Dehydration of Organic Solvents from Ternary Mixtures Containing Toluene/Methanol/Water by Pervaporation
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Pervaporation Experiments
2.3. Characterizations of PVA Membrane
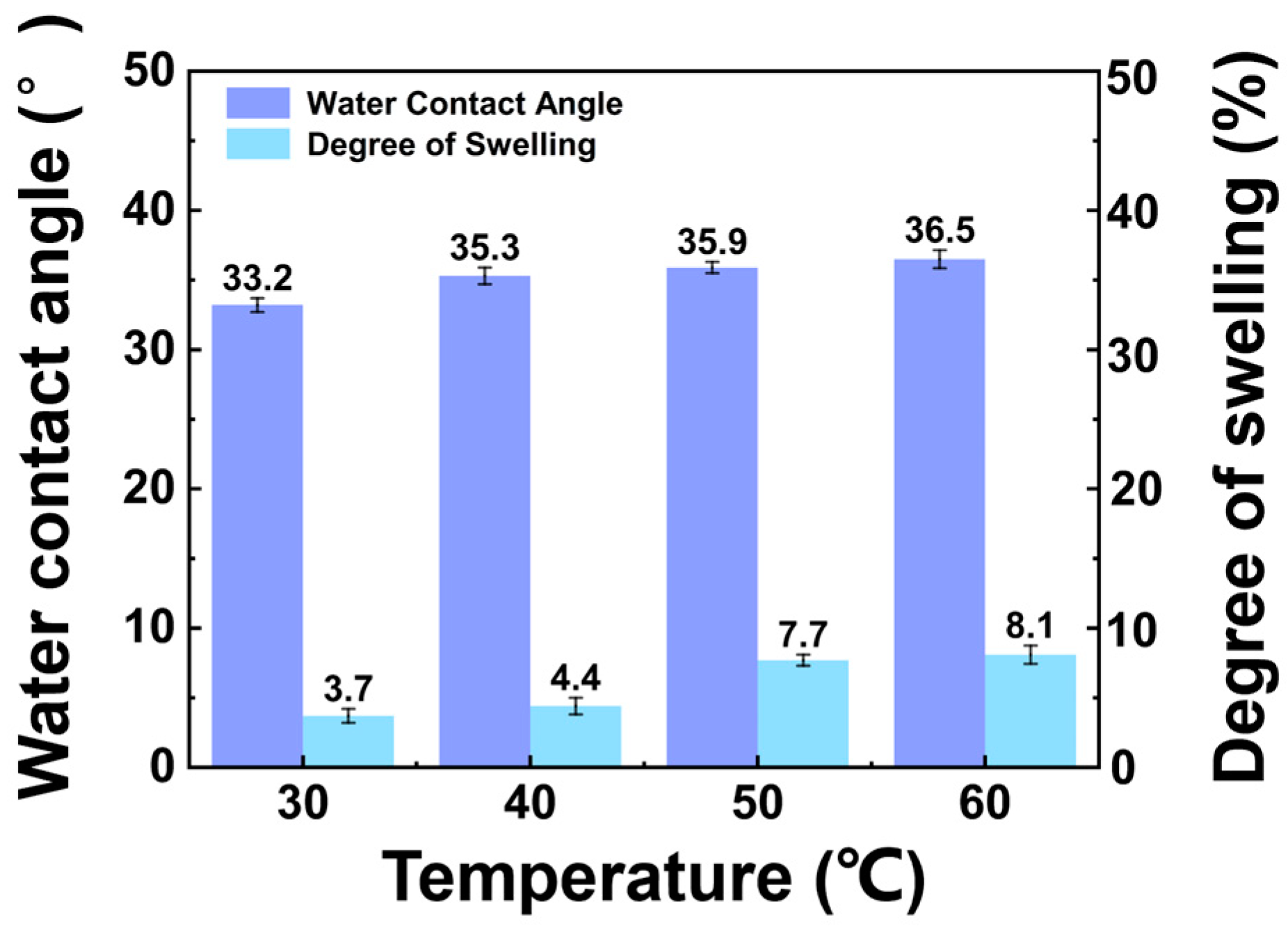
