Heavy Metal Detection and Removal by Composite Carbon Quantum Dots/Ionomer Membranes
Abstract
1. Introduction
2. Materials and Methods
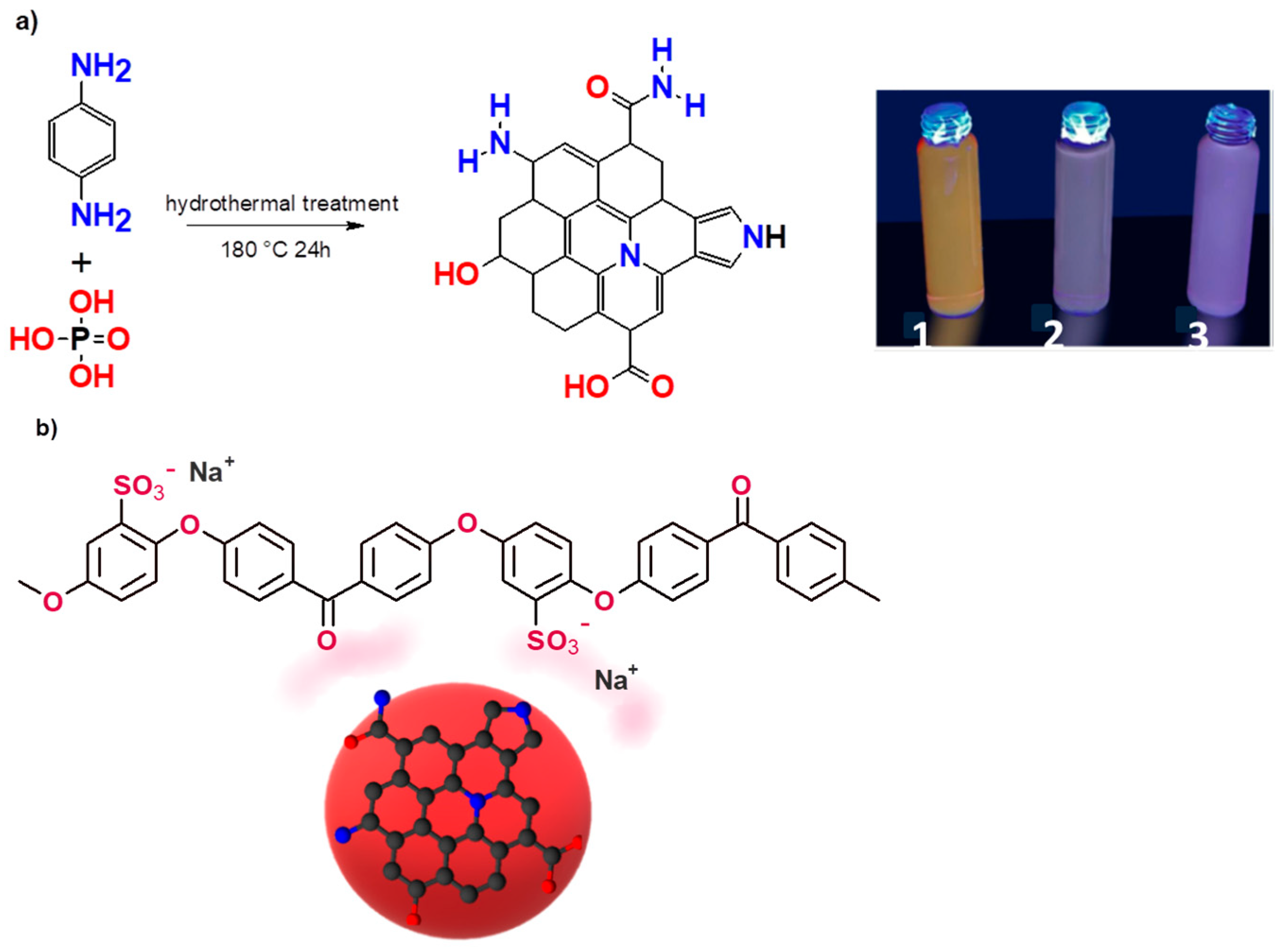
2.1. Synthesis of Red CQDs (rCQDs)
2.2. Preparation of SPEEK/rCQDs Composite
2.3. Ion Exchange Capacity (IEC)
2.4. Water Uptake (WU)
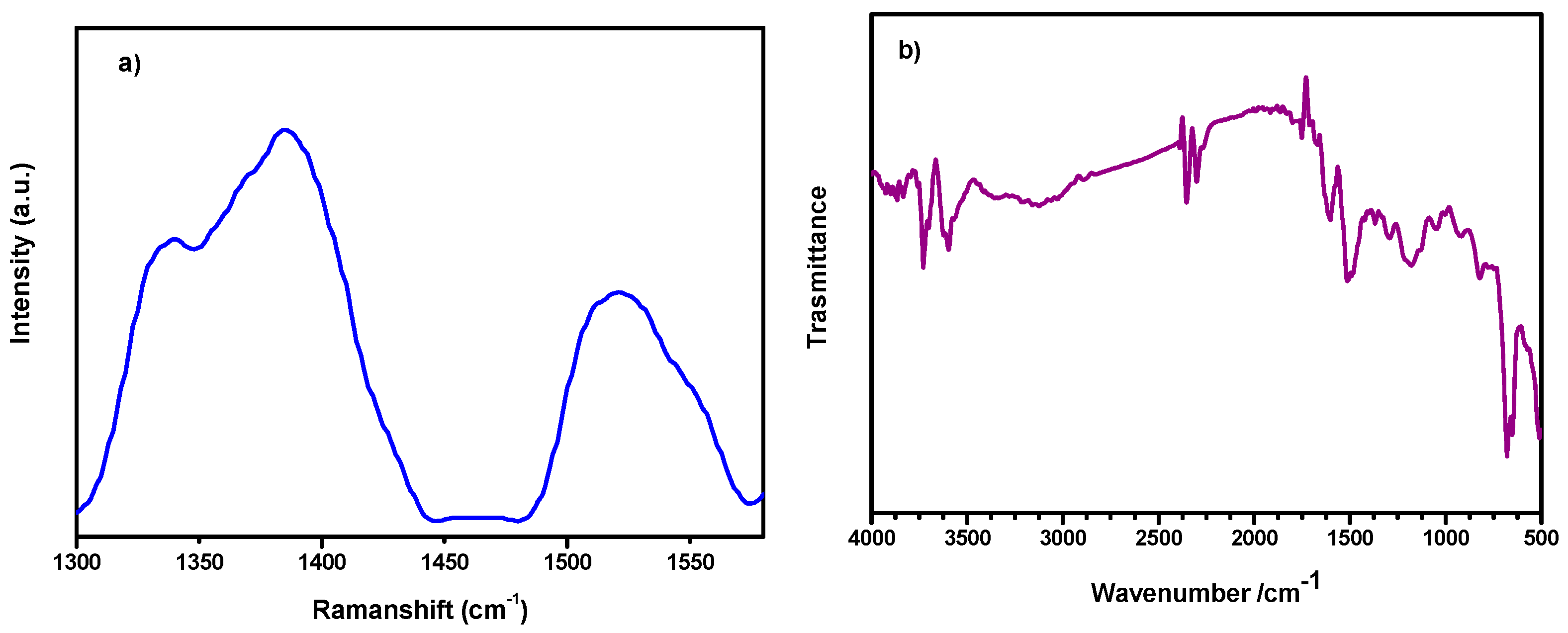
2.5. Raman Spectroscopy
2.6. Fourier-Transform Infrared Spectroscopy (FTIR)
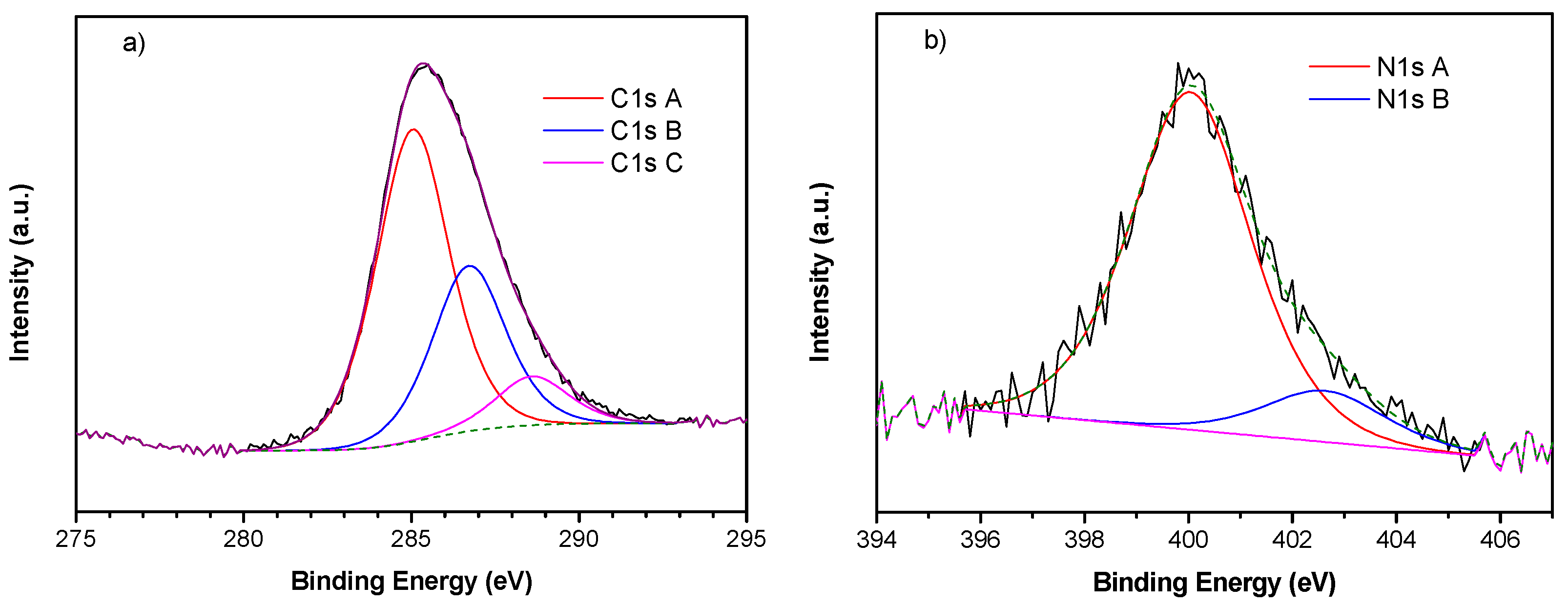
2.7. X-ray Photoemission Spectroscopy (XPS)
2.8. Atomic Force Microscopy (AFM)
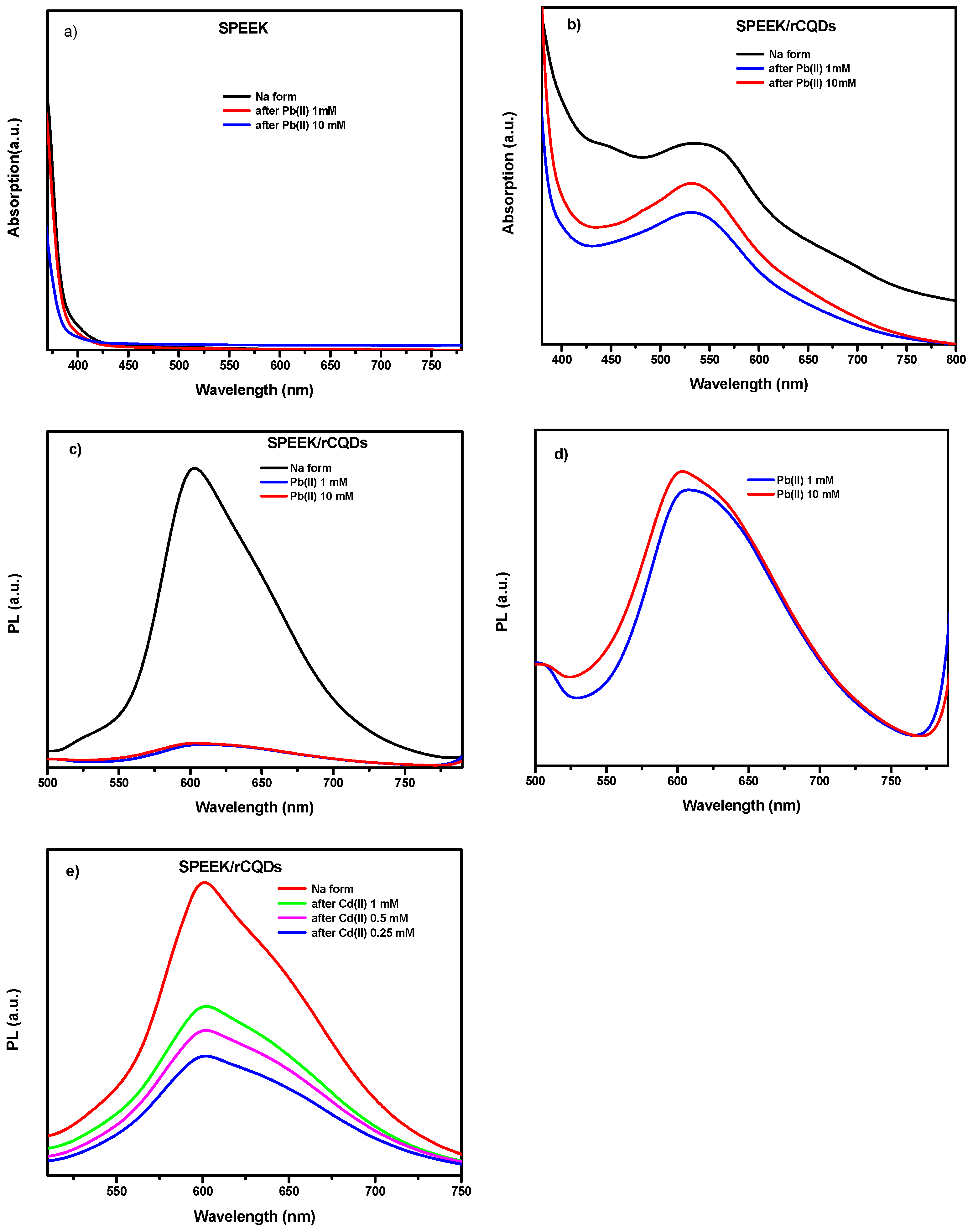
2.9. UV/VIS/NIR Absorption Spectroscopy
2.10. Photoluminescence Spectroscopy
2.11. Optical Microscopy
2.12. Inductively Coupled Plasma–Optical Emission Spectroscopy (ICP-OES)
2.13. Batch Sorption Studies
2.14. Stability Test of Composite Membranes
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hickner, M.A. Ion-containing polymers: New energy & clean water. Mater. Today 2010, 13, 34–41. [Google Scholar]
- Narducci, R.; Di Vona, M.L.; Knauth, P. Cation-conducting ionomers made by ion exchange of sulfonated poly-ether-ether-ketone: Hydration, mechanical and thermal properties and ionic conductivity. J. Membr. Sci. 2014, 465, 185–192. [Google Scholar] [CrossRef]
- Di Vona, M.L.; Licoccia, S.; Knauth, P. Organic-inorganic hybrid membranes based on sulfonated polyaryl-ether-ketones: Correlation between water uptake and electrical conductivity. Solid State Ion. 2008, 179, 1161–1165. [Google Scholar] [CrossRef]
