Recent Advances and Challenges in Anion Exchange Membranes Development/Application for Water Electrolysis: A Review
Abstract
1. Introduction
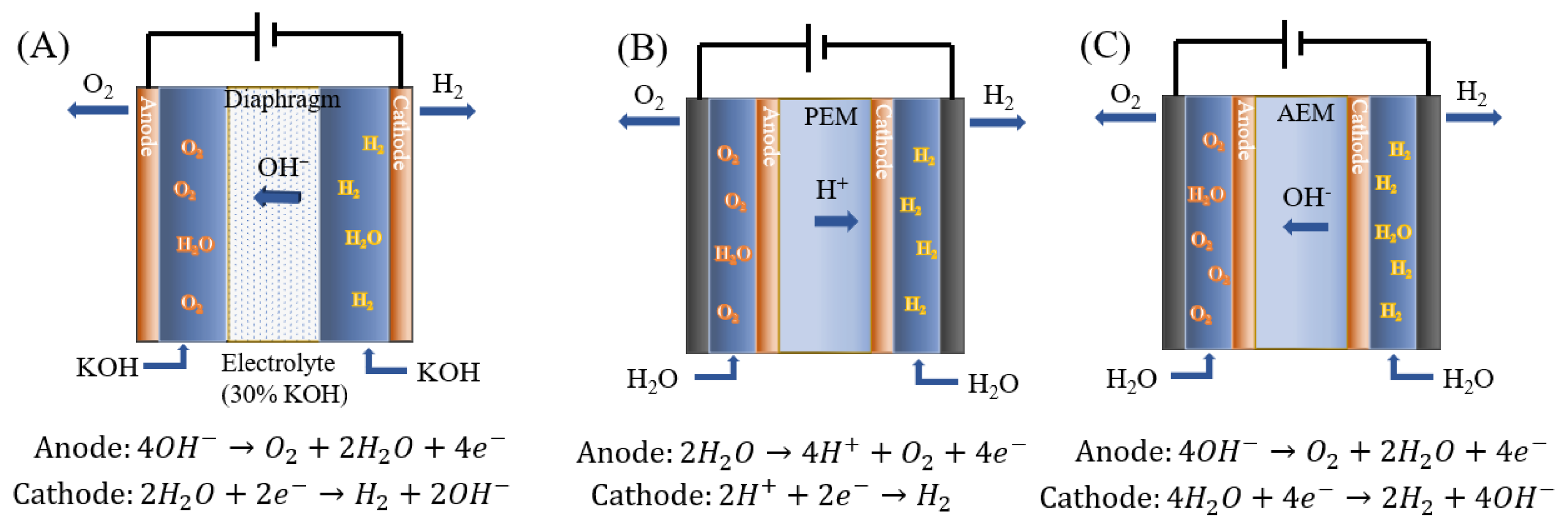
1.1. Water Electrolysis Technology
1.2. Alkaline Water Electrolysis
1.3. PEM Water Electrolysis
1.4. AEM Water Electrolysis
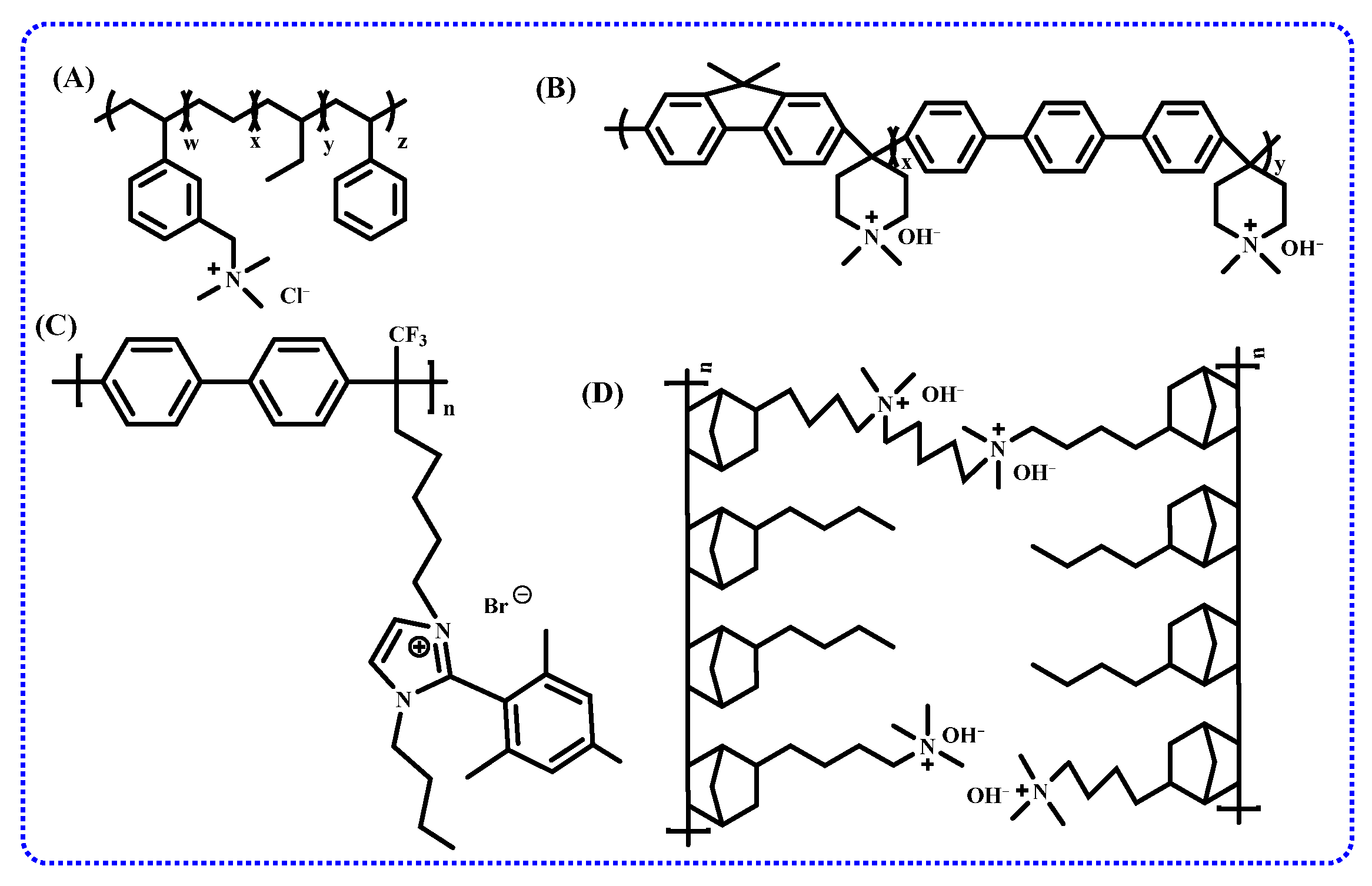
2. Progress in Academic Research of Anion Exchange Membrane
2.1. Degradation Mechanisms
2.2. Strategies to Improve AEM Alkaline Stability
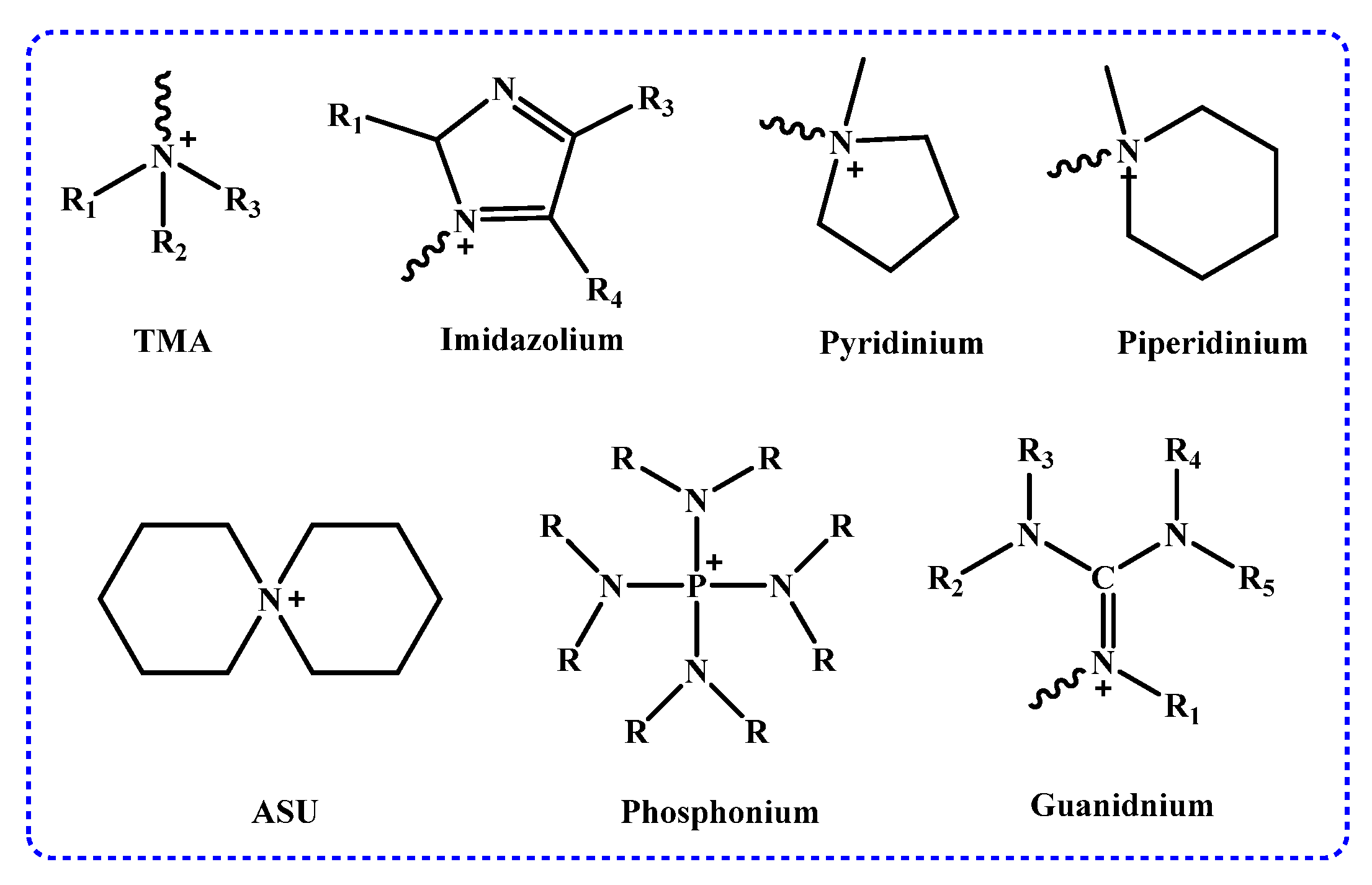
