Anion Exchange Membrane with Pendulous Piperidinium on Twisted All-Carbon Backbone for Fuel Cell
Abstract
1. Introduction
2. Result and Discussion
2.1. Synthesis and Structural Analysis
2.2. Morphology Characterization
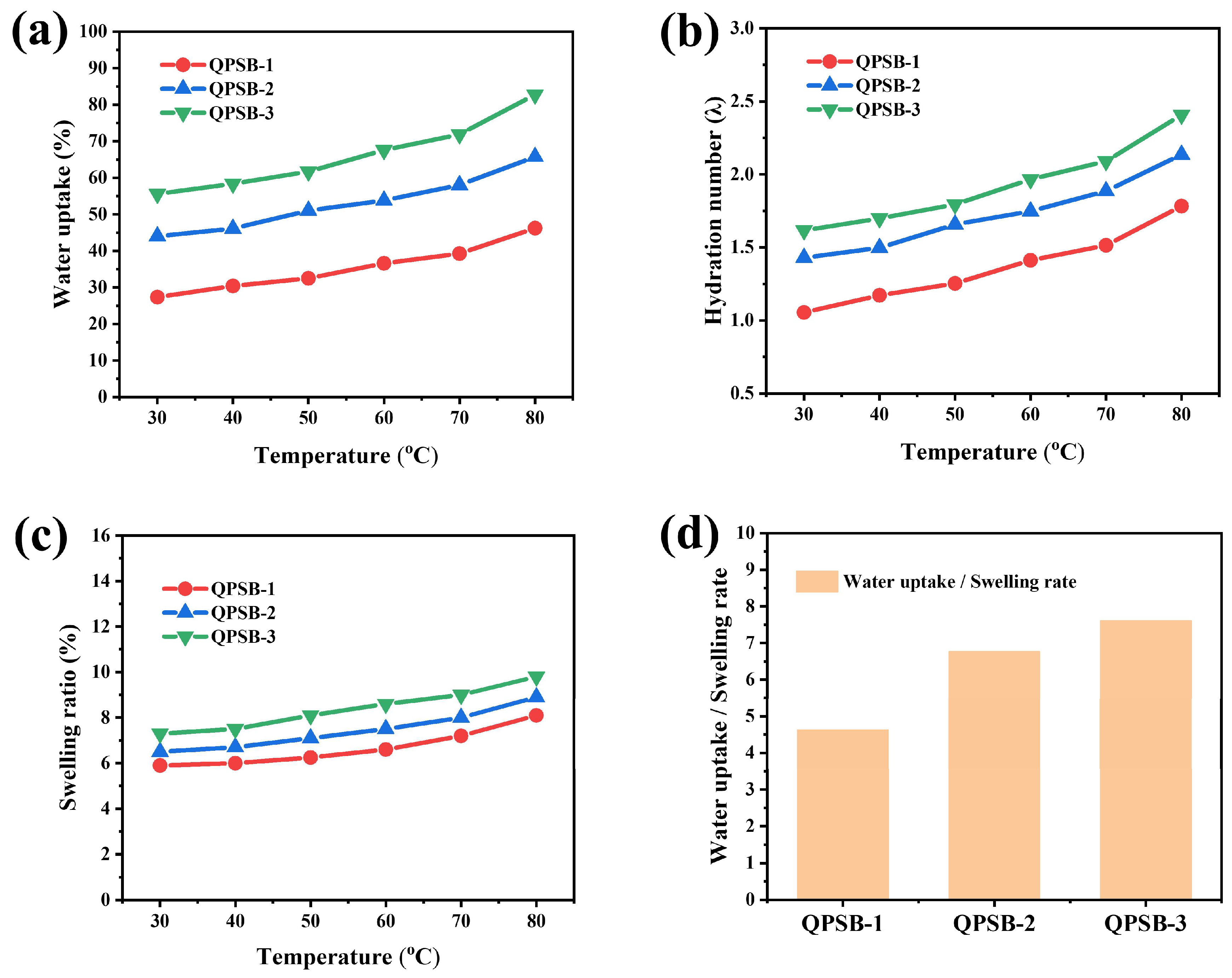
2.3. Ion Exchange Capacity (IEC), Water Uptake (WU) and Swelling Ratio (SR)
2.4. Hydroxide Conductivity
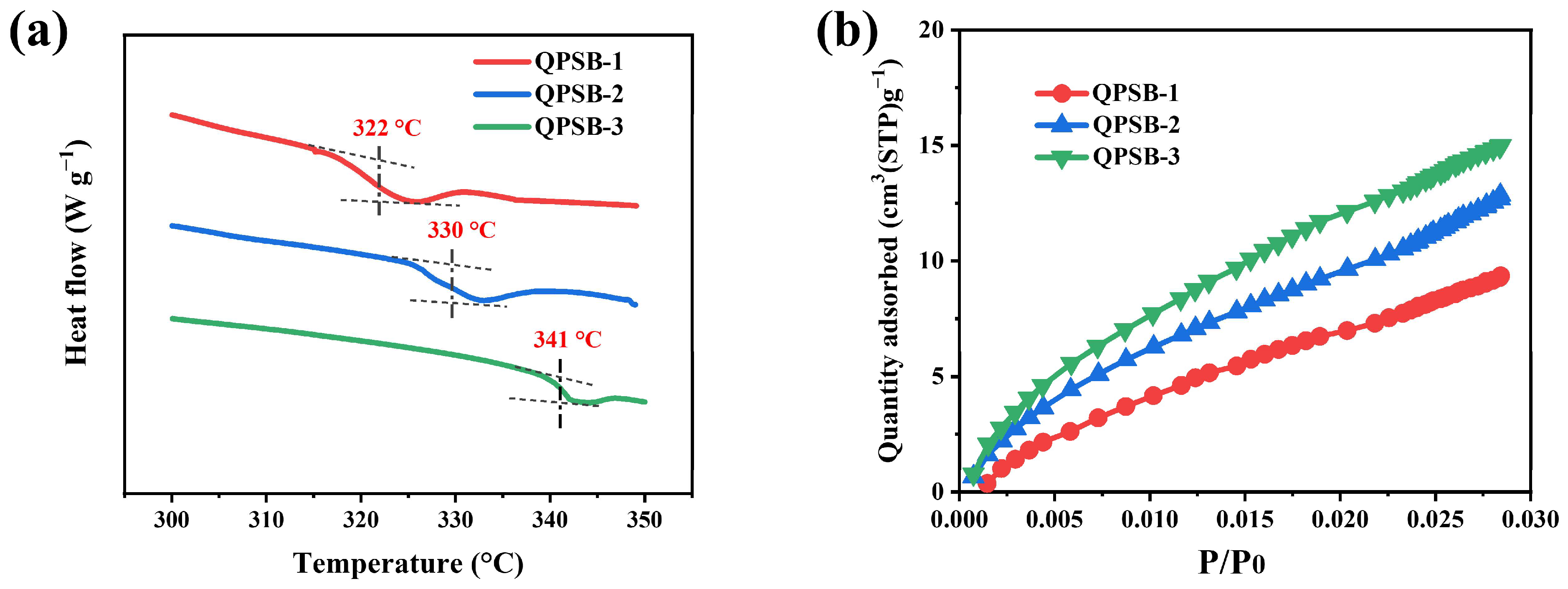
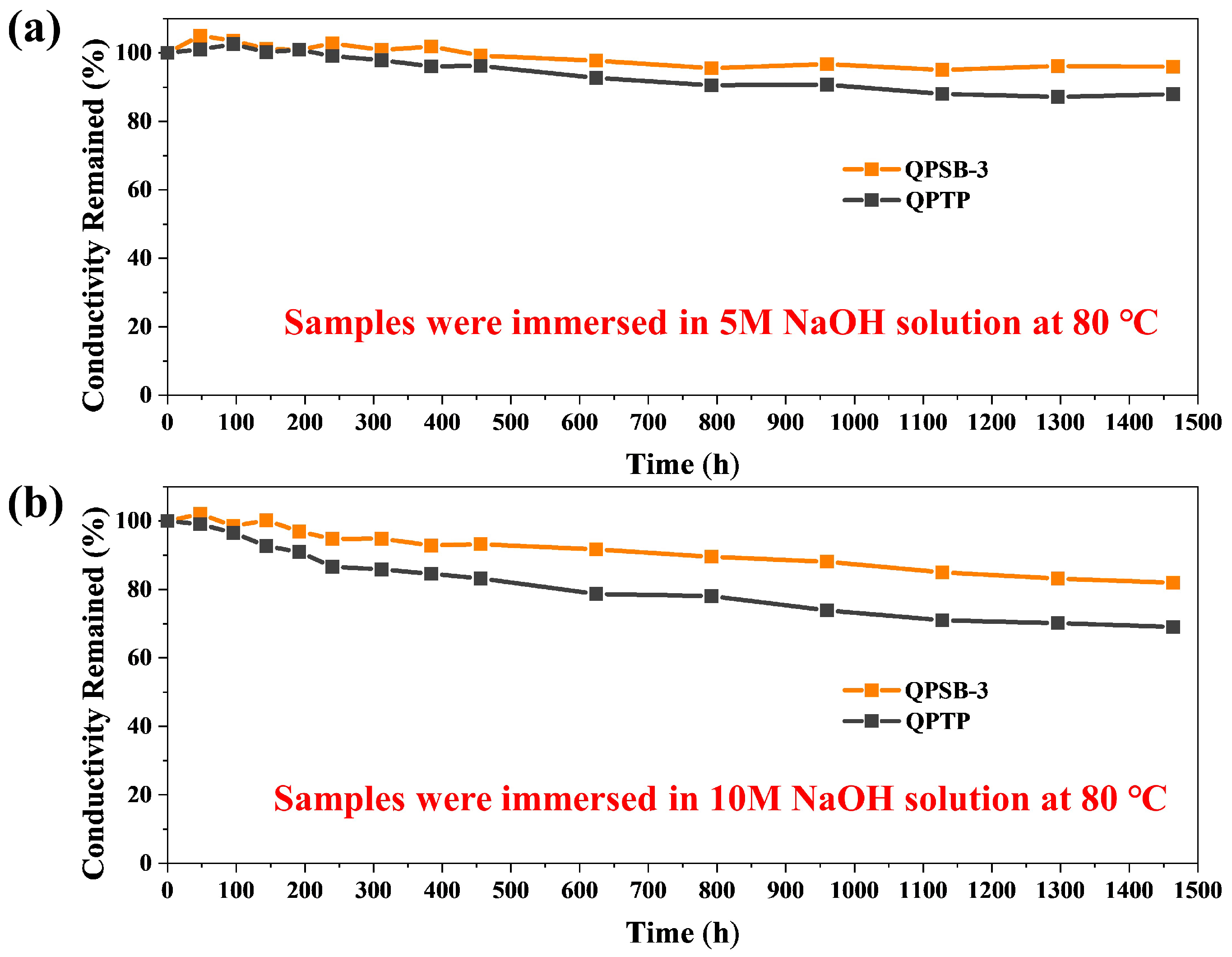
2.5. Mechanical, Thermal and Chemical Stability
2.6. Fuel Cell Performance
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Arsad, A.Z.; Hannan, M.A.; Al-Shetwi, A.Q.; Mansur, M.; Muttaqi, K.M.; Dong, Z.Y.; Blaabjerg, F. Hydrogen energy storage integrated hybrid renewable energy systems: A review analysis for future research directions. Int. J. Hydrogen Energy 2022, 47, 17285–17312. [Google Scholar] [CrossRef]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z.; et al. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Kim, H.-T. Powering the hydrogen future: Current status and challenges of anion exchange membrane fuel cells. Energy Environ. Sci. 2023, 16, 5633–5662. [Google Scholar] [CrossRef]
- Chen, H.; Tao, R.; Bang, K.-T.; Shao, M.; Kim, Y. Anion Exchange Membranes for Fuel Cells: State-of-the-Art and Perspectives. Adv. Energy Mater. 2022, 12, 2200934. [Google Scholar] [CrossRef]
- Yang, Y.; Peltier, C.R.; Zeng, R.; Schimmenti, R.; Li, Q.; Huang, X.; Yan, Z.; Potsi, G.; Selhorst, R.; Lu, X.; et al. Electrocatalysis in Alkaline Media and Alkaline Membrane-Based Energy Technologies. Chem. Rev. 2022, 122, 6117–6321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, K.; Han, J.; Zhang, X.; Wang, B.; Du, Q.; Jiao, K. Anion Exchange Membranes for Fuel Cells: Equilibrium Water Content and Conductivity Characterization. Adv. Funct. Mater. 2023, 33, 2303857. [Google Scholar] [CrossRef]
- Chen, N.; Hu, C.; Wang, H.H.; Kim, S.P.; Kim, H.M.; Lee, W.H.; Bae, J.Y.; Park, J.H.; Lee, Y.M. Poly(Alkyl-Terphenyl Piperidinium) Ionomers and Membranes with an Outstanding Alkaline-Membrane Fuel-Cell Performance of 2.58 W cm−2. Angew. Chem. Int. Ed. 2021, 60, 7710–7718. [Google Scholar] [CrossRef] [PubMed]