- Sgreccia, E.; Rogalska, C.; Gallardo Gonzalez, F.S.; Prosposito, P.; Burratti, L.; Knauth, P.; Di Vona, M.L. Heavy metal decontamination by ion exchange polymers for water purification: Counterintuitive cation removal by an anion exchange polymer. J. Mater. Sci. 2024, 59, 2776–2787. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; WHO: Geneva, Switzerland, 2022; ISBN 978-92-4-004506-4. [Google Scholar]
- Nordberg, M.; Nordberg, G.F.; Fowler, B.A.; Friberg, L. Handbook on the Toxicology of Metals; Elsevier Science: Burlington, NJ, USA, 2011. [Google Scholar]
- Medeiros, G.A.; da Silva Rodrigues, C.V.; Spencer, J.; Neto, B.A.D. 7-Carbon dots (C-dots): Fluorescence processes and bioimaging. In Quantum Materials, Devices, and Applications; Henini, M., Rodrigues, M.O., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 201–213. [Google Scholar]
- Li, L.; Wu, G.; Yang, G.; Peng, J.; Zhao, J.; Zhu, J.-J. Focusing on luminescent graphene quantum dots: Current status and future perspectives. Nanoscale 2013, 5, 4015–4039. [Google Scholar] [CrossRef] [PubMed]
- Gude, V.; Das, A.; Chatterjee, T.; Mandal, P.K. Molecular origin of photoluminescence of carbon dots: Aggregation-induced orange-red emission. Phys. Chem. Chem. Phys. 2016, 18, 28274–28280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, R.; Feng, B.; Zhong, X.; Ostrikov, K. Photoluminescence mechanism of carbon dots: Triggering high-color-purity red fluorescence emission through edge amino protonation. Nat. Commun. 