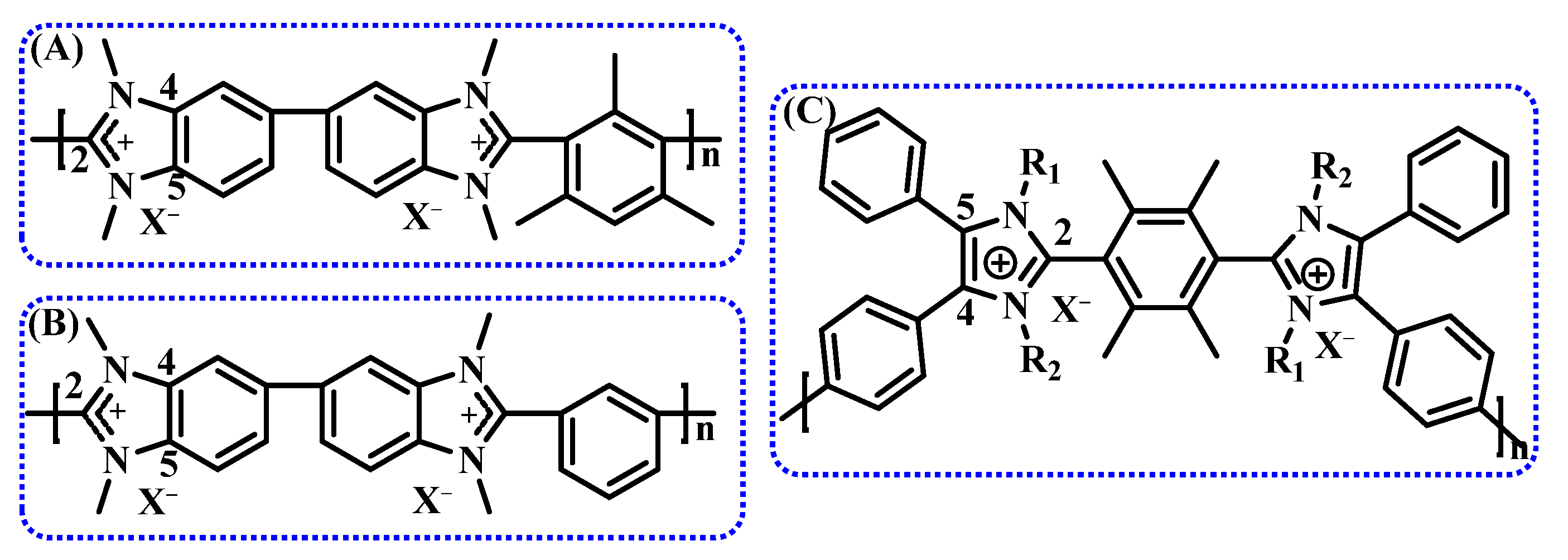
2.2.1. Cationic Functional Groups
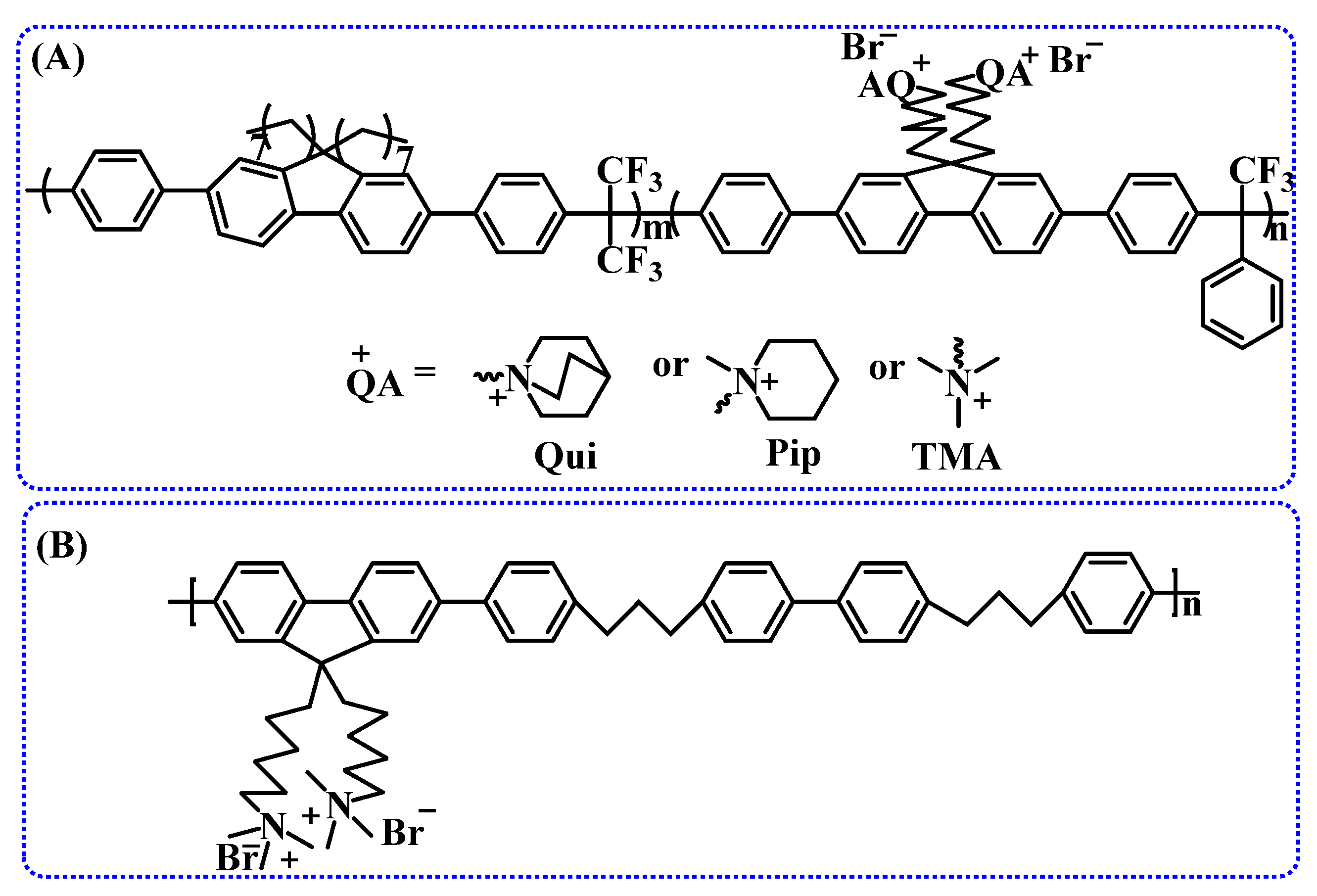
2.2.2. Polymer Backbones
2.3. Ion Conductivity of AEM
2.4. Other Strategies to Enhance AEM Ion Conductivity
2.5. Mechanical Properties of AEM
3. Progress in Patent Research of Anion Exchange Membranes
3.1. Composite Membranes
3.2. Ether-Free Main Chains
3.3. Microphase-Separated Structure
4. Conclusions and Outlook
- Ether-free polyaromatic main chains and N-cyclic quaternary ammonium are expected to meet the stability requirements of AEM.
