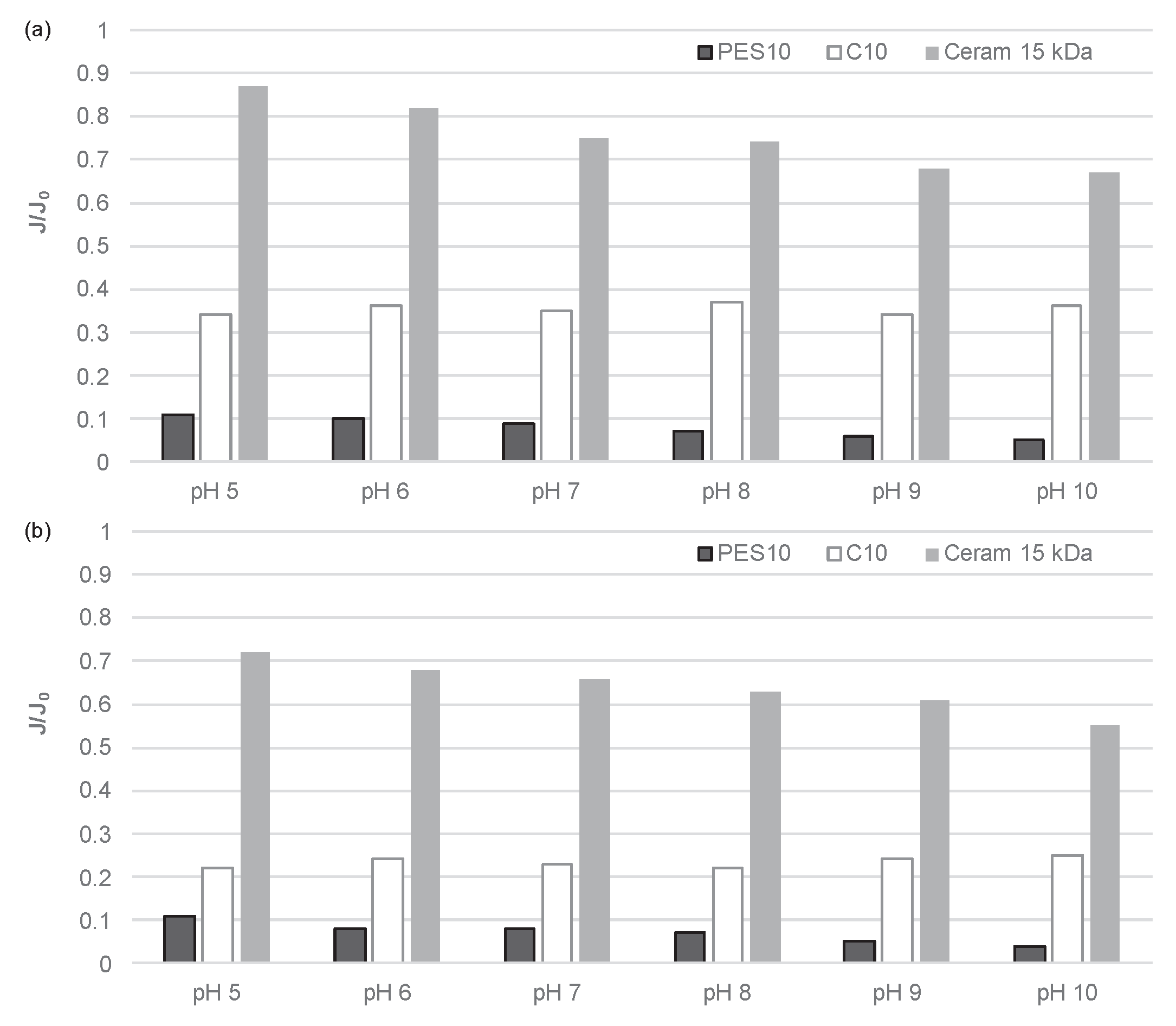
3.1. Transport Properties of Membranes
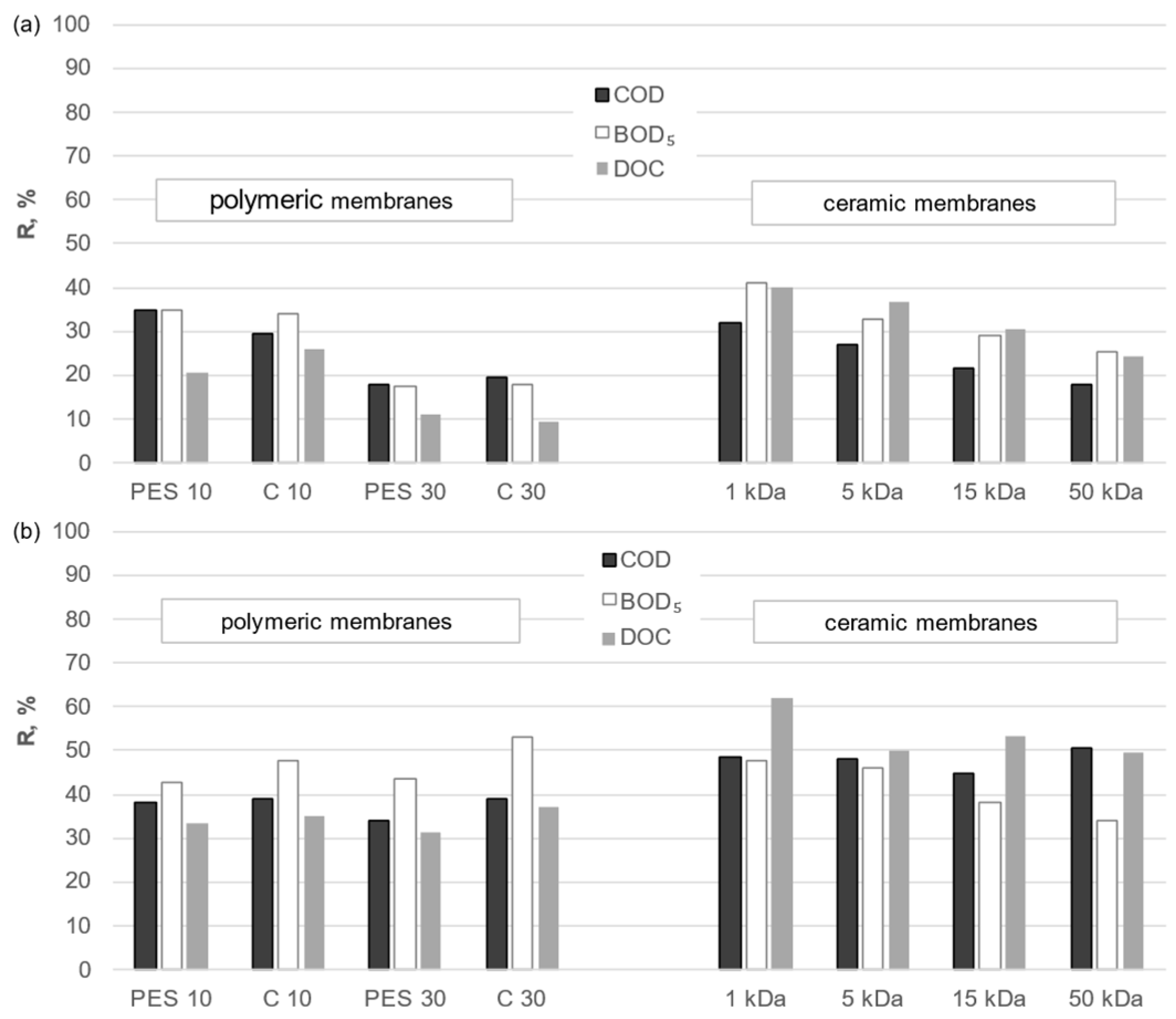
3.2. Separation Properties of Membranes
4. Conclusions
- Polymeric membranes are characterized by higher hydraulic permeability values compared to ceramic membranes.
- Permeate fluxes during membrane filtration (standalone or preceded by pretreatment) of the liquid fraction of agricultural digestate were lower than those measured for the liquid fraction of municipal digestate.
- Inorganic membranes are more susceptible to fouling when used in a standalone ultrafiltration (UF) process, whereas organic membranes are more susceptible in an integrated process.
