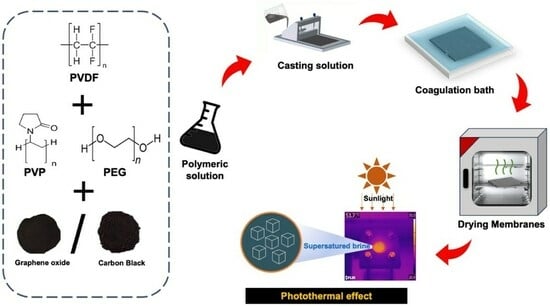
Environmentally Friendly Photothermal Membranes for Halite Recovery from Reverse Osmosis Brine via Solar-Driven Membrane Crystallization
Abstract
:1. Introduction
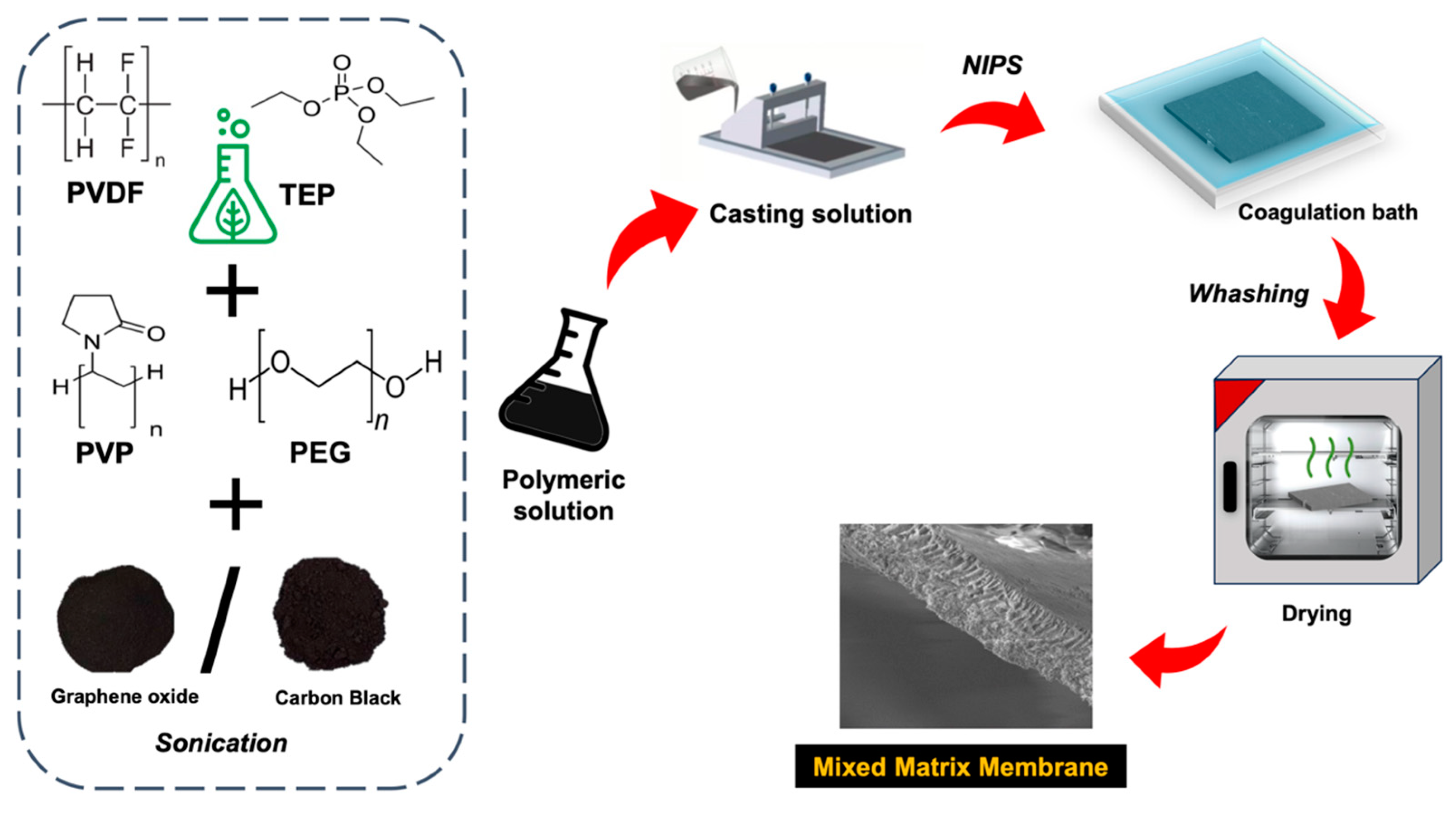
2. Experimental Section
2.1. Materials
2.2. Membrane Preparation
2.3. Membrane Characterization
2.4. Photothermal Membrane Crystallization Experiments
3. Results and Discussion
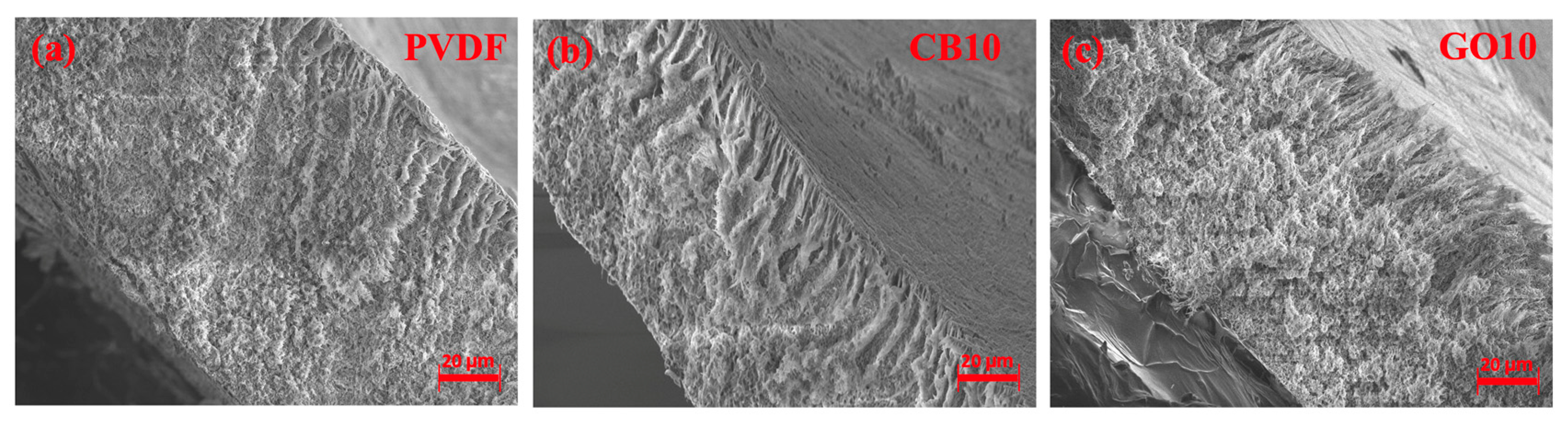
3.1. Membrane Properties
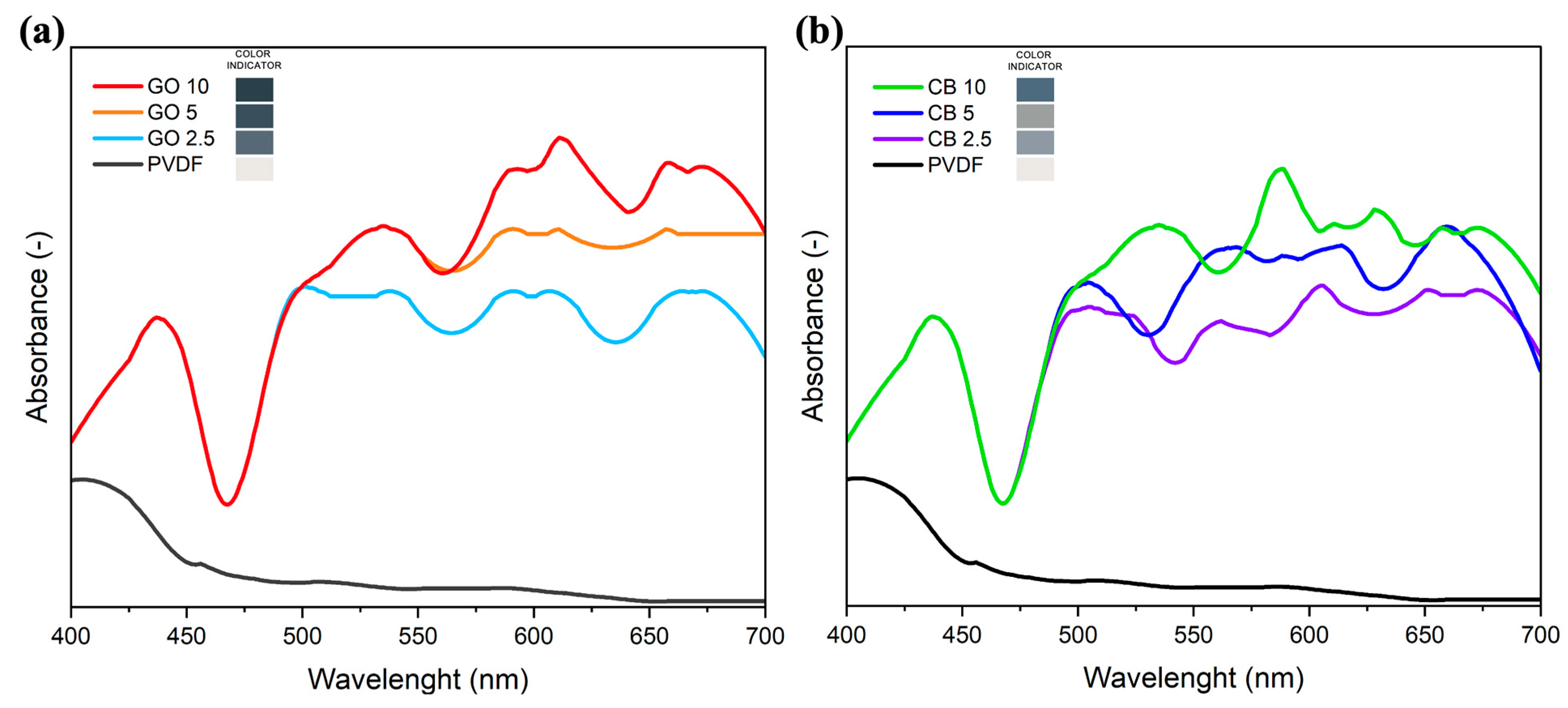
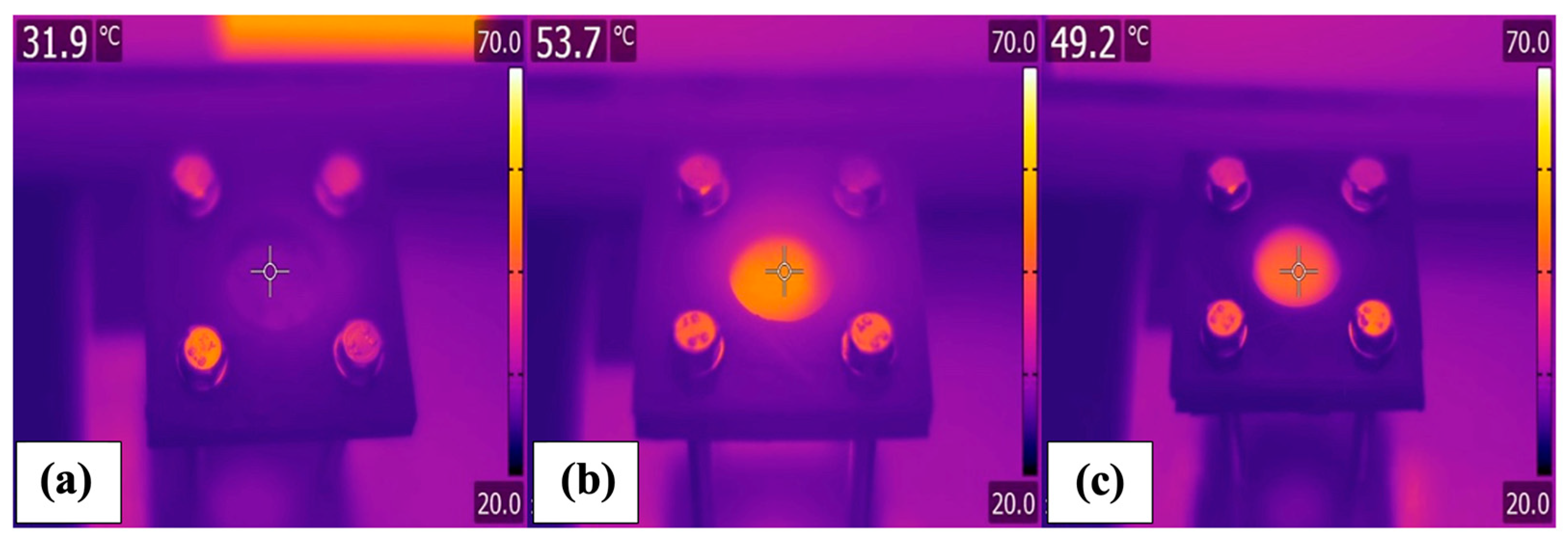
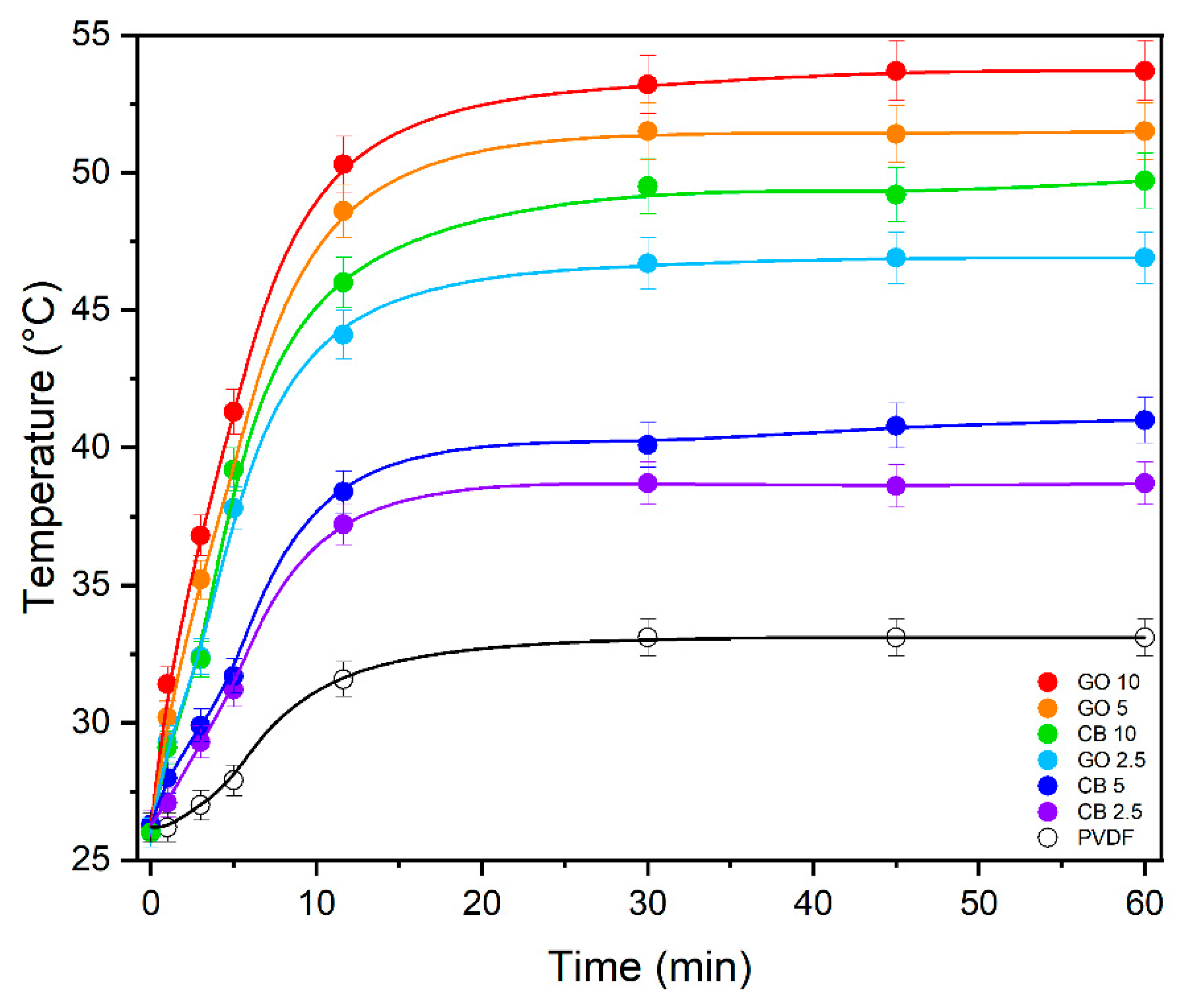
3.2. Photothermal Performance
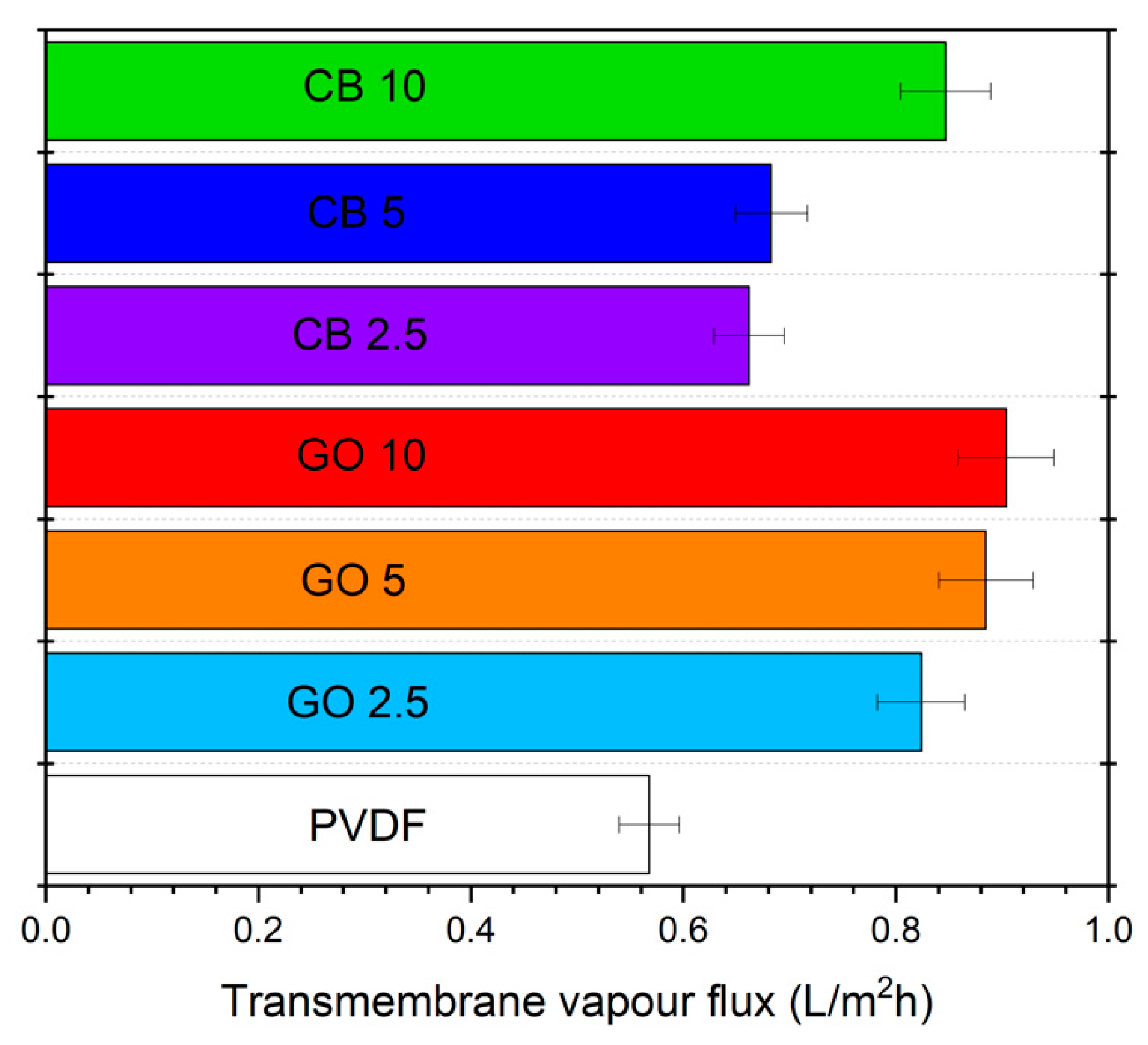
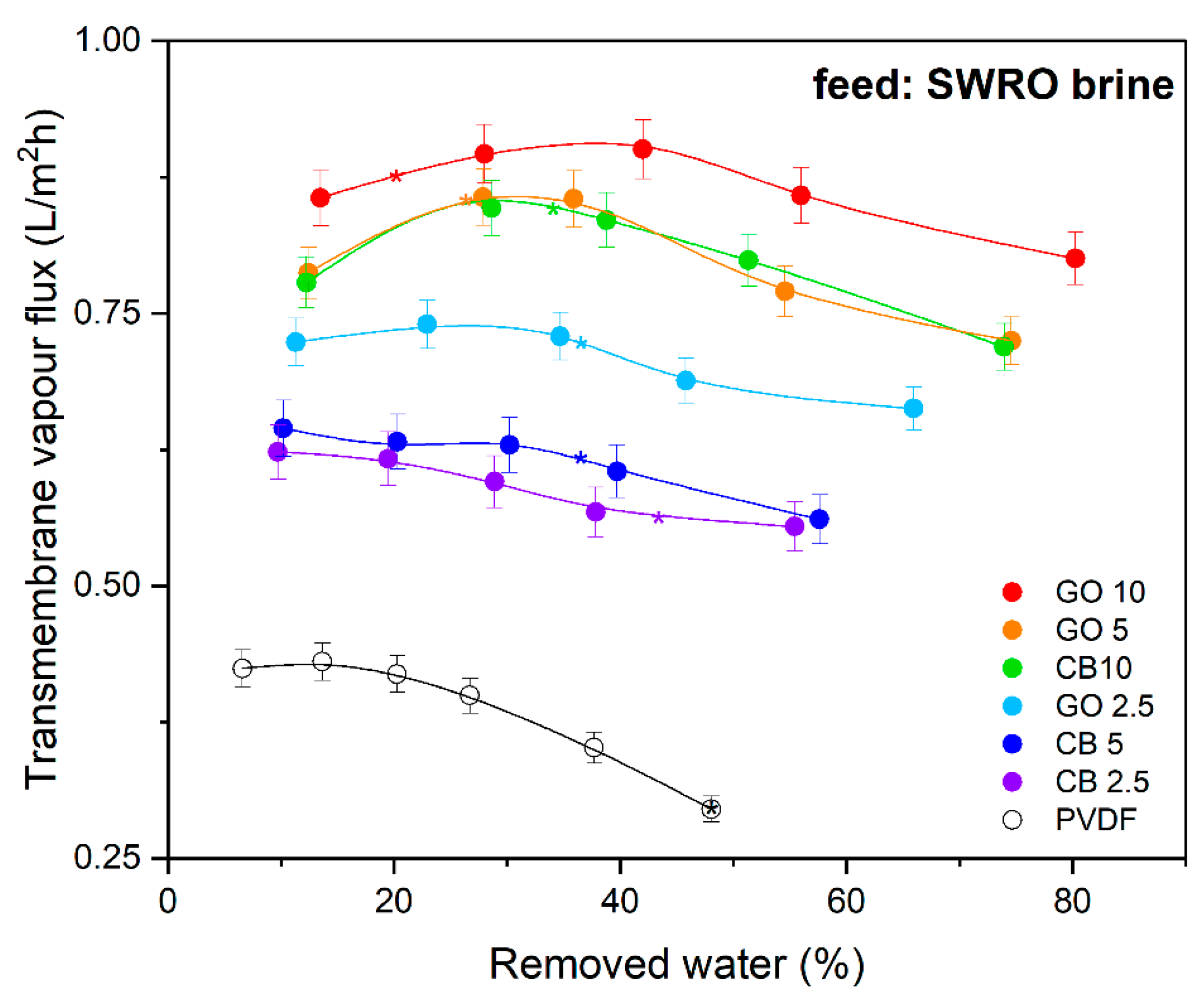
3.3. Photothermal Membrane Crystallization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Musie, W.; Gonfa, G. Fresh Water Resource, Scarcity, Water Salinity Challenges and Possible Remedies: A Review. Heliyon 2023, 9, e18685. [Google Scholar] [CrossRef] [PubMed]
- Subramani, A.; Jacangelo, J.G. Emerging Desalination Technologies for Water Treatment: A Critical Review. Water Res. 2015, 75, 164–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Recent Advance of MgO Expansive Agent in Cement and Concrete. J. Build. Eng. 2022, 45, 103633. [Google Scholar] [CrossRef]
- Cai, Y.; Wu, J.; Shi, S.Q.; Li, J.; Kim, K.-H. Advances in Desalination Technology and Its Environmental and Economic Assessment. J. Clean. Prod. 2023, 397, 136498. [Google Scholar] [CrossRef]
- Ghazi, Z.M.; Rizvi, S.W.F.; Shahid, W.M.; Abdulhameed, A.M.; Saleem, H.; Zaidi, S.J. An Overview of Water Desalination Systems Integrated with Renewable Energy Sources. Desalination 2022, 542, 116063. [Google Scholar] [CrossRef]
- IDA. GWI DesalData Desalination and Reuse Handbook 2022–2023. Available online: https://globalwatersecurity.org/handbook (accessed on 20 February 2024).