- Systematic studies of microphase separation structures at the molecular level, using molecular simulations to predict substance transport within the membrane, are likely to advance AEM development.
- AEM still requires sufficient ion exchange capacity to achieve high-performance AEMWE, and the reliability of hydrophilic/hydrophobic microphase separation structures remains crucial.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.A.; Choi, S.; Kim, C.; Yang, J.W.; Kim, S.Y.; Jang, H.W. Si-Based Water Oxidation Photoanodes Conjugated with Earth-Abundant Transition Metal-Based Catalysts. ACS Mater. Lett. 2020, 2, 107–126. [Google Scholar] [CrossRef]
- Rogelj, J.; Schaeffer, M.; Meinshausen, M.; Knutti, R.; Alcamo, J.; Riahi, K.; Hare, W. Zero Emission Targets as Long-Term Global Goals for Climate Protection. Environ. Res. Lett. 2015, 10, 105007. [Google Scholar] [CrossRef]
- Park, Y.S. High-Performance Anion Exchange Membrane Alkaline Seawater Electrolysis. J. Mater. Chem. A 2021, 9, 9586–9592. [Google Scholar] [CrossRef]
- Vincent, I.; Kruger, A.; Bessarabov, D. Development of Efficient Membrane Electrode Assembly for Low Cost Hydrogen Production by Anion Exchange Membrane Electrolysis. Int. J. Hydrogen Energy 2017, 42, 10752–10761. [Google Scholar] [CrossRef]
- Du, N.; Roy, C.; Peach, R.; Turnbull, M.; Thiele, S.; Bock, C. Anion-Exchange Membrane Water Electrolyzers. Chem. Rev. 2022, 122, 11830–11895. [Google Scholar] [CrossRef]
- Lee, B.; Heo, J.; Kim, S.; Sung, C.; Moon, C.; Moon, S.; Lim, H. Economic Feasibility Studies of High Pressure PEM Water Electrolysis for Distributed H2 Refueling Stations. Energy Convers. Manag. 2018, 162, 139–144. [Google Scholar] [CrossRef]
- Villagra, A.; Millet, P. An analysis of PEM water electrolysis cells operating at elevated current densities. Int. J. Hydrogen Energy 2019, 44, 9708–9717. [Google Scholar] [CrossRef]
- Li, Q.; Villarino, A.M.; Peltier, C.R.; Macbeth, A.J.; Yang, Y.; Kim, M.-J.; Shi, Z.; Krumov, M.R.; Lei, C.; Rodríguez-Calero, G.G.; et al. Anion Exchange Membrane Water Electrolysis: The Future of Green Hydrogen. J. Phys. Chem. C 2023, 127, 7901–7912. [Google Scholar] [CrossRef]
- Lopez, V.M.; Ziar, H.; Haverkort, J.W.; Zeman, M.; Isabella, O. Dynamic operation of water electrolyzers: A review for applications in photovoltaic systems integration. Renew. Sustain. Energy Rev. 2023, 182, 113407. [Google Scholar] [CrossRef]
- Yang, B.; Cunman, Z. Progress in Constructing High-Performance Anion Exchange Membrane: Molecular Design, Microphase Controllability and In-Device Property. Chem. Eng. J. 2023, 457, 141094. [Google Scholar] [CrossRef]
- Singh, M.R.; Xiang, C.; Lewis, N.S. Evaluation of Flow Schemes for Near-Neutral pH Electrolytes in Solar-Fuel Generators. Sustain. Energy Fuels 2017, 1, 458–466. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water Electrolysis Based on Renewable Energy for Hydrogen Production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Tong, W.; Forster, M.; Dionigi, F.; Dresp, S.; Sadeghi Erami, R.; Strasser, P.; Cowan, A.J.; Farràs, P. Electrolysis of Low-Grade and Saline Surface Water. Nat. Energy 2020, 5, 367–377. [Google Scholar] [CrossRef]
- Santoro, C.; Lavacchi, A.; Mustarelli, P.; Di Noto, V.; Elbaz, L.; Dekel, D.R.; Jaouen, F. What Is Next in Anion-Exchange Membrane Water Electrolyzers? Bottlenecks, Benefits, and Future. ChemSusChem 2022, 15, e202200027. [Google Scholar] [CrossRef]
- Hagesteijn, K.F.L.; Jiang, S.; Ladewig, B.P. A Review of the Synthesis and Characterization of Anion Exchange Membranes. J. Mater. Sci. 2018, 53, 11131–11150. [Google Scholar] [CrossRef]
- Buttler, A.; Spliethoff, H. Current Status of Water Electrolysis for Energy Storage, Grid Balancing and Sector Coupling via Power-to-Gas and Power-to-Liquids: A Review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Kjartansdóttir, C.K.; Nielsen, L.P.; Møller, P. Development of Durable and Efficient Electrodes for Large-Scale Alkaline Water Electrolysis. Int. J. Hydrogen Energy 2013, 38, 8221–8231. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A Comprehensive Review on PEM Water Electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Leng, Y.; Chen, G.; Mendoza, A.J.; Tighe, T.B.; Hickner, M.A.; Wang, C.-Y. Solid-State Water Electrolysis with an Alkaline Membrane. J. Am. Chem. Soc. 2012, 134, 9054–9057. [Google Scholar] [CrossRef]
- Cousins, I.T.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Ng, C.A.; Patton, S.; Scheringer, M.; Trier, X.; Vierke, L.; et al. The Concept of Essential Use for Determining When Uses of PFASs Can Be Phased Out. Environ. Sci. Process. Impacts 2019, 21, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Vincent, I. Low Cost Hydrogen Production by Anion Exchange Membrane Electrolysis: A Review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; et al. Anion-Exchange Membranes in Electrochemical Energy Systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef]
- Vinodh, R.; Kalanur, S.S.; Natarajan, S.K.; Pollet, B.G. Recent Advancements of Polymeric Membranes in Anion Exchange Membrane Water Electrolyzer (AEMWE): A Critical Review. Polymers 2023, 15, 2144. [Google Scholar] [CrossRef]
- Babic, U.; Suermann, M.; Büchi, F.N.; Gubler, L.; Schmidt, T.J. Critical Review—Identifying Critical Gaps for Polymer Electrolyte Water Electrolysis Development. J. Electrochem. Soc. 2017, 164, F387–F399. [Google Scholar] [CrossRef]
- Serov, A.; Kovnir, K.; Shatruk, M.; Kolen’ko, Y.V. Critical Review of Platinum Group Metal-Free Materials for Water Electrolysis: Transition from the Laboratory to the Market: Earth-Abundant Borides and Phosphides as Catalysts for Sustainable Hydrogen Production. Johns. Matthey Technol. Rev. 2021, 65, 207–226. [Google Scholar] [CrossRef]
- Henkensmeier, D.; Najibah, M.; Harms, C.; Žitka, J.; Hnát, J.; Bouzek, K. Overview: State-of-the art commercial membranes for anion exchange membrane water electrolysis. J. Electrochem. Energy Convers. Storage 2021, 18, 024001. [Google Scholar] [CrossRef]
- Li, C.; Baek, J.B. The promise of hydrogen production from alkaline anion exchange membrane electrolyzers. Nano Energy 2021, 87, 106162. [Google Scholar] [CrossRef]
- Marini, S.; Salvi, P.; Nelli, P.; Pesenti, R.; Villa, M.; Berrettoni, M.; Zangari, G.; Kiros, Y. Advanced alkaline water electrolysis. Electrochim. Acta 2012, 82, 384–391. [Google Scholar] [CrossRef]
- Miller, H.A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernäcker, C.I.; Weissgaerber, T.; Röntzsch, L.; Meier-Haack, J. Green hydrogen from anion exchange membrane water electrolysis: A review of recent developments in critical materials and operating conditions. Sustain. Energy Fuels 2020, 4, 2114–2133. [Google Scholar] [CrossRef]