- Hui Chen, J.; Ting Gao, W.; Shuen Lann Choo, Y.; Lang Gao, X.; Jie Liu, Y.; Bin Yue, X.; Hao Wang, X.; Mei Zhu, A.; Gen Zhang, Q.; Lin Liu, Q. Semi-interpenetrating anion exchange membranes using hydrophobic microporous linear poly(ether ketone). J. Colloid Interface Sci. 2023, 634, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fang, Z.; Zhang, M.; Xie, S.; Xie, D.; Liu, P.; Wang, S.; Cheng, F.; Xu, T. Macromolecular crosslinked poly(aryl piperidinium)-based anion exchange membranes with enhanced ion conduction for water electrolysis. J. Membr. Sci. 2024, 700, 122717. [Google Scholar] [CrossRef]
- Zhang, A.; Li, L.; Ma, L.; Ren, Y.; Bai, L.; He, G.; Zhang, F. Soft template promoted microphase separation in anion exchange membrane of electrodialysis. J. Membr. Sci. 2022, 658, 120758. [Google Scholar] [CrossRef]
- Gu, Y.; Dong, T.; Zhang, Y.; Li, Z.; Wang, Y.; Gao, J.; Lei, Y.; Wu, J.; Wang, Y.; Wang, Z. Microphase separation structures facilitated by dipole molecules and hydrogen bonding in poly (biphenyl piperidine-isatin) anion exchange membranes. J. Power Sources 2024, 592, 233918. [Google Scholar] [CrossRef]
- Li, N.; Yan, T.; Li, Z.; Thurn-Albrecht, T.; Binder, W.H. Comb-shaped polymers to enhance hydroxide transport in anion exchange membranes. Energy Environ. Sci. 2012, 5, 7888–7892. [Google Scholar] [CrossRef]
- Li, N.; Leng, Y.; Hickner, M.A.; Wang, C.-Y. Highly Stable, Anion Conductive, Comb-Shaped Copolymers for Alkaline Fuel Cells. J. Am. Chem. Soc. 2013, 135, 10124–10133. [Google Scholar] [CrossRef] [PubMed]
- Ponce-González, J.; Whelligan, D.K.; Wang, L.; Bance-Soualhi, R.; Wang, Y.; Peng, Y.; Peng, H.; Apperley, D.C.; Sarode, H.N.; Pandey, T.P.; et al. High performance aliphatic-heterocyclic benzyl-quaternary ammonium radiation-grafted anion-exchange membranes. Energy Environ. Sci. 2016, 9, 3724–3735. [Google Scholar] [CrossRef]