2021, 12, 6856. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Park, Y.; Cheon, B.; Park, M.H. Carbon Dots as an Effective Fluorescent Sensing Platform for Metal Ion Detection. Nanoscale Res. Lett. 2019, 14, 272. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Zhang, Z.L.; Tian, Z.Q.; Zhang, L.; Liu, C.; Lin, Y.; Qi, B.P.; Pang, D.W. Electrochemical Tuning of Luminescent Carbon Nanodots: From Preparation to Luminescence Mechanism. Adv. Mater. 2011, 23, 5801–5806. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.H.; Du, C.; Zhuang, Z.H.; Chen, W. Carbon quantum dot-based nanoprobes for metal ion detection. J. Mater. Chem. C 2016, 4, 6927–6945. [Google Scholar] [CrossRef]
- Dai, D.H.; Yang, J.; Wang, Y.; Yang, Y.W. Recent Progress in Functional Materials for Selective Detection and Removal of Mercury(II) Ions. Adv. Funct. Mater. 2021, 31, 2006168. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Wang, Y.X.; Meng, F.D.; Wang, B.X.; Cheng, Y.X.; Zhu, C.J. N-doped carbon dots synthesized by rapid microwave irradiation as highly fluorescent probes for Pb2+ detection. New J. Chem. 2015, 39, 3357–3360. [Google Scholar] [CrossRef]
- Wang, Z.X.; Yu, X.H.; Li, F.; Kong, F.Y.; Lv, W.X.; Fan, D.H.; Wang, W. Preparation of boron-doped carbon dots for fluorometric determination of Pb(II), Cu(II) and pyrophosphate ions. Microchim. Acta 2017, 184, 4775–4783. [Google Scholar] [CrossRef]