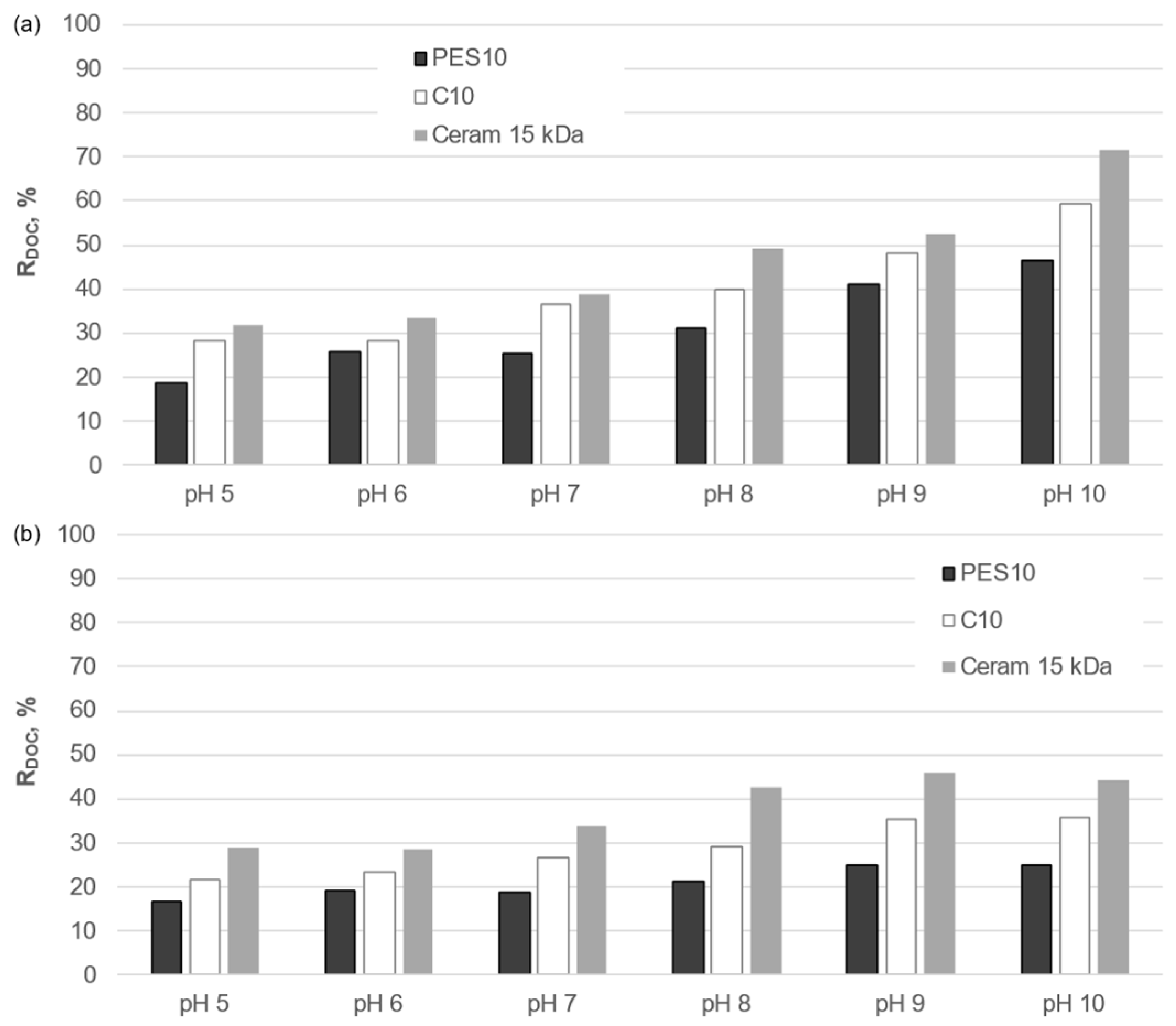
- The removal efficiency of organic macromolecules was significantly higher when using ceramic membranes than polymeric membranes with a comparable cut-off value.
- For both types of membranes tested, an increase in the pH value of the solution resulted in an increase in the intensity of membrane fouling, as well as an improvement in the separation efficiency of organic macromolecules.