- Tanaka, M.; Fukasawa, K.; Nishino, E.; Yamaguchi, S.; Yamada, K.; Tanaka, H.; Bae, B.; Miyatake, K.; Watanabe, M. Anion Conductive Block Poly(arylene ether)s: Synthesis, Properties, and Application in Alkaline Fuel Cells. J. Am. Chem. Soc. 2011, 133, 10646–10654. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Lu, C.; Li, Y.; Long, C.; Li, Z.; Zhu, H. Tunable multi-cations-crosslinked poly(arylene piperidinium)-based alkaline membranes with high ion conductivity and durability. J. Membr. Sci. 2019, 588, 117120. [Google Scholar] [CrossRef]
- Mandal, M.; Huang, G.; Hassan, N.U.; Mustain, W.E.; Kohl, P.A. Poly(norbornene) anion conductive membranes: Homopolymer, block copolymer and random copolymer properties and performance. J. Mater. Chem. A 2020, 8, 17568–17578. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Yang, Y.; Li, J.; Zheng, J.; Zhang, S. Microporous and low swelling branched poly(aryl piperidinium) anion exchange membranes for high-performed water electrolyzers. J. Membr. Sci. 2024, 698, 122587. [Google Scholar] [CrossRef]
- Hu, C.; Kang, H.W.; Jung, S.W.; Liu, M.-L.; Lee, Y.J.; Park, J.H.; Kang, N.Y.; Kim, M.-G.; Yoo, S.J.; Park, C.H.; et al. High Free Volume Polyelectrolytes for Anion Exchange Membrane Water Electrolyzers with a Current Density of 13.39 A cm−2 and a Durability of 1000 h (Adv. Sci. 5/2024). Adv. Sci. 2024, 11, 2470030. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, R.; Malpass-Evans, R.; Carta, M.; McKeown, N.B.; Guiver, M.D.; Wu, L.; Xu, T. Highly Conductive Anion-Exchange Membranes from Microporous Tröger’s Base Polymers. Angew. Chem. Int. Ed. 2016, 55, 11499–11502. [Google Scholar] [CrossRef]
- Tang, H.; Geng, K.; Wu, L.; Liu, J.; Chen, Z.; You, W.; Yan, F.; Guiver, M.D.; Li, N. Fuel cells with an operational range of –20 °C to 200 °C enabled by phosphoric acid-doped intrinsically ultramicroporous membranes. Nat. Energy 2022, 7, 153–162. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, J.; Pei, Y.; Liu, X.; Xue, J.; Jiang, H.; Qiu, X.; Yin, Y.; Wu, H.; Jiang, Z.; et al. Mechanically robust microporous anion exchange membranes with efficient anion conduction for fuel cells. Chem. Eng. J. 2021, 418, 129311. [Google Scholar] [CrossRef]