- Yang, Z.; Sun, P.-F.; Li, X.; Gan, B.; Wang, L.; Song, X.; Park, H.-D.; Tang, C.Y. A Critical Review on Thin-Film Nanocomposite Membranes with Interlayered Structure: Mechanisms, Recent Developments, and Environmental Applications. Environ. Sci. Technol. 2020, 54, 15563–15583. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.D.; Hankins, N.P. Osmotically Assisted Reverse Osmosis (OARO): Five Approaches to Dewatering Saline Brines Using Pressure-Driven Membrane Processes. Desalination 2019, 458, 1–13. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K.-J.; Loizidou, M. Desalination Brine Disposal Methods and Treatment Technologies—A Review. Sci. Total Environ. 2019, 693, 133545. [Google Scholar] [CrossRef]
- Avci, A.H.; Santoro, S.; Politano, A.; Propato, M.; Micieli, M.; Aquino, M.; Wenjuan, Z.; Curcio, E. Photothermal Sweeping Gas Membrane Distillation and Reverse Electrodialysis for Light-to-Heat-to-Power Conversion. Chem. Eng. Process.-Process Intensif. 2021, 164, 108382. [Google Scholar] [CrossRef]
- Liang, C.Z.; Askari, M.; Choong, L.T.; Chung, T.-S. Ultra-Strong Polymeric Hollow Fiber Membranes for Saline Dewatering and Desalination. Nat. Commun. 2021, 12, 2338. [Google Scholar] [CrossRef]
- Santoro, S.; Tufa, R.A.; Avci, A.H.; Fontananova, E.; Di Profio, G.; Curcio, E. Fouling Propensity in Reverse Electrodialysis Operated with Hypersaline Brine. Energy 2021, 228, 120563. [Google Scholar] [CrossRef]
- Mauter, M.S.; Fiske, P.S. Desalination for a Circular Water Economy. Energy Environ. Sci. 2020, 13, 3180–3184. [Google Scholar] [CrossRef]
- Choi, Y.; Naidu, G.; Nghiem, L.D.; Lee, S.; Vigneswaran, S. Membrane Distillation Crystallization for Brine Mining and Zero Liquid Discharge: Opportunities, Challenges, and Recent Progress. Environ. Sci. 2019, 5, 1202–1221. [Google Scholar] [CrossRef]
- Yaqub, M.; Lee, W. Zero-Liquid Discharge (ZLD) Technology for Resource Recovery from Wastewater: A Review. Sci. Total Environ. 2019, 681, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Edwie, F.; Chung, T.-S. Development of Simultaneous Membrane Distillation–Crystallization (SMDC) Technology for Treatment of Saturated Brine. Chem. Eng. Sci. 2013, 98, 160–172. [Google Scholar] [CrossRef]
- Gryta, M. Concentration of NaCl Solution by Membrane Distillation Integrated with Crystallization. Sep. Sci. Technol. 2002, 37, 3535–3558. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane Distillation: A Comprehensive Review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Das, P.; Dutta, S.; Singh, K.K. Insights into Membrane Crystallization: A Sustainable Tool for Value Added Product Recovery from Effluent Streams. Sep. Purif. Technol. 2021, 257, 117666. [Google Scholar] [CrossRef]