- Tan, Q.; Li, X.; Wang, L.; Zhao, J.; Yang, Q.; Sun, P.; Deng, Y.; Shen, G. One-step synthesis of highly fluorescent carbon dots as fluorescence sensors for the parallel detection of cadmium and mercury ions. Front. Chem. 2022, 10, 1005231. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, X.; Wu, Y.; Wei, C.; Zeng, H. Origin of green luminescence in carbon quantum dots: Specific emission bands originate from oxidized carbon groups. New J. Chem. 2018, 42, 4603–4611. [Google Scholar] [CrossRef]
- Devi, P.; Rajput, P.; Thakur, A.; Kim, K.-H.; Kumar, P. Recent advances in carbon quantum dot-based sensing of heavy metals in water. TrAC Trends Anal. Chem. 2019, 114, 171–195. [Google Scholar] [CrossRef]
- Han, Z.; Nan, D.; Yang, H.; Sun, Q.; Pan, S.; Liu, H.; Hu, X. Carbon quantum dots based ratiometric fluorescence probe for sensitive and selective detection of Cu2+ and glutathione. Sens. Actuators B Chem. 2019, 298, 126842. [Google Scholar] [CrossRef]
- He, Y.R.; Zhao, D.L.; Chung, T.S. Na+ functionalized carbon quantum dot incorporated thin-film nanocomposite membranes for selenium and arsenic removal. J. Membr. Sci. 2018, 564, 483–491. [Google Scholar] [CrossRef]
- Lu, H.Z.; Xu, S.F.; Liu, J.Q. One Pot Generation of Blue and Red Carbon Dots in One Binary Solvent System for Dual Channel Detection of Cr3+ and Pb2+ Based on Ion Imprinted Fluorescence Polymers. ACS Sens. 2019, 4, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Vogler, R.J.; Al Hasnine, S.M.A.; Hernandez, S.; Malekzadeh, N.; Hoelen, T.P.; Hatakeyama, E.S.; Bhattacharyya, D. Mercury Removal from Wastewater Using Cysteamine Functionalized Membranes. ACS Omega 2020, 5, 22255–22267. [Google Scholar] [CrossRef]