- Hu, C.; Kang, N.Y.; Kang, H.W.; Lee, J.Y.; Zhang, X.; Lee, Y.J.; Jung, S.W.; Park, J.H.; Kim, M.-G.; Yoo, S.J.; et al. Triptycene Branched Poly(aryl-co-aryl piperidinium) Electrolytes for Alkaline Anion Exchange Membrane Fuel Cells and Water Electrolyzers. Angew. Chem. Int. Ed. 2024, 63, e202316697. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, N.; Klok, H.A.; Lee, Y.M.; Hu, X. Branched Poly(Aryl Piperidinium) Membranes for Anion-Exchange Membrane Fuel Cells. Angew. Chem. Int. Ed. Eng. 2022, 61, e202114892. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, L.; Liu, J.; Chen, W.; Li, X.; Wu, X.; Jiang, X.; He, G.; Yan, X. Intrinsic microporous poly(phenyl-alkane)s with pendent piperazinium for high performance anion exchange membrane fuel cells. J. Membr. Sci. 2024, 700, 122673. [Google Scholar] [CrossRef]
- Zuo, P.; Xu, Z.; Zhu, Q.; Ran, J.; Ge, L.; Ge, X.; Wu, L.; Yang, Z.; Xu, T. Ion Exchange Membranes: Constructing and Tuning Ion Transport Channels. Adv. Funct. Mater. 2022, 32, 2207366. [Google Scholar] [CrossRef]
- Mustain, W.E.; Chatenet, M.; Page, M.; Kim, Y.S. Durability challenges of anion exchange membrane fuel cells. Energy Environ. Sci. 2020, 13, 2805–2838. [Google Scholar] [CrossRef]
- Pan, J.; Li, Y.; Han, J.; Li, G.; Tan, L.; Chen, C.; Lu, J.; Zhuang, L. A strategy for disentangling the conductivity–stability dilemma in alkaline polymer electrolytes. Energy Environ. Sci. 2013, 6, 2912–2915. [Google Scholar] [CrossRef]
- Noh, S.; Jeon, J.Y.; Adhikari, S.; Kim, Y.S.; Bae, C. Molecular Engineering of Hydroxide Conducting Polymers for Anion Exchange Membranes in Electrochemical Energy Conversion Technology. Acc. Chem. Res. 2019, 52, 2745–2755. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicki, S.; Douglin, J.C.; Kostuch, A.; Dekel, D.R.; Kruczała, K. Are Radicals Formed During Anion-Exchange Membrane Fuel Cell Operation? J. Phys. Chem. Lett. 2020, 11, 7630–7636. [Google Scholar] [CrossRef]
- Huang, J.; Yu, Z.; Tang, J.; Wang, P.; Tan, Q.; Wang, J.; Lei, X. A review on anion exchange membranes for fuel cells: Anion-exchange polyelectrolytes and synthesis strategies. Int. J. Hydrogen Energy 2022, 47, 27800–27820. [Google Scholar] [CrossRef]