- Shen, L.; Dang, M.; Han, X. Recent Advances in Membrane Crystallization. CrystEngComm 2023, 25, 2503–2517. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, G.; Li, Y.; Li, H.; McLellan, B.; Chen, S. Substitution Effect of Renewable Portfolio Standards and Renewable Energy Certificate Trading for Feed-in Tariff. Appl. Energy 2018, 227, 426–435. [Google Scholar] [CrossRef]
- López-Porfiri, P.; Ramos-Paredes, S.; Núñez, P.; Gorgojo, P. Towards the Technological Maturity of Membrane Distillation: The MD Module Performance Curve. NPJ Clean Water 2023, 6, 18. [Google Scholar] [CrossRef]
- Santoro, S.; Vidorreta, I.M.; Sebastian, V.; Moro, A.; Coelhoso, I.M.; Portugal, C.A.M.; Lima, J.C.; Desiderio, G.; Lombardo, G.; Drioli, E.; et al. A Non-Invasive Optical Method for Mapping Temperature Polarization in Direct Contact Membrane Distillation. J. Memb. Sci. 2017, 536, 156–166. [Google Scholar] [CrossRef]
- Anvari, A.; Azimi Yancheshme, A.; Kekre, K.M.; Ronen, A. State-of-the-Art Methods for Overcoming Temperature Polarization in Membrane Distillation Process: A Review. J. Memb. Sci. 2020, 616, 118413. [Google Scholar] [CrossRef]
- Russo, F.; Santoro, S.; Galiano, F.; Ursino, C.; Avruscio, E.; Di Nicolò, E.; Desiderio, G.; Lombardo, G.; Criscuoli, A.; Figoli, A. A Luminescent Thermosensitive Coating for a Non-Invasive and in-Situ Study of Thermal Polarization in Hollow Fiber Membranes. J. Memb. Sci. 2023, 685, 121928. [Google Scholar] [CrossRef]
- Martínez-Díez, L.; Vázquez-González, M.I. Temperature and Concentration Polarization in Membrane Distillation of Aqueous Salt Solutions. J. Memb. Sci. 1999, 156, 265–273. [Google Scholar] [CrossRef]
- Kabeel, A.E.; Abdelgaied, M.; El-Said, E.M.S. Study of a Solar-Driven Membrane Distillation System: Evaporative Cooling Effect on Performance Enhancement. Renew. Energy 2017, 106, 192–200. [Google Scholar] [CrossRef]