- Nallayagari, A.R.; Sgreccia, E.; Pizzoferrato, R.; Cabibbo, M.; Kaciulis, S.; Bolli, E.; Pasquini, L.; Knauth, P.; Di Vona, M.L. Tuneable properties of carbon quantum dots by different synthetic methods. J. Nanostructure Chem. 2022, 12, 565–580. [Google Scholar] [CrossRef]
- Chen, J.; Wei, J.-S.; Zhang, P.; Niu, X.-Q.; Zhao, W.; Zhu, Z.-Y.; Ding, H.; Xiong, H.-M. Red-Emissive Carbon Dots for Fingerprints Detection by Spray Method: Coffee Ring Effect and Unquenched Fluorescence in Drying Process. ACS Appl. Mater. Interfaces 2017, 9, 18429–18433. [Google Scholar] [CrossRef] [PubMed]
- Knauth, P.; Di Vona, M.L. Sulfonated aromatic ionomers: Analysis of proton conductivity and proton mobility. Solid State Ion. 2012, 225, 255–259. [Google Scholar] [CrossRef]
- Burratti, L.; Maranges, V.; Sisani, M.; Naryyev, E.; De Matteis, F.; Francini, R.; Prosposito, P. Determination of Pb(II) Ions in Water by Fluorescence Spectroscopy Based on Silver Nanoclusters. Chemosensors 2022, 10, 385. [Google Scholar] [CrossRef]
- Thang, P.N.; Hung, L.X.; Thuan, D.N.; Yen, N.H.; Hien, N.T.T.; Hanh, V.T.H.; Khang, N.C.; Laverdant, J.; Thu, P. Temperature-dependent Raman investigation and photoluminescence of graphene quantum dots with and without nitrogen-doping. J. Mater. Sci. 2021, 56, 4979–4990. [Google Scholar] [CrossRef]