- Arges, C.G.; Ramani, V. Two-dimensional NMR spectroscopy reveals cation-triggered backbone degradation in polysulfone-based anion exchange membranes. Proc. Natl. Acad. Sci. USA 2013, 110, 2490–2495. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, C.; Kim, D.-S.; Hibbs, M.; Wrobleski, D.; Kim, Y.S. Backbone stability of quaternized polyaromatics for alkaline membrane fuel cells. J. Membr. Sci. 2012, 423–424, 438–449. [Google Scholar] [CrossRef]
- Takamuku, S.; Jannasch, P. Properties and degradation of hydrocarbon fuel cell membranes: A comparative study of sulfonated poly(arylene ether sulfone)s with different positions of the acid groups. Polym. Chem. 2012, 3, 1202–1214. [Google Scholar] [CrossRef]
- Choe, Y.-K.; Fujimoto, C.; Lee, K.-S.; Dalton, L.T.; Ayers, K.; Henson, N.J.; Kim, Y.S. Alkaline Stability of Benzyl Trimethyl Ammonium Functionalized Polyaromatics: A Computational and Experimental Study. Chem. Mater. 2014, 26, 5675–5682. [Google Scholar] [CrossRef]
- Mohanty, A.D.; Tignor, S.E.; Krause, J.A.; Choe, Y.-K.; Bae, C. Systematic Alkaline Stability Study of Polymer Backbones for Anion Exchange Membrane Applications. Macromolecules 2016, 49, 3361–3372. [Google Scholar] [CrossRef]
- Song, W.; Peng, K.; Xu, W.; Liu, X.; Zhang, H.; Liang, X.; Ye, B.; Zhang, H.; Yang, Z.; Wu, L.; et al. Upscaled production of an ultramicroporous anion-exchange membrane enables long-term operation in electrochemical energy devices. Nat. Commun. 2023, 14, 2732. [Google Scholar] [CrossRef]
- Wang, J.J.; Gao, W.T.; Choo, Y.S.L.; Cai, Z.H.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Highly conductive branched poly(aryl piperidinium) anion exchange membranes with robust chemical stability. J. Colloid Interface Sci. 2023, 629, 377–387. [Google Scholar] [CrossRef]
- Bai, L.; Ma, L.; Li, L.; Zhang, A.; Yan, X.; Zhang, F.; He, G. Branched, Side-Chain Grafted Polyarylpiperidine Anion Exchange Membranes for Fuel Cell Application. ACS Appl. Energy Mater. 2021, 4, 6957–6967. [Google Scholar] [CrossRef]
- Chen, N.; Wang, H.H.; Kim, S.P.; Kim, H.M.; Lee, W.H.; Hu, C.; Bae, J.Y.; Sim, E.S.; Chung, Y.C.; Jang, J.H.; et al. Poly(fluorenyl aryl piperidinium) membranes and ionomers for anion exchange membrane fuel cells. Nat. Commun. 2021, 12, 2367. [Google Scholar] [CrossRef]