- Banat, F.; Jwaied, N.; Rommel, M.; Koschikowski, J.; Wieghaus, M. Performance Evaluation of the “Large SMADES” Autonomous Desalination Solar-Driven Membrane Distillation Plant in Aqaba, Jordan. Desalination 2007, 217, 17–28. [Google Scholar] [CrossRef]
- Santoro, S.; Avci, A.H.; Politano, A.; Curcio, E. The Advent of Thermoplasmonic Membrane Distillation. Chem. Soc. Rev. 2022, 51, 6087–6125. [Google Scholar] [CrossRef]
- Alessandro, F.; Macedonio, F.; Drioli, E. Plasmonic Phenomena in Membrane Distillation. Membranes 2023, 13, 254. [Google Scholar] [CrossRef]
- Politano, A.; Argurio, P.; Di Profio, G.; Sanna, V.; Cupolillo, A.; Chakraborty, S.; Arafat, H.A.; Curcio, E. Photothermal Membrane Distillation for Seawater Desalination. Adv. Mater. 2017, 29, 1603504. [Google Scholar] [CrossRef]
- Santoro, S.; Estay, H.; Avci, A.H.; Pugliese, L.; Ruby-Figueroa, R.; Garcia, A.; Aquino, M.; Nasirov, S.; Straface, S.; Curcio, E. Membrane Technology for a Sustainable Copper Mining Industry: The Chilean Paradigm. Clean. Eng. Technol. 2021, 2, 100091. [Google Scholar] [CrossRef]
- Loganathan, P.; Naidu, G.; Vigneswaran, S. Mining Valuable Minerals from Seawater: A Critical Review. Environ. Sci. 2017, 3, 37–53. [Google Scholar] [CrossRef]
- Almarzooqi, N.; Hong, S.; Verma, P.; Shaheen, A.F.M.; Schiffer, A.; AlMarzooqi, F. Photothermal Surface Heating Membrane Distillation Using 3D-Printed Ti3C2Tx MXene-Based Nanocomposite Spacers. ACS Appl. Mater. Interfaces 2023, 15, 20998–21007. [Google Scholar] [CrossRef]
- Abramovich, S.; Dutta, D.; Rizza, C.; Santoro, S.; Aquino, M.; Cupolillo, A.; Occhiuzzi, J.; Russa, M.F.L.; Ghosh, B.; Farias, D.; et al. NiSe and CoSe Topological Nodal-Line Semimetals: A Sustainable Platform for Efficient Thermoplasmonics and Solar-Driven Photothermal Membrane Distillation. Small 2022, 18, 2201473. [Google Scholar] [CrossRef]
- Santoro, S.; Aquino, M.; Rizza, C.; Cupolillo, A.; Boukhvalov, D.W.; D’Olimpio, G.; Abramovich, S.; Agarwal, A.; Sadan, M.B.; Politano, A.; et al. Plasmonic Nanofillers-Enabled Solar Membrane Crystallization for Mineral Recovery. Desalination 2023, 563, 116730. [Google Scholar] [CrossRef]
- Lin, C.; Hao, H.; Mei, L.; Wu, M. Metal-Free Two-Dimensional Nanomaterial-Mediated Photothermal Tumor Therapy. Smart Mater. Med. 2020, 1, 150–167. [Google Scholar] [CrossRef]