- Pan, D.Y.; Zhang, J.C.; Li, Z.; Wu, M.H. Hydrothermal Route for Cutting Graphene Sheets into Blue-Luminescent Graphene Quantum Dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Hallaji, Z.; Bagheri, Z.; Kalji, S.-O.; Ermis, E.; Ranjbar, B. Recent advances in the rational synthesis of red-emissive carbon dots for nanomedicine applications: A review. FlatChem 2021, 29, 100271. [Google Scholar] [CrossRef]
- Gao, D.; Zhao, H.; Chen, X.; Fan, H. Recent advance in red-emissive carbon dots and their photoluminescent mechanisms. Mater. Today Chem. 2018, 9, 103–113. [Google Scholar] [CrossRef]
- Barzetti, T.; Selli, E.; Moscotti, D.; Forni, L. Pyridine and ammonia as probes for FTIR analysis of solid acid catalysts. J. Chem. Soc. Faraday Trans. 1996, 92, 1401–1407. [Google Scholar] [CrossRef]
- Sciortino, A.; Cannas, M.; Messina, F. Temperature-Dependence of Solvent-Induced Stokes Shift and Fluorescence Tunability in Carbon Nanodots. C 2019, 5, 20. [Google Scholar] [CrossRef]
- Kasha, M. Characterization of electronic transitions in complex molecules. Discuss. Faraday Soc. 1950, 9, 14–19. [Google Scholar] [CrossRef]
- Dager, A.; Uchida, T.; Maekawa, T.; Tachibana, M. Synthesis and characterization of Mono-disperse Carbon Quantum Dots from Fennel Seeds: Photoluminescence analysis using Machine Learning. Sci. Rep. 2019, 9, 14004. [Google Scholar] [CrossRef] [PubMed]
- Okpalugo, T.I.T.; Papakonstantinou, P.; Murphy, H.; McLaughlin, J.; Brown, N.M.D. High resolution XPS characterization of chemical functionalised MWCNTs and SWCNTs. Carbon 2005, 43, 153–161. [Google Scholar] [CrossRef]