- Jin, H.; Ruqia, B.; Park, Y.; Kim, H.J.; Oh, H.; Choi, S.; Lee, K. Nanocatalyst Design for Long-Term Operation of Proton/Anion Exchange Membrane Water Electrolysis. Adv. Energy Mater. 2021, 11, 2003188. [Google Scholar] [CrossRef]
- Changkhamchom, S.; Kunanupatham, P.; Phasuksom, K.; Sirivat, A. Anion Exchange Membranes Composed of Quaternized Polybenzimidazole and Quaternized Graphene Oxide for Glucose Fuel Cell. Int. J. Hydrogen Energy 2021, 46, 5642–5652. [Google Scholar] [CrossRef]
- Lin, J. Thermoplastic Interpenetrating Polymer Networks Based on Polybenzimidazole and Poly (1, 2-Dimethy-3-Allylimidazolium) for Anion Exchange Membranes. Electrochim. Acta 2017, 257, 9–19. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, Z.; Tian, H.; Wang, S.; Zhang, B.; Cao, Y.; He, G.; Li, Z.; Wu, H. Preparing Alkaline Anion Exchange Membrane with Enhanced Hydroxide Conductivity via Blending Imidazolium-Functionalized and Sulfonated Poly(Ether Ether Ketone). J. Power Sources 2015, 288, 384–392. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Q.; Wang, C.; Lee, Y.M.; Guiver, M.D. Phenyltrimethylammonium Functionalized Polysulfone Anion Exchange Membranes. Macromolecules 2012, 45, 2411–2419. [Google Scholar] [CrossRef]
- Serbanescu, O.S.; Voicu, S.I.; Thakur, V.K. Polysulfone Functionalized Membranes: Properties and Challenges. Mater. Today Chem. 2020, 17, 100302. [Google Scholar] [CrossRef]
- Chen, W.; Hu, M.; Wang, H.; Wu, X.; Gong, X.; Yan, X.; Zhen, D.; He, G. Dimensionally Stable Hexamethylenetetramine Functionalized Polysulfone Anion Exchange Membranes. J. Mater. Chem. A 2017, 5, 15038–15047. [Google Scholar] [CrossRef]
- Mohanty, A.D.; Ryu, C.Y.; Kim, Y.S.; Bae, C. Stable Elastomeric Anion Exchange Membranes Based on Quaternary Ammonium-Tethered Polystyrene-b-Poly(Ethylene-Co-Butylene)-b-Polystyrene Triblock Copolymers. Macromolecules 2015, 48, 7085–7095. [Google Scholar] [CrossRef]
- Tuli, S.K.; Roy, A.L.; Elgammal, R.A.; Zawodzinski, T.A.; Fujiwara, T. Polystyrene-based Anion Exchange Membranes via Click Chemistry: Improved Properties and AEM Performance. Polym. Int. 2018, 67, 1302–1312. [Google Scholar] [CrossRef]
- Jheng, L.-C.; Hsu, C.-Y.; Yeh, H.-Y. Anion Exchange Membranes Based on Imidazoline Quaternized Polystyrene Copolymers for Fuel Cell Applications. Membranes 2021, 11, 901. [Google Scholar] [CrossRef]
- Tongwen, X.; Weihua, Y. Fundamental Studies of a New Series of Anion Exchange Membranes: Membrane Preparation and Characterization. J. Membr. Sci. 2001, 190, 159–166. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, J.; Liang, X.; Ge, X.; Wei, C.; Ge, Z.; Zhang, K.; Li, G.; Song, W.; Shehzad, M.A.; et al. Anion Exchange Membranes with Fast Ion Transport Channels Driven by Cation-Dipole Interactions for Alkaline Fuel Cells. J. Membr. Sci. 2021, 634, 119404. [Google Scholar] [CrossRef]
- Zhu, L.; Pan, J.; Christensen, C.M.; Lin, B.; Hickner, M.A. Functionalization of Poly(2,6-Dimethyl-1,4-Phenylene Oxide)s with Hindered Fluorene Side Chains for Anion Exchange Membranes. Macromolecules 2016, 49, 3300–3309. [Google Scholar] [CrossRef]
- Wang, C.; Mo, B.; He, Z.; Shao, Q.; Pan, D.; Wujick, E.; Guo, J.; Xie, X.; Xie, X.; Guo, Z. Crosslinked Norbornene Copolymer Anion Exchange Membrane for Fuel Cells. J. Membr. Sci. 2018, 556, 118–125. [Google Scholar] [CrossRef]
- Alsaiari, N.S.; Katubi, K.M.; Alzahrani, F.M.; Amari, A.; Osman, H.; Rebah, F.B.; Tahoon, M.A. Synthesis, Characterization and Application of Polypyrrole Functionalized Nanocellulose for the Removal of Cr(VI) from Aqueous Solution. Polymers 2021, 13, 3691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, W.; Liang, X.; Zhang, K.; Wang, H.; Ge, X.; Wei, C.; Song, W.; Ge, Z.; Wu, L.; et al. Flexible Bis-Piperidinium Side Chains Construct Highly Conductive and Robust Anion-Exchange Membranes. ACS Appl. Energy Mater. 2021, 4, 9701–9711. [Google Scholar] [CrossRef]
- Wu, X.; Chen, N.; Hu, C.; Klok, H.; Lee, Y.M.; Hu, X. Fluorinated Poly(Aryl Piperidinium) Membranes for Anion Exchange Membrane Fuel Cells. Adv. Mater. 2023, 35, 2210432. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Q.; Afsar, N.U.; Ge, L.; Xu, T. Poly(Alkyl-Biphenyl Pyridinium)-Based Anion Exchange Membranes with Alkyl Side Chains Enable High Anion Permselectivity and Monovalent Ion Flux. Membranes 2023, 13, 188. [Google Scholar] [CrossRef]
- Pham, T.H.; Jannasch, P. Aromatic Polymers Incorporating Bis-N-spirocyclic Quaternary Ammonium Moieties for Anion-Exchange Membranes. ACS Macro Lett. 2015, 4, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.-S. Alkali-Stable and Highly Anion Conducting Poly(Phenylene Oxide)s Carrying Quaternary Piperidinium Cations. J. Mater. Chem. A 2016, 4, 11924–11938. [Google Scholar] [CrossRef]
- Shang, L.; Yao, D.; Pang, B.; Zhao, C. Anion Exchange Membranes Based on Poly (Ether Ether Ketone) Containing N-Spirocyclic Quaternary Ammonium Cations in Phenyl Side Chain. Int. J. Hydrogen Energy 2021, 46, 19116–19128. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.; Li, T.; Yan, X.; Zhang, F.; Wang, X.; Wu, X.; Pang, B.; He, G. Tuning Hydrogen Bond and Flexibility of N-Spirocyclic Cationic Spacer for High Performance Anion Exchange Membranes. J. Membr. Sci. 2020, 613, 118507. [Google Scholar] [CrossRef]
- Noonan, K.J.T.; Hugar, K.M.; Kostalik, H.A.; Lobkovsky, E.B.; Abruña, H.D.; Coates, G.W. Phosphonium-Functionalized Polyethylene: A New Class of Base-Stable Alkaline Anion Exchange Membranes. J. Am. Chem. Soc. 2012, 134, 18161–18164. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J. Chemically Durable Polymer Electrolytes for Solid-State Alkaline Water Electrolysis. J. Power Sources. 2017, 375, 367–372. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Qiao, J.; Baker, R.; Zhang, J. Alkaline Polymer Electrolyte Membranes for Fuel Cell Applications. Chem. Soc. Rev. 2013, 42, 5768. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Hao, J.; Cheng, J.; Zhang, N.; He, G.; Zhang, F.; Hao, C. Hydroxide Ion Transfer in Anion Exchange Membrane: A Density Functional Theory Study. Int. J. Hydrogen Energy 2016, 41, 6877–6884. [Google Scholar] [CrossRef]
- Marino, M.G.; Kreuer, K.D. Alkaline Stability of Quaternary Ammonium Cations for Alkaline Fuel Cell Membranes and Ionic Liquids. ChemSusChem 2015, 8, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Haj-Bsoul, S.; Varcoe, J.R.; Dekel, D.R. Measuring the Alkaline Stability of Anion-Exchange Membranes. J. Electroanal. Chem. 2022, 908, 116112. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, J.; Zhang, K.; Ma, L.; Qaisrani, N.A.; Zhang, F.; He, G. Hybrid Anion Exchange Membrane of Hydroxyl-Modified Polysulfone Incorporating Guanidinium-Functionalized Graphene Oxide. Ionics 2017, 23, 3085–3096. [Google Scholar] [CrossRef]