- Santoro, S.; Aquino, M.; Rizza, C.; Occhiuzzi, J.; Mastrippolito, D.; D’Olimpio, G.; Avci, A.H.; De Santis, J.; Paolucci, V.; Ottaviano, L.; et al. Lithium Recovery through WS2 Nanofillers-Promoted Solar Photothermal Membrane Crystallization of LiCl. Desalination 2023, 546, 116186. [Google Scholar] [CrossRef]
- Santoro, S.; Aquino, M.; Han Seo, D.; Van Der Laan, T.; Lee, M.; Sung Yun, J.; Jun Park, M.; Bendavid, A.; Kyong Shon, H.; Halil Avci, A.; et al. Dimensionally Controlled Graphene-Based Surfaces for Photothermal Membrane Crystallization. J. Colloid. Interface Sci. 2022, 623, 607–616. [Google Scholar] [CrossRef]
- Lou, M.; Li, J.; Zhu, X.; Chen, J.; Zhang, X.; Fang, X.; Li, F. Difunctional MOF-Wrapped Graphene Membranes for Efficient Photothermal Membrane Distillation and VOCs Interception. J. Memb. Sci. 2023, 676, 121592. [Google Scholar] [CrossRef]
- Alyarnezhad, S.; Marino, T.; Parsa, J.B.; Galiano, F.; Ursino, C.; Garcìa, H.; Puche, M.; Figoli, A. Polyvinylidene Fluoride-Graphene Oxide Membranes for Dye Removal under Visible Light Irradiation. Polymers 2020, 12, 1509. [Google Scholar] [CrossRef] [PubMed]
- Seraj, S.; Mohammadi, T.; Tofighy, M.A. Graphene-Based Membranes for Membrane Distillation Applications: A Review. J. Environ. Chem. Eng. 2022, 10, 107974. [Google Scholar] [CrossRef]
- Marino, T.; Blefari, S.; Di Nicolò, E.; Figoli, A. A More Sustainable Membrane Preparation Using Triethyl Phosphate as Solvent. Green Process. Synth. 2017, 6, 295–300. [Google Scholar] [CrossRef]
- Tan, X.; Rodrigue, D. A Review on Porous Polymeric Membrane Preparation. Part I: Production Techniques with Polysulfone and Poly (Vinylidene Fluoride). Polymers 2019, 11, 1160. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA). Corrigendum to Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 Con-Cerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemi-Cals Agency, Amending Directive 1999/45/EC and Repealing Council Regulation (EEC) No 793/93 and Commission Regula-Tion (EC) No 1488/94 as Well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32006R1907R(01) (accessed on 20 February 2024).