- Kingsbury, R.S.; Bruning, K.; Zhu, S.; Flotron, S.; Miller, C.T.; Coronell, O. Influence of Water Uptake, Charge, Manning Parameter, and Contact Angle on Water and Salt Transport in Commercial Ion Exchange Membranes. Ind. Eng. Chem. Res. 2019, 58, 18663–18674. [Google Scholar] [CrossRef]
- Xing, P.; Robertson, G.P.; Guiver, M.D.; Mikhailenko, S.D.; Wang, K.; Kaliaguine, S. Synthesis and characterization of sulfonated poly(ether ether ketone) for proton exchange membranes. J. Membr. Sci. 2004, 229, 95–106. [Google Scholar] [CrossRef]
- Lofrano, G.; Carotenuto, M.; Libralato, G.; Domingos, R.F.; Markus, A.; Dini, L.; Gautam, R.K.; Baldantoni, D.; Rossi, M.; Sharma, S.K.; et al. Polymer functionalized nanocomposites for metals removal from water and wastewater: An overview. Water Res. 2016, 92, 22–37. [Google Scholar] [CrossRef]
- Conway, B.E.; Ayranci, E. Effective Ionic Radii and Hydration Volumes for Evaluation of Solution Properties and Ionic Adsorption. J. Solut. Chem. 1999, 28, 163–192. [Google Scholar] [CrossRef]


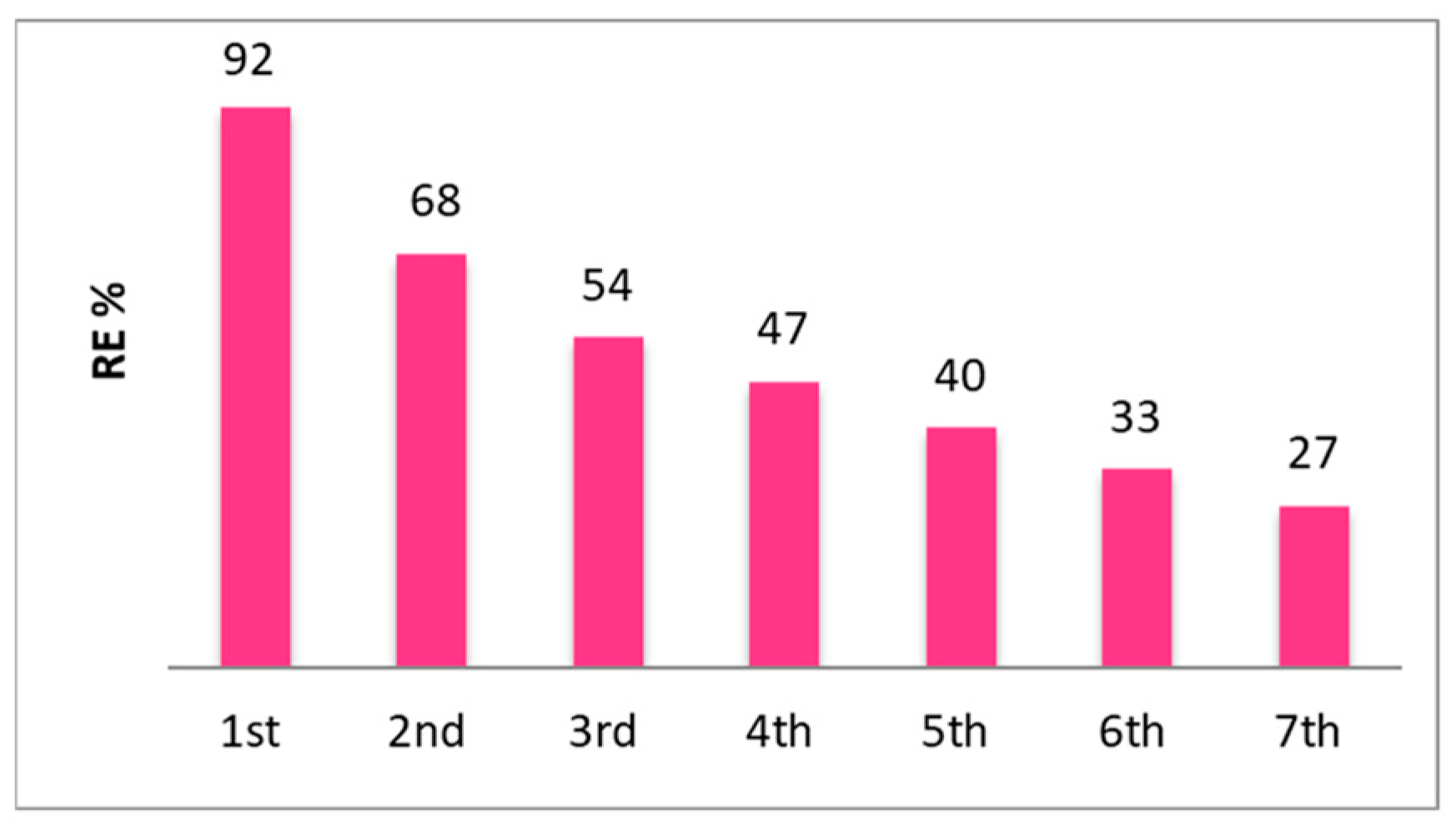

| Pb(II) | ||
|---|---|---|
| Ci mg/L (mM) | Cf mg/L | RE% |
| 2238 (10.8) | 1253 | 44 |
| 1036 (5.0) | 83 | 92 |
| 207 (1.0) | 0 | 100 |
| Cd(II) | ||
| 1130 (10) | 346 | 41 |
| 125 (1.1) | 3.8 | 97 |
| 63 (0.55) | 0 | 100 |
| 31 (0.27) | 0 | 100 |
| Time (h) | Pb(II) Concentration/mg/L (mM) | RE (%) |
|---|---|---|