- Olsson, J.S.; Pham, T.H.; Jannasch, P. Poly(arylene piperidinium) Hydroxide Ion Exchange Membranes: Synthesis, Alkaline Stability, and Conductivity. Adv. Funct. Mater. 2018, 28, 1702758. [Google Scholar] [CrossRef]
- Ertem, S.P.; Coughlin, E.B. Alkaline Stability Evaluation of Polymerizable Hexyl-Tethered Ammonium Cations. Macromol. Rapid Commun. 2022, 43, 2100610. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Willdorf-Cohen, S.; Schibli, E.M.; Paula, Z.; Li, W.; Skalski, T.J.G.; Sergeenko, A.T.; Hohenadel, A.; Frisken, B.J.; Magliocca, E.; et al. Poly(bis-arylimidazoliums) possessing high hydroxide ion exchange capacity and high alkaline stability. Nat. Commun. 2019, 10, 2306. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.G.; Kreuer, K.D. Alkaline Stability of Quaternary Ammonium Cations for Alkaline Fuel Cell Membranes and Ionic Liquids. ChemSusChem 2015, 8, 513–523. [Google Scholar] [CrossRef]
- Noonan, K.J.T.; Hugar, K.M.; Kostalik, H.A.I.V.; Lobkovsky, E.B.; Abruña, H.D.; Coates, G.W. Phosphonium-Functionalized Polyethylene: A New Class of Base-Stable Alkaline Anion Exchange Membranes. J. Am. Chem. Soc. 2012, 134, 18161–18164. [Google Scholar] [CrossRef] [PubMed]
- Zha, Y.; Disabb-Miller, M.L.; Johnson, Z.D.; Hickner, M.A.; Tew, G.N. Metal-Cation-Based Anion Exchange Membranes. J. Am. Chem. Soc. 2012, 134, 4493–4496. [Google Scholar] [CrossRef]
- Chen, N.; Jin, Y.; Liu, H.; Hu, C.; Wu, B.; Xu, S.; Li, H.; Fan, J.; Lee, Y.M. Insight into the Alkaline Stability of N-Heterocyclic Ammonium Groups for Anion-Exchange Polyelectrolytes. Angew. Chem. Int. Ed. 2021, 60, 19272–19280. [Google Scholar] [CrossRef]
- Zeng, M.; He, X.; Wen, J.; Zhang, G.; Zhang, H.; Feng, H.; Qian, Y.; Li, M. N-Methylquinuclidinium-Based Anion Exchange Membrane with Ultrahigh Alkaline Stability. Adv. Mater. 2023, 35, 2306675. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Y.; Setzler, B.P.; Rojas-Carbonell, S.; Ben Yehuda, C.; Amel, A.; Page, M.; Wang, L.; Hu, K.; Shi, L.; et al. Poly(aryl piperidinium) membranes and ionomers for hydroxide exchange membrane fuel cells. Nat. Energy 2019, 4, 392–398. [Google Scholar] [CrossRef]