- Chang, J.; Zuo, J.; Zhang, L.; O’Brien, G.S.; Chung, T.-S. Using Green Solvent, Triethyl Phosphate (TEP), to Fabricate Highly Porous PVDF Hollow Fiber Membranes for Membrane Distillation. J. Memb. Sci. 2017, 539, 295–304. [Google Scholar] [CrossRef]
- Molinari, R.; Avci, A.H.; Argurio, P.; Curcio, E.; Meca, S.; Plà-Castellana, M.; Cortina, J.L. Selective Precipitation of Calcium Ion from Seawater Desalination Reverse Osmosis Brine. J. Clean. Prod. 2021, 328, 129645. [Google Scholar] [CrossRef]
- Hu, X.; Chen, X.; Giagnorio, M.; Wu, C.; Luo, Y.; Hélix-Nielsen, C.; Yu, P.; Zhang, W. Beaded Electrospun Polyvinylidene Fluoride (PVDF) Membranes for Membrane Distillation (MD). J. Memb. Sci. 2022, 661, 120850. [Google Scholar] [CrossRef]
- Dimakis, N.; Salas, I.; Gonzalez, L.; Vadodaria, O.; Ruiz, K.; Bhatti, M.I. Li and Na Adsorption on Graphene and Graphene Oxide Examined by Density Functional Theory, Quantum Theory of Atoms in Molecules, and Electron Localization Function. Molecules 2019, 24, 754. [Google Scholar] [CrossRef]
- Han, Y.; Guo, Y.; Gu, J. Chapter 5—Thermally Conductive Fillers. In Thermally Conductive Polymer Composites; Gu, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 111–147. ISBN 978-0-323-95231-6. [Google Scholar]
- Garside, J.; Mersmann, A.; Nývlt, J. Measurement of Crystal Growth and Nucleation Rates, 2nd ed.; IChemE: Rugby, UK; Institution of Chemical Engineers, 2002; ISBN 9780852954492. [Google Scholar]
- Wang, D.-M.; Lai, J.-Y. Recent Advances in Preparation and Morphology Control of Polymeric Membranes Formed by Nonsolvent Induced Phase Separation. Curr. Opin. Chem. Eng. 2013, 2, 229–237. [Google Scholar] [CrossRef]
- Marino, T.; Russo, F.; Figoli, A. The Formation of Polyvinylidene Fluoride Membranes with Tailored Properties via Vapour/Non-Solvent Induced Phase Separation. Membranes 2018, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Basko, A.; Lebedeva, T.; Yurov, M.; Ilyasova, A.; Elyashevich, G.; Lavrentyev, V.; Kalmykov, D.; Volkov, A.; Pochivalov, K. Mechanism of PVDF Membrane Formation by NIPS Revisited: Effect of Precipitation Bath Nature and Polymer–Solvent Affinity. Polymers 2023, 15, 4307. [Google Scholar] [CrossRef]
- Silva, T.L.S.; Morales-Torres, S.; Figueiredo, J.L.; Silva, A.M.T. Multi-Walled Carbon Nanotube/PVDF Blended Membranes with Sponge- and Finger-like Pores for Direct Contact Membrane Distillation. Desalination 2015, 357, 233–245. [Google Scholar] [CrossRef]
- Pagliero, M.; Comite, A.; Soda, O.; Costa, C. Influence of Carbon-Based Fillers on Photoactive Mixed Matrix Membranes Formation. J. Memb. Sci. 2022, 658, 120752. [Google Scholar] [CrossRef]
- Figoli, A.; Simone, S.; Criscuoli, A.; AL-Jlil, S.A.; Al Shabouna, F.S.; Al-Romaih, H.S.; Di Nicolò, E.; Al-Harbi, O.A.; Drioli, E. Hollow Fibers for Seawater Desalination from Blends of PVDF with Different Molecular Weights: Morphology, Properties and VMD Performance. Polymer 2014, 55, 1296–1306. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, B.; Wang, L.; Zhang, Z.; Li, M. Preparation and Characterization of Superhydrophobic PVDF/HMSNs Hybrid Membrane for CO2 Absorption. Polymer 2021, 214, 123242. [Google Scholar] [CrossRef]
- Mortaheb, H.R.; Baghban Salehi, M.; Rajabzadeh, M. Optimized Hybrid PVDF/Graphene Membranes for Enhancing Performance of AGMD Process in Water Desalination. J. Ind. Eng. Chem. 2021, 99, 407–421. [Google Scholar] [CrossRef]