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Grafton, R.Q.; Williams, J.; Jiang, Q. Food and Water Gaps to 2050: Preliminary Results from the Global Food and Water System (GFWS) Platform. Food Secur. 2015, 7, 209–220. [Google Scholar] [CrossRef]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Planetary Boundaries: Guiding Human Development on a Changing Planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef]
- Hertwich, E.; Lifset, R.; Pauliuk, S.; Heeren, N. Material Efficiency Strategies for a Low-Carbon Future Summary for Policymakers; UN Environment Programme: Cambridge, UK, 2020. [Google Scholar]
- Franco-García, M.-L.; Carpio-Aguilar, J.C.; Bressers, H. Towards Zero Waste, Circular Economy Boost: Waste to Resources; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–8. [Google Scholar]
- Ma, H.; Guo, Y.; Qin, Y.; Li, Y.-Y. Nutrient Recovery Technologies Integrated with Energy Recovery by Waste Biomass Anaerobic Digestion. Bioresour. Technol. 2018, 269, 520–531. [Google Scholar] [CrossRef]
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popiel, P. The Future of Anaerobic Digestion and Biogas Utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef]
- Drosg, B.; Fuchs, W.; Al Seadi, T.; Madsen, M.; Linke, B. Nutrient Recovery by Biogas Digestate Processing; Tamarind: London, UK, 2015. [Google Scholar]
- Sikora, J.; Sadowska, U.; Klimek-Kopyra, A.; Gliniak, M. The Impact of Biochar Dosing with Simultaneous Fertilization with a Biogas Plant Digestate on the Volumetric Density of Soil. E3S Web Conf. 2019, 132, 02006. [Google Scholar] [CrossRef]
- Kumar, A.; Samadder, S.R. Performance Evaluation of Anaerobic Digestion Technology for Energy Recovery from Organic Fraction of Municipal Solid Waste: A Review. Energy 2020, 197, 117253. [Google Scholar] [CrossRef]
- Hosseini Koupaie, E.; Azizi, A.; Bazyar Lakeh, A.A.; Hafez, H.; Elbeshbishy, E. Comparison of Liquid and Dewatered Digestate as Inoculum for Anaerobic Digestion of Organic Solid Wastes. Waste Manag. 2019, 87, 228–236. [Google Scholar] [CrossRef]
- Carlsson, M.; Lagerkvist, A.; Morgan-Sagastume, F. The Effects of Substrate Pre-Treatment on Anaerobic Digestion Systems: A Review. Waste Manag. 2012, 32, 1634–1650. [Google Scholar] [CrossRef]
- Kovačić, Đ.; Lončarić, Z.; Jović, J.; Samac, D.; Popović, B.; Tišma, M. Digestate Management and Processing Practices: A Review. Appl. Sci. 2022, 12, 9216. [Google Scholar] [CrossRef]
- Baştabak, B.; Koçar, G. A Review of the Biogas Digestate in Agricultural Framework. J. Mater. Cycles Waste Manag. 2020, 22, 1318–1327. [Google Scholar] [CrossRef]
- Nowak, M.; Czekała, W. Sustainable Use of Digestate from Biogas Plants: Separation of Raw Digestate and Liquid Fraction Processing. Sustainability 2024, 16, 5461. [Google Scholar] [CrossRef]
- Makara, A.; Kowalski, Z.; Fela, K. Disposal of After-Fermentation Substance in the Aspect of Ecological Safety. Prace Naukowe Akademii im. Jana Długosza w Częstochowie. Tech. Inform. Inżynieria Bezpieczeństwa 2017, 5, 177–190. [Google Scholar] [CrossRef]