- Carbone, A.; Pedicini, R.; Gatto, I.; Saccà, A.; Patti, A.; Bella, G.; Cordaro, M. Development of Polymeric Membranes Based on Quaternized Polysulfones for AMFC Applications. Polymers 2020, 12, 283. [Google Scholar] [CrossRef]
- Zuo, X.; Chang, K.; Zhao, J.; Xie, Z.; Tang, H.; Li, B.; Chang, Z. Bubble-Template-Assisted Synthesis of Hollow Fullerene-like MoS2 Nanocages as a Lithium Ion Battery Anode Material. J. Mater. Chem. A 2016, 4, 51–58. [Google Scholar] [CrossRef]
- Zuo, P.; Xu, Z.; Zhu, Q.; Ran, J.; Ge, L.; Ge, X.; Wu, L.; Yang, Z.; Xu, T. Ion Exchange Membranes: Constructing and Tuning Ion Transport Channels. Adv. Funct. Mater. 2022, 32, 2207366. [Google Scholar] [CrossRef]
- Arges, C.G.; Ramani, V. Two-Dimensional NMR Spectroscopy Reveals Cation-Triggered Backbone Degradation in Polysulfone-Based Anion Exchange Membranes. Proc. Natl. Acad. Sci. USA 2013, 110, 2490–2495. [Google Scholar] [CrossRef]
- Mahmoud, A.M.A.; Elsaghier, A.M.M.; Otsuji, K.; Miyatake, K. High Hydroxide Ion Conductivity with Enhanced Alkaline Stability of Partially Fluorinated and Quaternized Aromatic Copolymers as Anion Exchange Membranes. Macromolecules 2017, 50, 4256–4266. [Google Scholar] [CrossRef]
- Hibbs, M.R. Alkaline Stability of Poly(Phenylene)-based Anion Exchange Membranes with Various Cations. J. Polym. Sci. B Polym. Phys. 2013, 51, 1736–1742. [Google Scholar] [CrossRef]
- Olsson, J.S.; Pham, T.H.; Jannasch, P. Poly(Arylene Piperidinium) Hydroxide Ion Exchange Membranes: Synthesis, Alkaline Stability, and Conductivity. Adv. Funct. Mater. 2018, 28, 1702758. [Google Scholar] [CrossRef]
- Aili, D.; Wright, A.G.; Kraglund, M.R.; Jankova, K.; Holdcroft, S.; Jensen, J.O. Towards a Stable Ion-Solvating Polymer Electrolyte for Advanced Alkaline Water Electrolysis. J. Mater. Chem. A 2017, 5, 5055–5066. [Google Scholar] [CrossRef]
- Liu, L.; Li, Q.; Dai, J.; Wang, H.; Jin, B.; Bai, R. A Facile Strategy for the Synthesis of Guanidinium-Functionalized Polymer as Alkaline Anion Exchange Membrane with Improved Alkaline Stability. J. Membr. Sci. 2014, 453, 52–60. [Google Scholar] [CrossRef]
- Xue, B.; Dong, X.; Li, Y.; Zheng, J.; Li, S.; Zhang, S. Synthesis of Novel Guanidinium-Based Anion-Exchange Membranes with Controlled Microblock Structures. J. Membr. Sci. 2017, 537, 151–159. [Google Scholar] [CrossRef]
- Fan, J.; Willdorf-Cohen, S.; Schibli, E.M.; Paula, Z.; Li, W.; Skalski, T.J.G.; Sergeenko, A.T.; Hohenadel, A.; Frisken, B.J.; Magliocca, E.; et al. Poly(Bis-Arylimidazoliums) Possessing High Hydroxide Ion Exchange Capacity and High Alkaline Stability. Nat. Commun. 2019, 10, 2306. [Google Scholar] [CrossRef]
- Fan, J.; Wright, A.G.; Britton, B.; Weissbach, T.; Skalski, T.J.G.; Ward, J.; Peckham, T.J.; Holdcroft, S. Cationic Polyelectrolytes, Stable in 10 M KOHaq at 100 °C. ACS Macro Lett. 2017, 6, 1089–1093. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, L.; Zhang, G.; Li, R.; Hu, X.; Chang, X.; Shen, Y.; Liu, L.; Li, N. Rational Design of Comb-Shaped Poly(Arylene Indole Piperidinium) to Enhance Hydroxide Ion Transport for H2/O2 Fuel Cell. J. Membr. Sci. 2021, 631, 119335. [Google Scholar] [CrossRef]
- Hu, E.N.; Lin, C.X.; Liu, F.H.; Wang, X.Q.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Poly(Arylene Ether Nitrile) Anion Exchange Membranes with Dense Flexible Ionic Side Chain for Fuel Cells. J. Membr. Sci. 2018, 550, 254–265. [Google Scholar] [CrossRef]
- Akiyama, R.; Yokota, N.; Miyatake, K. Chemically Stable, Highly Anion Conductive Polymers Composed of Quinquephenylene and Pendant Ammonium Groups. Macromolecules 2019, 52, 2131–2138. [Google Scholar] [CrossRef]
- Lin, C.; Liu, X.; Yang, Q.; Wu, H.; Liu, F.; Zhang, Q.; Zhu, A.; Liu, Q. Hydrophobic Side Chains to Enhance Hydroxide Conductivity and Physicochemical Stabilities of Side-Chain-Type Polymer AEMs. J. Membr. Sci. 2019, 585, 90–98. [Google Scholar] [CrossRef]
- Long, H.; Kim, K.; Pivovar, B.S. Hydroxide Degradation Pathways for Substituted Trimethylammonium Cations: A DFT Study. J. Phys. Chem. C 2012, 116, 9419–9426. [Google Scholar] [CrossRef]
- Chen, J.; Li, C.; Wang, J.; Li, L.; Wei, Z. A General Strategy to Enhance the Alkaline Stability of Anion Exchange Membranes. J. Mater. Chem. A 2017, 5, 6318–6327. [Google Scholar] [CrossRef]
- Koronka, D.; Miyatake, K. Anion Exchange Membranes Containing No β-Hydrogen Atoms on Ammonium Groups: Synthesis, Properties, and Alkaline Stability. RSC Adv. 2021, 11, 1030–1038. [Google Scholar] [CrossRef]
- Thomas, O.D.; Soo, K.J.W.Y.; Peckham, T.J.; Kulkarni, M.P.; Holdcroft, S. A Stable Hydroxide-Conducting Polymer. J. Am. Chem. Soc. 2012, 134, 10753–10756. [Google Scholar] [CrossRef]
- Wright, A.G.; Fan, J.; Britton, B.; Weissbach, T.; Lee, H.-F.; Kitching, E.A.; Peckham, T.J.; Holdcroft, S. Hexamethyl-p-Terphenyl Poly(Benzimidazolium): A Universal Hydroxide-Conducting Polymer for Energy Conversion Devices. Energy Environ. Sci. 2016, 9, 2130–2142. [Google Scholar] [CrossRef]
- Hugar, K.M.; Kostalik, H.A.; Coates, G.W. Imidazolium Cations with Exceptional Alkaline Stability: A Systematic Study of Structure–Stability Relationships. J. Am. Chem. Soc. 2015, 137, 8730–8737. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.; Yan, X.; Zhang, F.; Wang, X.; Wu, X.; Pang, B.; Wang, J.; He, G. Ether Spaced N-Spirocyclic Quaternary Ammonium Functionalized Crosslinked Polysulfone for High Alkaline Stable Anion Exchange Membranes. J. Membr. Sci. 2020, 598, 117650. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, J.; Wang, S.; Hou, J.; Wu, L.; Xu, T. A Strategy to Construct Alkali-Stable Anion Exchange Membranes Bearing Ammonium Groups via Flexible Spacers. J. Mater. Chem. A 2015, 3, 15015–15019. [Google Scholar] [CrossRef]
- Olsson, J.S.; Pham, T.H.; Jannasch, P. Poly(N,N-Diallylazacycloalkane)s for Anion-Exchange Membranes Functionalized with N-Spirocyclic Quaternary Ammonium Cations. Macromolecules 2017, 50, 2784–2793. [Google Scholar] [CrossRef]
- Tao, Z.; Wang, C.; Zhao, X.; Li, J.; Guiver, M.D. Progress in High-Performance Anion Exchange Membranes Based on the Design of Stable Cations for Alkaline Fuel Cells. Adv. Mater. Technol. 2021, 6, 2001220. [Google Scholar] [CrossRef]
- Choe, Y.-K.; Fujimoto, C.; Lee, K.-S.; Dalton, L.T.; Ayers, K.; Henson, N.J.; Kim, Y.S. Alkaline Stability of Benzyl Trimethyl Ammonium Functionalized Polyaromatics: A Computational and Experimental Study. Chem. Mater. 2014, 26, 5675–5682. [Google Scholar] [CrossRef]
- Meek, K.M.; Antunes, C.M.; Strasser, D.; Owczarczyk, Z.R.; Neyerlin, A.; Pivovar, B.S. High-Throughput Anion Exchange Membrane Characterization at NREL. ECS Trans. 2019, 92, 723–731. [Google Scholar] [CrossRef]
- Pan, D.; Olsson, J.S.; Jannasch, P. Poly(Fluorene Alkylene) Anion Exchange Membranes with Pendant Spirocyclic and Bis-Spirocyclic Quaternary Ammonium Cations. ACS Appl. Energy Mater. 2022, 5, 981–991. [Google Scholar] [CrossRef]