- Yang, Y.; Rigdon, W.; Huang, X.; Li, X. Enhancing Graphene Reinforcing Potential in Composites by Hydrogen Passivation Induced Dispersion. Sci. Rep. 2013, 3, 2086. [Google Scholar] [CrossRef] [PubMed]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. Membrane Synthesis for Membrane Distillation: A Review. Sep. Purif. Technol. 2017, 182, 36–51. [Google Scholar] [CrossRef]
- Han, B.; Zhang, Y.-L.; Chen, Q.-D.; Sun, H.-B. Carbon-Based Photothermal Actuators. Adv. Funct. Mater. 2018, 28, 1802235. [Google Scholar] [CrossRef]
- Noguchi, T.; Kimura, M.; Hashimoto, T.; Konno, M.; Nakamura, T.; Zolensky, M.E.; Tsuchiyama, A.; Matsumoto, T.; Matsuno, J.; Okazaki, R.; et al. Sylvite and Halite on Particles Recovered from 25143 Itokawa: A Preliminary Report. Meteorit Planet Sci 2014, 49, 1305–1314. [Google Scholar] [CrossRef]
- Christie, K.S.S.; McGaughey, A.; McBride, S.A.; Xu, X.; Priestley, R.D.; Ren, Z.J. Membrane Distillation–Crystallization for Sustainable Carbon Utilization and Storage. Environ. Sci. Technol. 2023, 57, 16628–16640. [Google Scholar] [CrossRef]
- Sparenberg, M.-C.; Chergaoui, S.; Sang Sefidi, V.; Luis, P. Crystallization Control via Membrane Distillation-Crystallization: A Review. Desalination 2021, 519, 115315. [Google Scholar] [CrossRef]
- Zimmermann, N.E.R.; Vorselaars, B.; Quigley, D.; Peters, B. Nucleation of NaCl from Aqueous Solution: Critical Sizes, Ion-Attachment Kinetics, and Rates. J. Am. Chem. Soc. 2015, 137, 13352–13361. [Google Scholar] [CrossRef] [PubMed]
- Chergaoui, S.; Lauzer, J.; Debecker, D.P.; Leyssens, T.; Luis, P. Tuning Membrane Properties to Control Supersaturation of Antisolvent Crystallization. J. Memb. Sci. 2024, 694, 122415. [Google Scholar] [CrossRef]
- Polino, M.; Portugal, C.A.M.; Di Profio, G.; Coelhoso, I.M.; Crespo, J.G. Protein Crystallization by Membrane-Assisted Technology. Cryst. Growth Des. 2019, 19, 4871–4883. [Google Scholar] [CrossRef]
- Tsai, J.-H.; Perrotta, M.L.; Gugliuzza, A.; Macedonio, F.; Giorno, L.; Drioli, E.; Tung, K.-L.; Tocci, E. Membrane-Assisted Crystallization: A Molecular View of NaCl Nucleation and Growth. Appl. Sci. 2018, 8, 2145. [Google Scholar] [CrossRef]
- Eljaddi, T.; Cabassud, C. Wetting of Photoplasmonic PVDF/Silver Membranes in Photothermal Membrane Distillation: Identification of Wetting Mechanisms and Comparison of Wetting Dynamics. Desalination 2022, 540, 116019. [Google Scholar] [CrossRef]
- Gryta, M. Degradation of Polypropylene Membranes Applied in Membrane Distillation Crystallizer. Crystals 2016, 6, 33. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Thangavadivel, K.; Shu, L.; Jegatheesan, V. A Critical Review of Membrane Crystallization for the Purification of Water and Recovery of Minerals. Rev. Environ. Sci. Biotechnol. 2016, 15, 411–439. [Google Scholar] [CrossRef]
- Jiang, X.; Shao, Y.; Sheng, L.; Li, P.; He, G. Membrane Crystallization for Process Intensification and Control: A Review. Engineering 2021, 7, 50–62. [Google Scholar] [CrossRef]
- Jafari, M.; Vanoppen, M.; van Agtmaal, J.M.C.; Cornelissen, E.R.; Vrouwenvelder, J.S.; Verliefde, A.; van Loosdrecht, M.C.M.; Picioreanu, C. Cost of Fouling in Full-Scale Reverse Osmosis and Nanofiltration Installations in the Netherlands. Desalination 2021, 500, 114865. [Google Scholar] [CrossRef]