| 0 | 145 (0.7) | 0 |
| 0.25 | 116 | 20 |
| 0.5 | 109 | 33 |
| 1 | 89 | 39 |
| 2 | 75 | 48 |
| 4 | 55 | 62 |
| Cd(II) concentration/mg/L (mM) | ||
| 0 | 85 (0.7) | 0 |
| 0.25 | 72 | 15 |
| 0.5 | 65 | 23 |
| 1 | 52 | 38 |
| 2 | 39 | 53 |
| 4 | 33 | 61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sgreccia, E.; Gallardo Gonzalez, F.S.; Prosposito, P.; Burratti, L.; Sisani, M.; Bastianini, M.; Knauth, P.; Di Vona, M.L. Heavy Metal Detection and Removal by Composite Carbon Quantum Dots/Ionomer Membranes. Membranes 2024, 14, 134. https://doi.org/10.3390/membranes14060134
Sgreccia E, Gallardo Gonzalez FS, Prosposito P, Burratti L, Sisani M, Bastianini M, Knauth P, Di Vona ML. Heavy Metal Detection and Removal by Composite Carbon Quantum Dots/Ionomer Membranes. Membranes. 2024; 14(6):134. https://doi.org/10.3390/membranes14060134
Chicago/Turabian StyleSgreccia, Emanuela, Francia Sarhaly Gallardo Gonzalez, Paolo Prosposito, Luca Burratti, Michele Sisani, Maria Bastianini, Philippe Knauth, and Maria Luisa Di Vona. 2024. "Heavy Metal Detection and Removal by Composite Carbon Quantum Dots/Ionomer Membranes" Membranes 14, no. 6: 134. https://doi.org/10.3390/membranes14060134
APA StyleSgreccia, E., Gallardo Gonzalez, F. S., Prosposito, P., Burratti, L., Sisani, M., Bastianini, M., Knauth, P., & Di Vona, M. L. (2024). Heavy Metal Detection and Removal by Composite Carbon Quantum Dots/Ionomer Membranes. Membranes, 14(6), 134. https://doi.org/10.3390/membranes14060134