- Żakowicz, A.; Zimiński, K.; Obolewicz, J. Analysis of Structural Solutions of Reinforced Concrete Tanks Used in Agricultural Farms. Inżynieria Bezpieczeństwa Obiektów Antropog. 2023, 1, 22–32. [Google Scholar] [CrossRef]
- Delzeit, R.; Kellner, U. The Impact of Plant Size and Location on Profitability of Biogas Plants in Germany under Consideration of Processing Digestates. Biomass Bioenergy 2013, 52, 43–53. [Google Scholar] [CrossRef]
- Kasprzycka, A.; Lalak, J.; Tys, J.; Chmielewska, M. Selected Methods for Management of Post-Fermentation Sediment. In The Use of Extrusion Processing in Digested Sludge Management (A Review); Acta Agrophysica: Lublin, Poland, 2016; Volume 23. [Google Scholar]
- Yue, C.; Dong, H.; Chen, Y.; Shang, B.; Wang, Y.; Wang, S.; Zhu, Z. Direct Purification of Digestate Using Ultrafiltration Membranes: Influence of Pore Size on Filtration Behavior and Fouling Characteristics. Membranes 2021, 11, 179. [Google Scholar] [CrossRef]
- Baker, R.W. Ultrafiltration. In Membrane Technology and Applications; Wiley: Hoboken, NJ, USA, 2023; pp. 241–286. [Google Scholar]
- Li, X.; Jiang, L.; Li, H. Application of Ultrafiltration Technology in Water Treatment. IOP Conf. Ser. Earth Environ. Sci. 2018, 186, 012009. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.; Sun, R.; Zhang, W. Preparation of Ceramic Membranes and Their Application in Wastewater and Water Treatment. Water 2023, 15, 3344. [Google Scholar] [CrossRef]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-Based Membranes for Water and Wastewater Treatment. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123513. [Google Scholar] [CrossRef]
- Gerardo, M.L.; Aljohani, N.H.M.; Oatley-Radcliffe, D.L.; Lovitt, R.W. Moving towards Sustainable Resources: Recovery and Fractionation of Nutrients from Dairy Manure Digestate Using Membranes. Water Res. 2015, 80, 80–89. [Google Scholar] [CrossRef]
- Chiumenti, R.; Chiumenti, A.; Da Borso, F. Digestate Treatment by Means of a Full Scale Membrane System: An Innovative Method for Managing Surplus Nitrogen and for Valorising Farm Effluents. In Proceedings of the 14th Ramiran International Conference, Lisbon, Portugal, 13–15 September 2010. [Google Scholar]
- Camilleri-Rumbau, M.S.; Norddahl, B.; Wei, J.; Christensen, K.V.; Søtoft, L.F. Microfiltration and Ultrafiltration as a Post-Treatment of Biogas Plant Digestates for Producing Concentrated Fertilizers. Desalination Water Treat 2015, 55, 1639–1653. [Google Scholar] [CrossRef]
- Baird, R.; Eaton, A.D.; Rice, E.W.; Bridgewater, L.; Federation, W.E. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017; ISBN 9780875532875. [Google Scholar]
- MANN+HUMMEL. Water & Fluid Solutions Flat Sheet Membrane Data Sheets. Available online: https://water-membrane-solutions.mann-hummel.com/en/downloads.html (accessed on 12 August 2024).
- Sterlitech. Ceramic Membrane Filters. Available online: https://www.sterlitech.com/media/wysiwyg/pdfs/Sterlitech_Catalog2016_Ceramic_.pdf (accessed on 14 June 2024).