- Soni, R.; Miyanishi, S.; Kuroki, H.; Yamaguchi, T. Pure Water Solid Alkaline Water Electrolyzer Using Fully Aromatic and High-Molecular-Weight Poly(Fluorene-Alt-Tetrafluorophenylene)-Trimethyl Ammonium Anion Exchange Membranes and Ionomers. ACS Appl. Energy Mater. 2021, 4, 1053–1058. [Google Scholar] [CrossRef]
- Xu, H.; Hu, X. Preparation of Anion Exchangers by Reductive Amination of Acetylated Crosslinked Polystyrene. React. Funct. Polym. 1999, 42, 235–242. [Google Scholar] [CrossRef]
- Lee, W.-H.; Kim, Y.S.; Bae, C. Robust Hydroxide Ion Conducting Poly(Biphenyl Alkylene)s for Alkaline Fuel Cell Membranes. ACS Macro Lett. 2015, 4, 814–818. [Google Scholar] [CrossRef]
- Lim, H.; Jeong, I.; Choi, J.; Shin, G.; Kim, J.; Kim, T.-H.; Park, T. Anion Exchange Membranes and Ionomer Properties of a Polyfluorene-Based Polymer with Alkyl Spacers for Water Electrolysis. Appl. Surf. Sci. 2023, 610, 155601. [Google Scholar] [CrossRef]
- Kraglund, M.R.; Carmo, M.; Schiller, G.; Ansar, S.A.; Aili, D.; Christensen, E.; Jensen, J.O. Ion-solvating membranes as a new approach towards high rate alkaline electrolyzers. Energy Environ. Sci. 2019, 12, 3313–3318. [Google Scholar] [CrossRef]
- Jung, J.; Park, Y.S.; Hwang, D.J.; Choi, G.H.; Choi, D.H.; Park, H.J.; Ahn, C.-H.; Hwang, S.S.; Lee, A.S. Polydiallylammonium interpenetrating cationic network ion-solvating membranes for anion exchange membrane water electrolyzers. J. Mater. Chem. A 2023, 11, 10891–10900. [Google Scholar] [CrossRef]
- Li, D.; Motz, A.R.; Bae, C.; Fujimoto, C.; Yang, G.; Zhang, F.-Y.; Ayers, K.E.; Kim, Y.S. Durability of anion exchange membrane water electrolyzers. Energy Environ. Sci. 2021, 14, 3393–3419. [Google Scholar] [CrossRef]
- Jensen, J.O.; Kraglund, M.R.; Gellrich, F.; Serhiichuk, D.; Xia, Y.; Chatzichristodoulou, C.; Li, Q.; Aili, D. Perspectives of ion-solvating membranes for alkaline electrolysis. In 3rd International Conference on Electrolysis; Colorado School of Mines: Golden, CO, USA, 2021; Volume 2022, p. 108. [Google Scholar]
- Hu, X.; Liu, M.; Huang, Y.; Liu, L.; Li, N. Sulfonate-functionalized polybenzimidazole as ion-solvating membrane toward high-performance alkaline water electrolysis. J. Membr. Sci. 2022, 663, 121005. [Google Scholar] [CrossRef]
- Allushi, A.; Pham, T.H.; Olsson, J.S.; Jannasch, P. Ether-Free Polyfluorenes Tethered with Quinuclidinium Cations as Hydroxide Exchange Membranes. J. Mater. Chem. A 2019, 7, 27164–27174. [Google Scholar] [CrossRef]
- Pérez-Prior, M.T.; Várez, A.; Levenfeld, B. Synthesis and characterization of benzimidazolium-functionalized polysulfones as anion-exchange membranes. J. Polym. Sci. A Polym. Chem. 2015, 53, 2363–2373. [Google Scholar] [CrossRef]
- Guo, D.; Lai, A.N.; Lin, C.X.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Imidazolium-Functionalized Poly(Arylene Ether Sulfone) Anion-Exchange Membranes Densely Grafted with Flexible Side Chains for Fuel Cells. ACS Appl. Mater. Interfaces 2016, 8, 25279–25288. [Google Scholar] [CrossRef]
- Wang, X.; Lammertink, R.G.H. Dimensionally Stable Multication-Crosslinked Poly(Arylene Piperidinium) Membranes for Water Electrolysis. J. Mater. Chem. A 2022, 10, 8401–8412. [Google Scholar] [CrossRef]
- Du, X.; Wang, Z.; Zhang, H.; Liu, W.; Xu, J. Constructing Micro-Phase Separation Structure by Multi-Arm Side Chains to Improve the Property of Anion Exchange Membrane. Int. J. Hydrogen Energy 2020, 45, 17916–17926. [Google Scholar] [CrossRef]
- Weiber, E.A.; Jannasch, P. Anion-Conducting Polysulfone Membranes Containing Hexa-Imidazolium Functionalized Biphenyl Units. J. Membr. Sci. 2016, 520, 425–433. [Google Scholar] [CrossRef]
- Hou, J.; Wang, X.; Liu, Y.; Ge, Q.; Yang, Z.; Wu, L.; Xu, T. Wittig Reaction Constructed an Alkaline Stable Anion Exchange Membrane. J. Membr. Sci. 2016, 518, 282–288. [Google Scholar] [CrossRef]
- Dang, H.-S.; Weiber, E.A.; Jannasch, P. Poly(Phenylene Oxide) Functionalized with Quaternary Ammonium Groups via Flexible Alkyl Spacers for High-Performance Anion Exchange Membranes. J. Mater. Chem. A 2015, 3, 5280–5284. [Google Scholar] [CrossRef]
- Lin, C.X.; Huang, X.L.; Guo, D.; Zhang, Q.G.; Zhu, A.M.; Ye, M.L.; Liu, Q.L. Side-Chain-Type Anion Exchange Membranes Bearing Pendant Quaternary Ammonium Groups via Flexible Spacers for Fuel Cells. J. Mater. Chem. A 2016, 4, 13938–13948. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, P.; Shi, Q.; Li, S.; Gong, F.; Chen, X.; An, Z. Block Poly(Arylene Ether Sulfone) Copolymers Bearing Quaterinized Aromatic Pendants: Synthesis, Property and Stability. Int. J. Hydrogen Energy 2017, 42, 26320–26332. [Google Scholar] [CrossRef]
- Tanaka, M.; Fukasawa, K.; Nishino, E.; Yamaguchi, S.; Yamada, K.; Tanaka, H.; Bae, B.; Miyatake, K.; Watanabe, M. Anion Conductive Block Poly(Arylene Ether)s: Synthesis, Properties, and Application in Alkaline Fuel Cells. J. Am. Chem. Soc. 2011, 133, 10646–10654. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.W.; Jung, J.; Choi, Y.-M.; Choi, J.H.; Yu, J.; Lee, J.K.; You, N.-H.; Goh, M. Enhancement of the Crosslink Density, Glass Transition Temperature, and Strength of Epoxy Resin by Using Functionalized Graphene Oxide Co-Curing Agents. Polym. Chem. 2016, 7, 36–43. [Google Scholar] [CrossRef]
- Chen, X.; Zhan, Y.; Tang, J.; Yang, X.; Sun, A.; Lin, B.; Zhu, F.; Jia, H.; Lei, X. Advances in High Performance Anion Exchange Membranes: Molecular Design, Preparation Methods, and Ion Transport Dynamics. J. Environ. Chem. Eng. 2023, 11, 110749. [Google Scholar] [CrossRef]
- Lee, K.H.; Chu, J.Y.; Kim, A.R.; Kim, H.G.; Yoo, D.J. Functionalized TiO2 Mediated Organic-Inorganic Composite Membranes Based on Quaternized Poly(Arylene Ether Ketone) with Enhanced Ionic Conductivity and Alkaline Stability for Alkaline Fuel Cells. J. Membr. Sci. 2021, 634, 119435. [Google Scholar] [CrossRef]
- Msomi, P.F.; Nonjola, P.T.; Ndungu, P.G.; Ramontja, J. Poly (2, 6-Dimethyl-1, 4-Phenylene)/Polysulfone Anion Exchange Membrane Blended with TiO2 with Improved Water Uptake for Alkaline Fuel Cell Application. Int. J. Hydrogen Energy 2020, 45, 29465–29476. [Google Scholar] [CrossRef]
- Chu, J.Y.; Lee, K.H.; Kim, A.R.; Yoo, D.J. Graphene-Mediated Organic-Inorganic Composites with Improved Hydroxide Conductivity and Outstanding Alkaline Stability for Anion Exchange Membranes. Compos. B Eng. 2019, 164, 324–332. [Google Scholar] [CrossRef]
- Shukla, G.; Shahi, V.K. Sulfonated Poly(Ether Ether Ketone)/Imidized Graphene Oxide Composite Cation Exchange Membrane with Improved Conductivity and Stability for Electrodialytic Water Desalination. Desalination 2019, 451, 200–208. [Google Scholar] [CrossRef]
- Yu, B.-C.; Wang, Y.-C.; Lu, H.-C.; Lin, H.-L.; Shih, C.-M.; Kumar, S.R.; Lue, S.J. Hydroxide-Ion Selective Electrolytes Based on a Polybenzimidazole/Graphene Oxide Composite Membrane. Energy 2017, 134, 802–812. [Google Scholar] [CrossRef]