- Liu, L.; He, H.; Wang, Y.; Tong, T.; Li, X.; Zhang, Y.; He, T. Mitigation of Gypsum and Silica Scaling in Membrane Distillation by Pulse Flow Operation. J. Memb. Sci. 2021, 624, 119107. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, M.; Hu, X.; Chang, H.; Yan, Z.; Liang, Y.; Meng, Y.; Liang, H. Evaluation of the Performance of Ultrasound-Assisted Membrane Distillation Crystallization Process for Water and Sodium Chloride Recovery in Hypersaline Solution. Desalination 2022, 531, 115727. [Google Scholar] [CrossRef]
- Julian, H.; Ye, Y.; Li, H.; Chen, V. Scaling Mitigation in Submerged Vacuum Membrane Distillation and Crystallization (VMDC) with Periodic Air-Backwash. J. Memb. Sci. 2018, 547, 19–33. [Google Scholar] [CrossRef]
- Jiang, X.; Niu, Y.; Du, S.; He, G. Membrane Crystallization: Engineering the Crystallization via Microscale Interfacial Technology. Chem. Eng. Res. Des. 2022, 178, 454–465. [Google Scholar] [CrossRef]
- Jiang, X.; Tuo, L.; Lu, D.; Hou, B.; Chen, W.; He, G. Progress in Membrane Distillation Crystallization: Process Models, Crystallization Control and Innovative Applications. Front. Chem. Sci. Eng. 2017, 11, 647–662. [Google Scholar] [CrossRef]
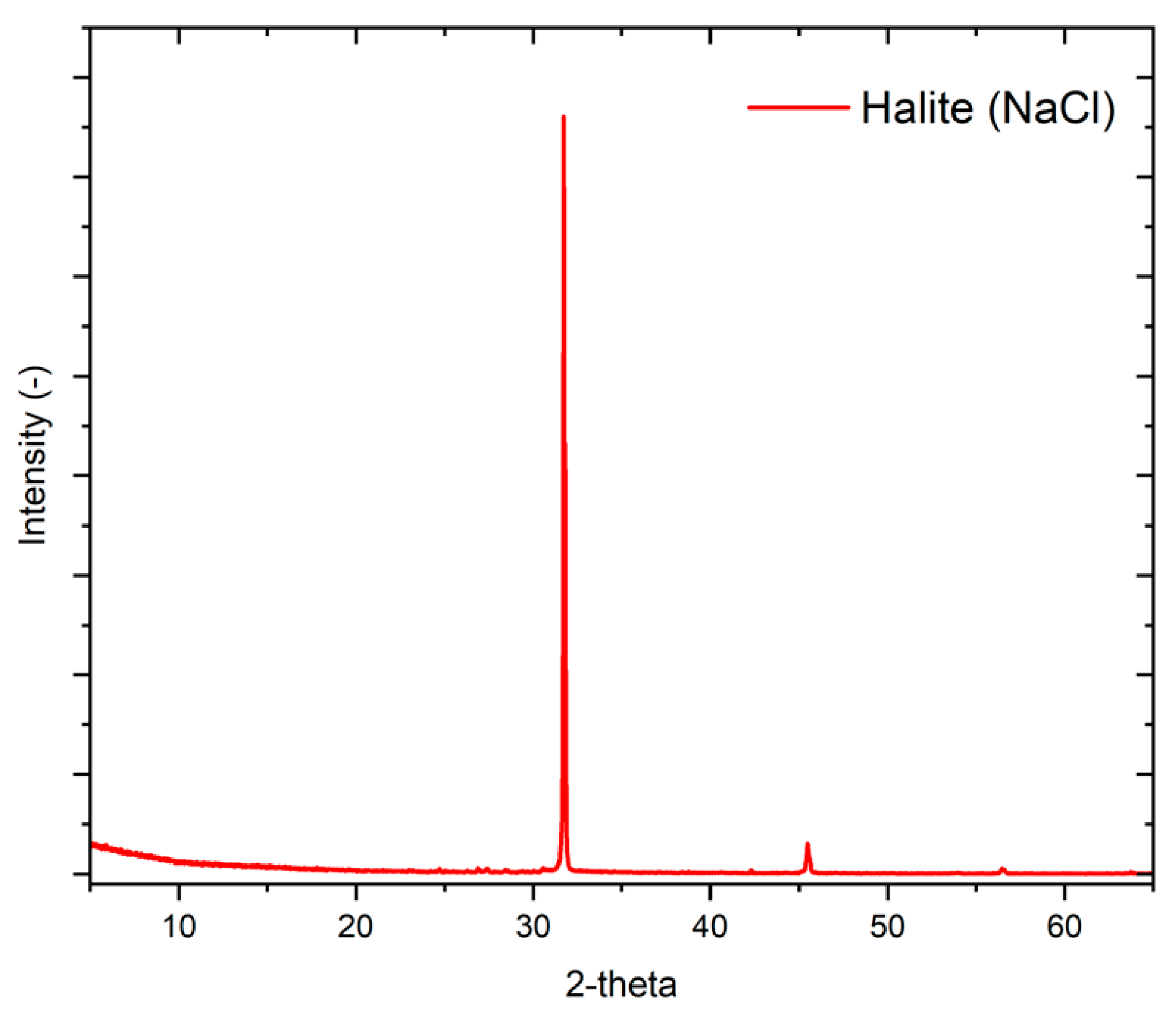
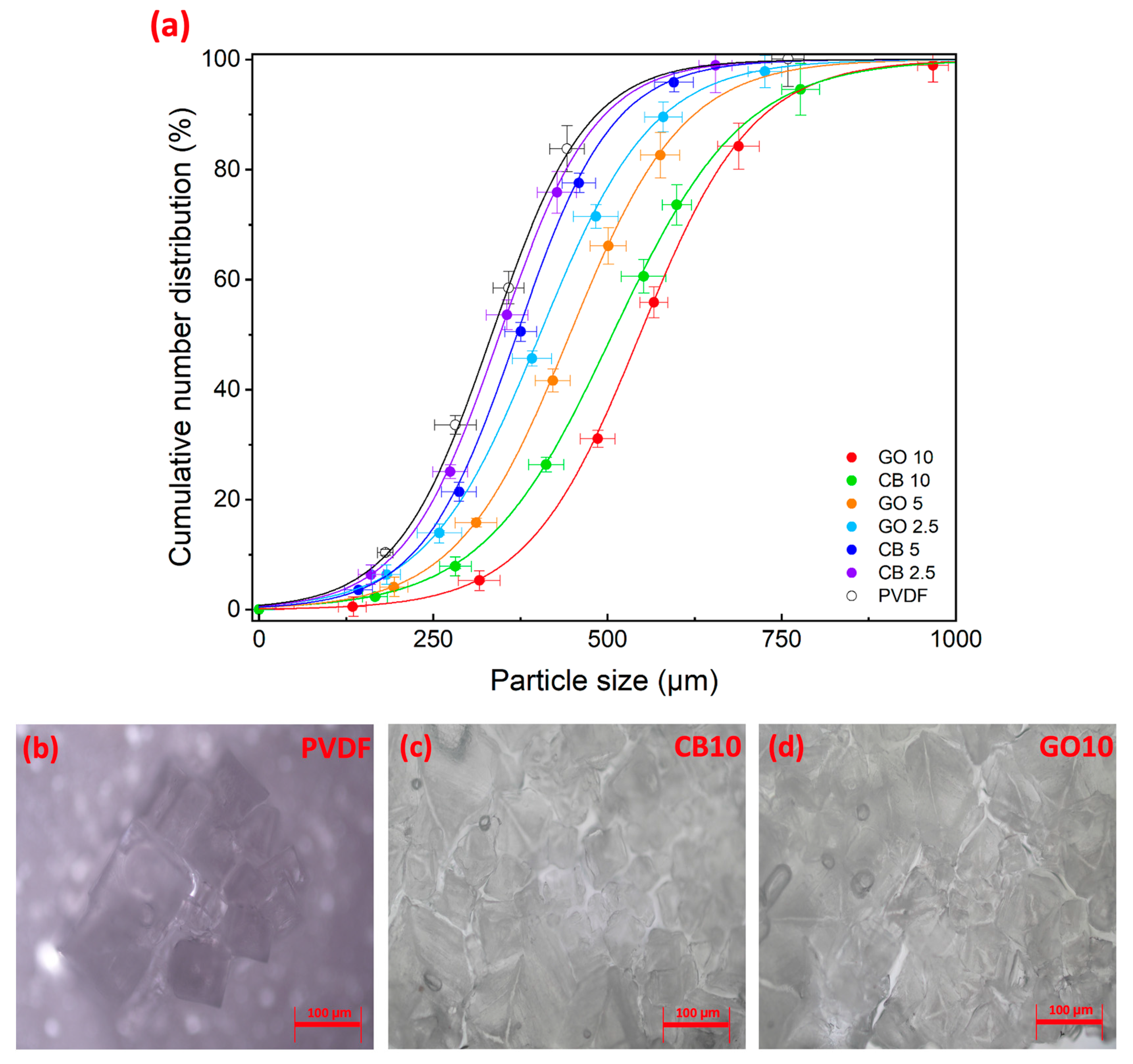
| Ion | Na+ | Mg2+ | K+ | Ca2+ | Cl− | SO42− | CO32− | NO3− |
|---|---|---|---|---|---|---|---|---|
| Concentration (ppm) | 35,902 | 4236 | 1356 | 980 | 41,642 | 4398 | 320 | 130 |
| Membrane | PVDF | GO/CB 2.5 | GO/CB 5 | GO/CB 10 |
|---|---|---|---|---|
| TEP (wt.%) | 60 | 59.625 | 59.25 | 58.5 |
| PVDF (wt.%) | 15 | 15 | 15 | 15 |
| PEG (wt.%) | 20 | 20 | 20 | 20 |
| PVP (wt.%) | 5 | 5 | 5 | 5 |
| NPs: GO/CB (wt.%) | - | 0.375 | 0.75 | 1.5 |
| Membrane | Thickness (μm) | Mean Pore (μm) | Porosity (%) | Contact Angle Bottom (°) | Contact Angle Top (°) |
|---|---|---|---|---|---|
| Bare PVDF | 161 ± 3 | 0.092 ± 0.007 | 70.6 ± 0.8 | 97 ± 2 | 92 ± 2 |
| CB 2.5 | 152 ± 1 | 0.112 ± 0.004 | 78.1 ± 0.6 | 96 ± 2 | 93 ± 2 |
| CB 5 | 166 ± 2 | 0.092 ± 0.006 | 82.6 ± 1.0 | 110 ± 1 | 92 ± 1 |
| CB 10 | 150 ± 3 | 0.071 ± 0.005 | 67.0 ± 0.6 | 113 ± 2 | 93 ± 2 |
| GO 2.5 | 150 ± 2 | 0.184 ± 0.009 | 84.6 ± 0.7 | 96 ± 2 | 94 ± 2 |
| GO 5 | 136 ± 1 | 0.126 ± 0.008 | 80.6 ± 0.4 | 120 ± 2 | 96 ± 2 |
| GO 10 | 133 ± 1 | 0.075 ± 0.006 | 85.2 ± 0.6 | 119 ± 2 | 99 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aquino, M.; Santoro, S.; Politano, A.; D’Andrea, G.; Siciliano, A.; Straface, S.; La Russa, M.F.; Curcio, E. Environmentally Friendly Photothermal Membranes for Halite Recovery from Reverse Osmosis Brine via Solar-Driven Membrane Crystallization. Membranes 2024, 14, 87. https://doi.org/10.3390/membranes14040087
Aquino M, Santoro S, Politano A, D’Andrea G, Siciliano A, Straface S, La Russa MF, Curcio E. Environmentally Friendly Photothermal Membranes for Halite Recovery from Reverse Osmosis Brine via Solar-Driven Membrane Crystallization. Membranes. 2024; 14(4):87. https://doi.org/10.3390/membranes14040087
Chicago/Turabian StyleAquino, Marco, Sergio Santoro, Antonio Politano, Giuseppe D’Andrea, Alessio Siciliano, Salvatore Straface, Mauro Francesco La Russa, and Efrem Curcio. 2024. "Environmentally Friendly Photothermal Membranes for Halite Recovery from Reverse Osmosis Brine via Solar-Driven Membrane Crystallization" Membranes 14, no. 4: 87. https://doi.org/10.3390/membranes14040087
APA StyleAquino, M., Santoro, S., Politano, A., D’Andrea, G., Siciliano, A., Straface, S., La Russa, M. F., & Curcio, E. (2024). Environmentally Friendly Photothermal Membranes for Halite Recovery from Reverse Osmosis Brine via Solar-Driven Membrane Crystallization. Membranes, 14(4), 87. https://doi.org/10.3390/membranes14040087