- Urbanowska, A.; Kabsch-Korbutowicz, M. Properties of Flat Ceramic Membranes and Their Application for Municipal Digestate Liquid Fraction Purification. J. Membr. Sci. Res. 2023, 9, 556692. [Google Scholar] [CrossRef]
- Elimelech, M.; Chen, W.H.; Waypa, J.J. Measuring the Zeta (Electrokinetic) Potential of Reverse Osmosis Membranes by a Streaming Potential Analyzer. Desalination 1994, 95, 269–286. [Google Scholar] [CrossRef]






| Liquid Digestate Fraction from the Municipal Waste Biogas Plant | Liquid Digestate Fraction from the Agricultural Biogas Plant | |
|---|---|---|
| pH | 7.17 | 7.21 |
| Conductivity, mS/cm | 25.21 | 14.95 |
| Total suspended solids, mg/dm3 | 670 | 3950 |
| Chemical oxygen demand (COD), mg O2/dm3 | 29,360 | 38,595 |
| 5-day biochemical oxygen demand (BOD5), mg O2/dm3 | 8690 | 12,320 |
| Dissolved organic carbon (DOC), mg C/dm3 | 8650 | 23,070 |
| Na, mg/dm3 | 487.2 | 521.3 |
| K, mg/dm3 | 1678.4 | 1966.5 |
| Ca, mg/dm3 | 89.2 | 104.7 |
| Mg, mg/dm3 | 672.2 | 101.9 |
| Fe, mg/dm3 | 6.2 | 15.9 |
| Mn, mg/dm3 | 4.4 | 1.5 |
| Cu, mg/dm3 | 0.230 | 0.545 |
| Zn, mg/dm3 | 1.434 | 3.977 |
| Hg, mg/dm3 | 0.0040 | 0.0029 |
| Co, mg/dm3 | 0.156 | 0.069 |
| Ni, mg/dm3 | 0.320 | 0.147 |
| Examined Parameter | Method | Apparatus |
|---|---|---|
| pH | Potentiometric method | Digital multimeter HQ40D with IntelliCALTM PHC 101 electrode (Hach, Ames, IA, USA) |
| Conductivity | Conductometric method | |
| Total suspended solids | Weight-based method | - |
| COD | Bichromate method | |
| BOD5 | Dilution method | |
| DOC | NPOC high temperature oxidation method; thermal method | Hach IL550 carbon analyzer (Hach, Ames, IA, USA) |
| Na, K | Ion chromatography method | Thermo Scientific Dionex Aquion ion chromatograph with a conductometric detector for anions or cations analysis (Thermo Fisher Scientific, Waltham, MA, USA) |
| Ca, Mg | Titration method | - |
| Fe, Mn | Spectrophotometric method | Shimadzu UV-VIS 1800 (Shimadzu Corporation, Kyoto, Japan) |
| Cu, Zn, Co, Ni | Atomic absorption spectroscopy (ASA) with flame atomization | Atomic absorption spectrometer iCE 3500 (Thermo Fisher Scientific, Waltham, MA, USA) |
| Hg | Atomic absorption spectroscopy (ASA)—selective for Hg with concentration by amalgamation | AMA 254 mercury analyzer (Leco Corporation, St. Joseph, MI, USA) |
| Membrane Type | Membrane Material | Cut-Off | Max. Press., MPa | Max Temp., °C | pH Range | Active Filtration Area, cm2 | |
|---|---|---|---|---|---|---|---|
| Flat polymeric membranes | |||||||
| PES 10 kDa | UF | Polyethersulfone | 10 kDa | - | 95 | 1–14 | 38.5 |
| PES 30 kDa | UF | 30 kDa | |||||
| C 10 kDa | UF | Regenerated cellulose | 10 kDa | 55 | 1–11 | ||
| C 30 kDa | UF | 30 kDa | |||||
| Flat ceramic membranes | |||||||
| Ceram 1 kDa | FINE UF | TiO2 | 1 kDa | 0.4 | 350 | 2–14 | 56 |
| Ceram 5 kDa | FINE UF | 5 kDa | |||||
| Ceram 15 kDa | UF | ZrO2 | 15 kDa | 0–14 | |||
| Ceram 50 kDa | UF | 50 kDa | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbanowska, A. Purification of Liquid Fraction of Digestates from Different Origins—Comparison of Polymeric and Ceramic Ultrafiltration Membranes Used for This Purpose. Membranes 2024, 14, 203. https://doi.org/10.3390/membranes14100203
Urbanowska A. Purification of Liquid Fraction of Digestates from Different Origins—Comparison of Polymeric and Ceramic Ultrafiltration Membranes Used for This Purpose. Membranes. 2024; 14(10):203. https://doi.org/10.3390/membranes14100203
Chicago/Turabian StyleUrbanowska, Agnieszka. 2024. "Purification of Liquid Fraction of Digestates from Different Origins—Comparison of Polymeric and Ceramic Ultrafiltration Membranes Used for This Purpose" Membranes 14, no. 10: 203. https://doi.org/10.3390/membranes14100203
APA StyleUrbanowska, A. (2024). Purification of Liquid Fraction of Digestates from Different Origins—Comparison of Polymeric and Ceramic Ultrafiltration Membranes Used for This Purpose. Membranes, 14(10), 203. https://doi.org/10.3390/membranes14100203