- Gahlot, S.; Kulshrestha, V. Dramatic Improvement in Water Retention and Proton Conductivity in Electrically Aligned Functionalized CNT/SPEEK Nanohybrid PEM. ACS Appl. Mater. Interfaces 2015, 7, 264–272. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, C.; Shan, Y.; Chen, T.; Zhao, Y.; Yu, C.; Pang, H. Metal-Organic Frameworks Nanocomposites with Different Dimensionalities for Energy Conversion and Storage. Adv. Energy Mater. 2022, 12, 2100346. [Google Scholar] [CrossRef]
- He, G.; Xu, M.; Li, Z.; Wang, S.; Jiang, S.; He, X.; Zhao, J.; Li, Z.; Wu, X.; Huang, T.; et al. Highly Hydroxide-Conductive Nanostructured Solid Electrolyte via Predesigned Ionic Nanoaggregates. ACS Appl. Mater. Interfaces 2017, 9, 28346–28354. [Google Scholar] [CrossRef]
- Chen, W.; Mandal, M.; Huang, G.; Wu, X.; He, G.; Kohl, P.A. Highly Conducting Anion-Exchange Membranes Based on Cross-Linked Poly(Norbornene): Ring Opening Metathesis Polymerization. ACS Appl. Energy Mater. 2019, 2, 2458–2468. [Google Scholar] [CrossRef]
- Xu, Z.; Wilke, V.; Chmielarz, J.J.; Tobias, M.; Atanasov, V.; Gago, A.S.; Friedrich, K.A. Novel Piperidinium-Functionalized Crosslinked Anion Exchange Membrane with Flexible Spacers for Water Electrolysis. J. Membr. Sci. 2023, 670, 121302. [Google Scholar] [CrossRef]
- Lu, W.; Yang, Z.; Huang, H.; Wei, F.; Li, W.; Yu, Y.; Gao, Y.; Zhou, Y.; Zhang, G. Piperidinium-Functionalized Poly(Vinylbenzyl Chloride) Cross-Linked by Polybenzimidazole as an Anion Exchange Membrane with a Continuous Ionic Transport Pathway. Ind. Eng. Chem. Res. 2020, 59, 21077–21087. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; You, W. Dithiol Cross-Linked Polynorbornene-Based Anion-Exchange Membranes with High Hydroxide Conductivity and Alkaline Stability. J. Membr. Sci. 2023, 685, 121916. [Google Scholar] [CrossRef]
- Guo, M.; Ban, T.; Wang, Y.; Wang, X.; Zhu, X. “Thiol-Ene” Crosslinked Polybenzimidazoles Anion Exchange Membrane with Enhanced Performance and Durability. J. Colloid Interface Sci. 2023, 638, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, C.; Pan, J.; Sotto, A.; Shen, J. Constructing an Internally Cross-Linked Structure for Polysulfone to Improve Dimensional Stability and Alkaline Stability of High Performance Anion Exchange Membranes. Int. J. Hydrogen Energy 2019, 44, 8279–8289. [Google Scholar] [CrossRef]
- Zhu, L.; Zimudzi, T.J.; Li, N.; Pan, J.; Lin, B.; Hickner, M.A. Crosslinking of comb-shaped polymer anion exchange membranes via thiol–ene click chemistry. Polym. Chem. 2016, 7, 2464–2475. [Google Scholar] [CrossRef]
- Tian, L.; Li, J.; Liu, Q.; Ma, W.; Wang, F.; Zhu, H.; Wang, Z. Cross-linked anion-exchange membranes with dipole-containing cross-linkers based on poly (terphenyl isatin piperidinium) copolymers. ACS Appl. Mater. Interfaces 2022, 14, 39343–39353. [Google Scholar] [CrossRef]
- Fujifilm Manufacturing Europe, B.V.; Fujifilm Corporation. Membrane Stacks and Their Uses. US17904999, 30 March 2023. [Google Scholar]
- Junji, F.; Hajime, U.; Naoya, K.; Keiichiro, H. Pure Water Making Method and Electric Regeneration Type Pure Water Making Apparatus. JP2003024948, 28 January 2003. [Google Scholar]
- Wilfred, G.S.; Cavell, M.T.T.; Paul, G. Anion Exchange Membranes. EP0382439, 16 August 1990. [Google Scholar]
- Evoqua Water Tech LLC. Anion Exchange Membrane and Manufacturing Method. JP2017018947A, 26 January 2017.
- Arthur, L.G.; Wayne, A.M.; Keith, J.S. Electrodialysis Including Filled Cell Electrodialysis (Electrodeionization). US5679229A, 21 October 1997. [Google Scholar]
- Sun, Y.-M.; Huang, L.-F.; Chuang, J.-N.; Huang, W.-S. Homogeneous Anion Exchange Membrane and Biosensing Membrane Prepared from the Same. US20220259392, 12 November 2021. [Google Scholar]
- Gambaro, C.; Meda, L.; Di Noto, V.; Vezzu’, K.; Sun, C. Zipped Ion-Exchange Membrane. EP4008036, 30 July 2020. [Google Scholar]
- Takeshi, M. Electrodialytic Method. JPS61192312, 21 February 1985. [Google Scholar]
- Kim, D.J.; Ahn, Y.H. Anion Exchange Membrane Based on Aromatic Polymer Functionalized with Imidazolium Group, Preparation Method Thereof, and Vanadium Redox Flow Battery Including the Membrane. US17011095, 4 March 2021. [Google Scholar]
- Lee, C.H.H. Chemically Modified Anion Exchange Membrane and Manufacturing Method Therefor. EP18787265, 31 March 2021. [Google Scholar]
- Lin, J.R. High-Performance Anion Exchange Membranes and Methods of Making Same. EP2903737, 15 March 2013. [Google Scholar]
- Qin, H.; Zhu, C.; Hu, Y.; Chen, K.; Liu, J.; Kong, Z.; Wang, H.; He, Y.; Ji, Z. Preparation Method for Alkaline Anion Exchange Membrane and Use Thereof in Fuel Cell. US10797333, 22 November 2016. [Google Scholar]
- Hoon, K.J.; Jun, C.B.; Young, M.S. Homogeneous Anion-Exchange Composite Membrane Having Excellent Chemical Resistance and Method for Producing the Same. US2018326364, 10 May 2018. [Google Scholar]
- Gu, B.B.T. Anionic Membranes Incorporating Functional Additives. US20200406248, 31 December 2020. [Google Scholar]
- Schübel-Hopf, S. Hydrophilic Composite Microporous Membrane and Method for Producing Same. EP1961784, 27 August 2008. [Google Scholar]
- Roelofs, M.G.C. Improved Composite Polymer Electrolyte Membrane. EP2721676, 23 April 2014. [Google Scholar]
- Staser, J.A.; Movil-Cabrera, O. Ionic Liquid-Functionalized Graphene Oxide-Based Nanocomposite Anion Exchange Membranes. US20190044169, 2 February 2017. [Google Scholar]
- Eguchi, T.; Mori, S.; Shimokawa, M. Process for Preparing a Composite Amphoteric Ion Exchange Membrane. US4262041, 2 February 1978. [Google Scholar]
- Xiangchun, Y. Resilient Anion Exchange Membranes Prepared by Polymerizing a Composition. US9636642, 16 October 2015. [Google Scholar]
- Seung-Hyeon, M.; Sung-Hee, S.; Yekyung, K.; Won, S.K. Organic-Inorganic Composite Anion Exchange Membrane Containing Polyvinylidene Fluoride Polymer for Non-Aqueous Redox Flow Battery and Method for Preparing the Same. US10249901, 30 December 2014. [Google Scholar]
- Li, L.-F.; Zeng, S.; Liu, C.; Jung, H.Y. Systems Including Ion Exchange Membranes and Methods of Making the Same. WO2022/147497, 3 January 2022. [Google Scholar]
- Nicoloso, N.; Kerres, J.; Schafer, G. Proton-Conducting Ceramics/Polymer Composite Membrane for the Temperature Range up to 300 °C. CA2372693, 2 May 2000. [Google Scholar]
- Haering, T.; Haering Thomas, D.; Kerres, J.; Kerres Jochen, D.; Ullrich, A.; Ullrich Andreas, D. Organisch-Anorganische Komposites und Kompositmembranen aus Ionomeren Oder Ionomerblends und Aus Schicht- Oder Gerätsilicaten. DE000019919881, 30 April 1999. [Google Scholar]
- ASTM B907-16; Standard Specification for Zinc, Tin and Cadmium Base Alloys Used as Solders. ASTM International: West Conshohocken, PA, USA, 2016.