- Miyanishi, S.; Yamaguchi, T. Ether cleavage-triggered degradation of benzyl alkylammonium cations for polyethersulfone anion exchange membranes. Phys. Chem. Chem. Phys. 2016, 18, 12009–12023. [Google Scholar] [CrossRef]
- Han, J.; Song, W.; Cheng, X.; Cheng, Q.; Zhang, Y.; Liu, C.; Zhou, X.; Ren, Z.; Hu, M.; Ning, T.; et al. Conductivity and Stability Properties of Anion Exchange Membranes: Cation Effect and Backbone Effect. ChemSusChem 2021, 14, 5021–5031. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhou, J.; Wang, S.; Hou, J.; Wu, L.; Xu, T. A strategy to construct alkali-stable anion exchange membranes bearing ammonium groups via flexible spacers. J. Mater. Chem. A 2015, 3, 15015–15019. [Google Scholar] [CrossRef]
- Pham, T.H.; Olsson, J.S.; Jannasch, P. Effects of the N-alicyclic cation and backbone structures on the performance of poly(terphenyl)-based hydroxide exchange membranes. J. Mater. Chem. A 2019, 7, 15895–15906. [Google Scholar] [CrossRef]
- Ling, Q.; Wang, C.; Wang, T.; Yang, S.; Li, X.; Wei, H.; Ding, Y. Beyond Small Molecular Cations: Elucidating the Alkaline Stability of Cationic Moieties at the Membrane Scale. ChemSusChem 2024, 17, e202301656. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Pan, D.; Gong, H.; Jannasch, P. Hydroxide Conducting Membranes with Quaternary Ammonium Cations Tethered to Poly(arylene alkylene)s via Flexible Phenylpropyl Spacers. Chem. Mater. 2023, 36, 371–381. [Google Scholar] [CrossRef]
- Huang, J.; Yu, Z.; Tang, J.; Wang, P.; Zhang, X.; Wang, J.; Lei, X. A non-cationic crosslinking strategy to improve the performance of anion exchange membranes based on poly(aryl piperidinium) for fuel cells. Colloid. Surface. A 2023, 674, 131890. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, Y.; Wang, Z.; Liu, D.; Wang, Y.; Dong, T.; Wang, S.; Li, Z.; Wu, J.; Lei, Y. Synergistic functionalization of poly(p-terphenyl isatin) anion exchange membrane with quaternary ammonium and piperidine cations for fuel cells. Ind. Chem. Mater. 