- Peter, N.P.; Andrew, P.; Jason, B. Composite Membranes, Methods of Making Same, and Applications of Same. US10141593, 23 May 2016. [Google Scholar]
- Kazuki, K.; Junji, S. Composite Polymer Electrolyte Membrane. JP2017249141, 18 January 2022. [Google Scholar]
- Wang, Z.; Parrondo, J.; Ramani, V.K. Triblock Copolymer Based Anion Exchange Membranes (AEMs) as Separators in Electrochemical Devices. US17113973, 15 April 2021. [Google Scholar]
- Bahar, B.; Gu, T.; Yellamilli, S.N. Anion Exchange Ionomer with a Poyarylene Backbone and Anion Exchange Membrane Incorporating Same. US20210347956, 2 July 2021. [Google Scholar]
- Lee, Y.M.; Chen, N.; Wang, H.H.; Kim, S.P. Novel Polyfluorene-Based Ionomer, Anion Exchange Membrane, Method for Preparing the Polyfluorene-Based Ionomer and Method for Fabricating the Anion Exchange Membrane. US20230038279, 9 November 2020. [Google Scholar]
- Junfeng, Z.; Paul A., K.; Murat, U. Anion Exchange Polyelectrolytes. KR20120082007, 24 September 2010. [Google Scholar]
- Yoshimura, K.; Koshikawa, H.; Yamaki, T.; Asano, M.; Maekawa, Y.; Shishitani, H.; Asazawa, K.; Yamaguchi, S.; Tanaka, H. Anion Exchange Membrane and Producing Method Thereof. US201314012071, 6 March 2014. [Google Scholar]
- Yan, Y.H. Polymers Having Stable Cationic Pendant Groups for Use as Anion Exchange Membranes. EP20778028.9, 28 December 2022. [Google Scholar]
- He, S.S.; Frank, C.W. Anion Transport Membrane. US9233345, 12 February 2014. [Google Scholar]
- Mandal, M.; Huang, G.; Hassan, N.U.; Peng, X.; Gu, T.; Brooks-Starks, A.H.; Bahar, B.; Mustain, W.E.; Kohl, P.A. The Importance of Water Transport in High Conductivity and High-Power Alkaline Fuel Cells. J. Electrochem. Soc. 2020, 167, 054501. [Google Scholar] [CrossRef]
- Kohl, P.A.; Mandal, M.; Barchok, M.L.; Skilskyj, D.; Rhodes, L.F. Polycyloolefinic Polymers and Anion Exchange Membranes Derived Therefrom. US20220033571, 30 July 2021. [Google Scholar]
- Wu, X.; He, G.; Wang, X.; Yan, X.; Li, T.; Chen, W.; Li, X.; Xiao, W.; Jiang, X.; Cui, F.; et al. Comb-Shaped Structure Polybenzimidazole Anion Exchange Membrane with High Conductivity and Preparation Method Thereof. US20210202972, 19 March 2020. [Google Scholar]
- Kerres, J. Cross-Linked High Stable Anion Exchange Blend Membranes with Polyethyleneglycols as Hydrophilic Membrane Phase. US11278879, 22 June 2017. [Google Scholar]
- Liberatore, M.; Ozioko, G.A.; Schoeps, K. Crosslinked Membrane for Anion Exchange Applications. US11600837, 8 October 2019. [Google Scholar]
- Harada, M.; Takamoto, T. Functional Polymer Membrane, Production Method Thereof, and Stack or Device Provided with Functional Polymer Membrane. US20170152361, 13 February 2017. [Google Scholar]
- Harada, M.; Takamoto, T. Polymer Functional Film, Production Method Thereof, and Stack or Device Provided with Polymer Functional Film. EP3181619, 22 July 2015. [Google Scholar]
- Hyun, K.T.; Hyun, K.S. Terminally-Crosslinked Methyl Morpholinium-Functionalized Block Copolymers, and Anion Exchange Membranes Using the Same. US2018345269, 4 September 2017. [Google Scholar]
- Bae, C.; Ryu, C.Y.; Tian, D. Methods of Making Anion Exchange Membrane via Simultaneous Post-Functionalization and Crosslinking of Epoxidized Sbs. US20210309818, 6 April 2021. [Google Scholar]
- Bae, C.; Jeon, J.; Han, J.; Noh, S. Crosslinking of Aromatic Polymers for Anion Exchange Membranes. EP3784369, 24 April 2019. [Google Scholar]
- Sung, S.; Mayadevi, T.S.; Min, K.; Lee, J.; Chae, J.E.; Kim, T.-H. Crosslinked PPO-Based Anion Exchange Membranes: The Effect of Crystallinity versus Hydrophilicity by Oxygen-Containing Crosslinker Chain Length. J. Membr. Sci. 2021, 619, 118774. [Google Scholar] [CrossRef]
- Motealleh, B.; Liu, Z.; Masel, R.I.; Sculley, J.P.; Richard Ni, Z.; Meroueh, L. Next-Generation Anion Exchange Membrane Water Electrolyzers Operating for Commercially Relevant Lifetimes. Int. J. Hydrogen Energy 2021, 46, 3379–3386. [Google Scholar] [CrossRef]






| Name | Type | IEC (mmol/g) | Tensile Strength (MPa) | Water Uptake (%) | Swelling Ratio (%) | Ref. |
|---|---|---|---|---|---|---|
| CAPSF-5 | uncrosslinked | 1.92 | 22.98 | 13.04 | 9.42 | [123] |
| Neosepta AMX | crosslinked | 2.16 | 35.07 | 44.23 | 4.22 | [123] |
| V-2-H-1 | crosslinked | 2.93 | ~40 MPa | <60 (90 °C) | <15 | [121] |
| X60Y30C6 | crosslinked | 2.38 | - | 93 (80 °C) | 35 | [124] |
| PcPBI-Nb-2.33 | uncrosslinked | 2.37 | 25.51 | 83.0 (80 °C) | 6.9 | [122] |
| PcPBI-Nb-C2 | crosslinked | 2.25 | 39.76 | 100.3 (80 °C) | 5.9 | [122] |
| PBI-PVBC-NMPD/OH | crosslinked | 2.31 | 37.5 | 48 (80 °C) | 11 | [120] |
| PTPBHIN-O19 | crosslinked | 1.64 | 64.8 | 133 (80 °C) | 10.53 | [125] |
| m-TPNPiQA | uncrosslinked | 2.54 | <20 | 65.2 (80 °C) | 25.7 | [100] |
| C-IL-100 | crosslinked | 2.99 | 22.91 | 97.0 | 35.9 | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Ma, H.; Khan, M.; Hsiao, B.S. Recent Advances and Challenges in Anion Exchange Membranes Development/Application for Water Electrolysis: A Review. Membranes 2024, 14, 85. https://doi.org/10.3390/membranes14040085
Liu L, Ma H, Khan M, Hsiao BS. Recent Advances and Challenges in Anion Exchange Membranes Development/Application for Water Electrolysis: A Review. Membranes. 2024; 14(4):85. https://doi.org/10.3390/membranes14040085
Chicago/Turabian StyleLiu, Lu, Hongyang Ma, Madani Khan, and Benjamin S. Hsiao. 2024. "Recent Advances and Challenges in Anion Exchange Membranes Development/Application for Water Electrolysis: A Review" Membranes 14, no. 4: 85. https://doi.org/10.3390/membranes14040085
APA StyleLiu, L., Ma, H., Khan, M., & Hsiao, B. S. (2024). Recent Advances and Challenges in Anion Exchange Membranes Development/Application for Water Electrolysis: A Review. Membranes, 14(4), 85. https://doi.org/10.3390/membranes14040085