2024, 2, 141–153. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Guo, M.; Ban, T.; Zhu, X. Poly(isatin-piperidinium-terphenyl) anion exchange membranes with improved performance for direct borohydride fuel cells. Int. J. Hydrog. Energy 2023, 48, 14837–14852. [Google Scholar] [CrossRef]
- Qi, L.; Wang, X.; Chao, G.; Li, N.; Zhang, X. Enhancement in the alkaline stability of poly(arylene ether ketone)-typed anion-exchange membranes via phenylene piperidinium blocks. J. Membr. Sci. 2023, 687, 122057. [Google Scholar] [CrossRef]
- Liu, Y.J.; Gao, W.T.; Zhu, A.M.; Zhang, Q.G.; Liu, Q.L. High-performance di-piperidinium-crosslinked poly(p-terphenyl piperidinium) anion exchange membranes. J. Membr. Sci. 2023, 687, 122045. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, W.; Yin, T.; Chen, S.; Zhang, X.; Li, N.; Liu, L. Oligo (ethylene glycol)-grafted poly(terphenyl indole piperidinium) with high water diffusivity for anion exchange membrane fuel cells. J. Membr. Sci. 2024, 694, 122424. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Song, W.; Sun, L.; Yang, C.; Zhang, X.; Wu, M.; Wu, L.; Ge, X.; Xu, T. Anion Exchange Membrane with Pendulous Piperidinium on Twisted All-Carbon Backbone for Fuel Cell. Membranes 2024, 14, 121. https://doi.org/10.3390/membranes14060121
Zhang H, Song W, Sun L, Yang C, Zhang X, Wu M, Wu L, Ge X, Xu T. Anion Exchange Membrane with Pendulous Piperidinium on Twisted All-Carbon Backbone for Fuel Cell. Membranes. 2024; 14(6):121. https://doi.org/10.3390/membranes14060121
Chicago/Turabian StyleZhang, Huaqing, Wanjie Song, Lixuan Sun, Cui Yang, Xin Zhang, Mingyue Wu, Liang Wu, Xiaolin Ge, and Tongwen Xu. 2024. "Anion Exchange Membrane with Pendulous Piperidinium on Twisted All-Carbon Backbone for Fuel Cell" Membranes 14, no. 6: 121. https://doi.org/10.3390/membranes14060121
APA StyleZhang, H., Song, W., Sun, L., Yang, C., Zhang, X., Wu, M., Wu, L., Ge, X., & Xu, T. (2024). Anion Exchange Membrane with Pendulous Piperidinium on Twisted All-Carbon Backbone for Fuel Cell. Membranes, 14(6), 121. https://doi.org/10.3390/membranes14060121