2.3.1. Contact Angle
2.3.2. Measurement of Membrane Swelling
2.4. Data Analysis
3. Results and Discussion
3.1. Influence of Operating Conditions on Pervaporation
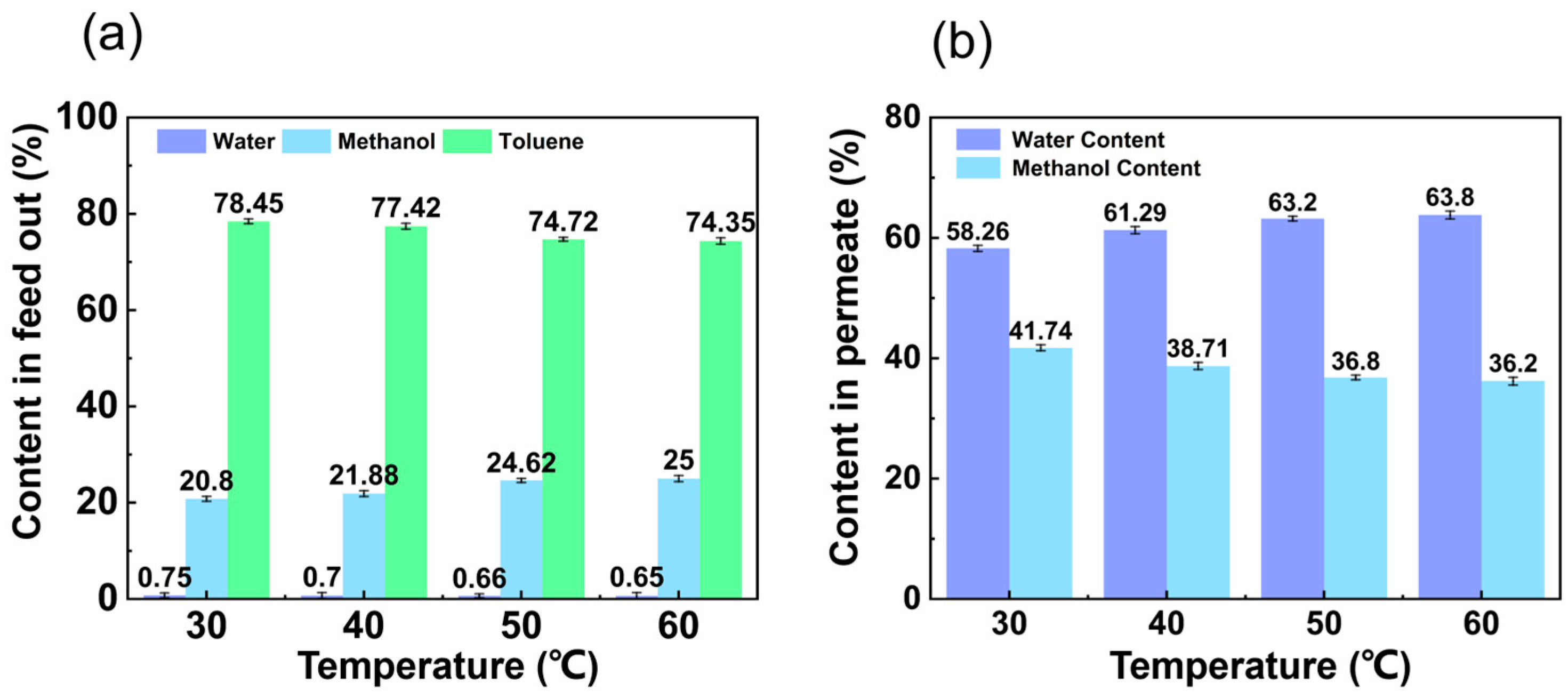
3.1.1. Influence of Feed Temperature
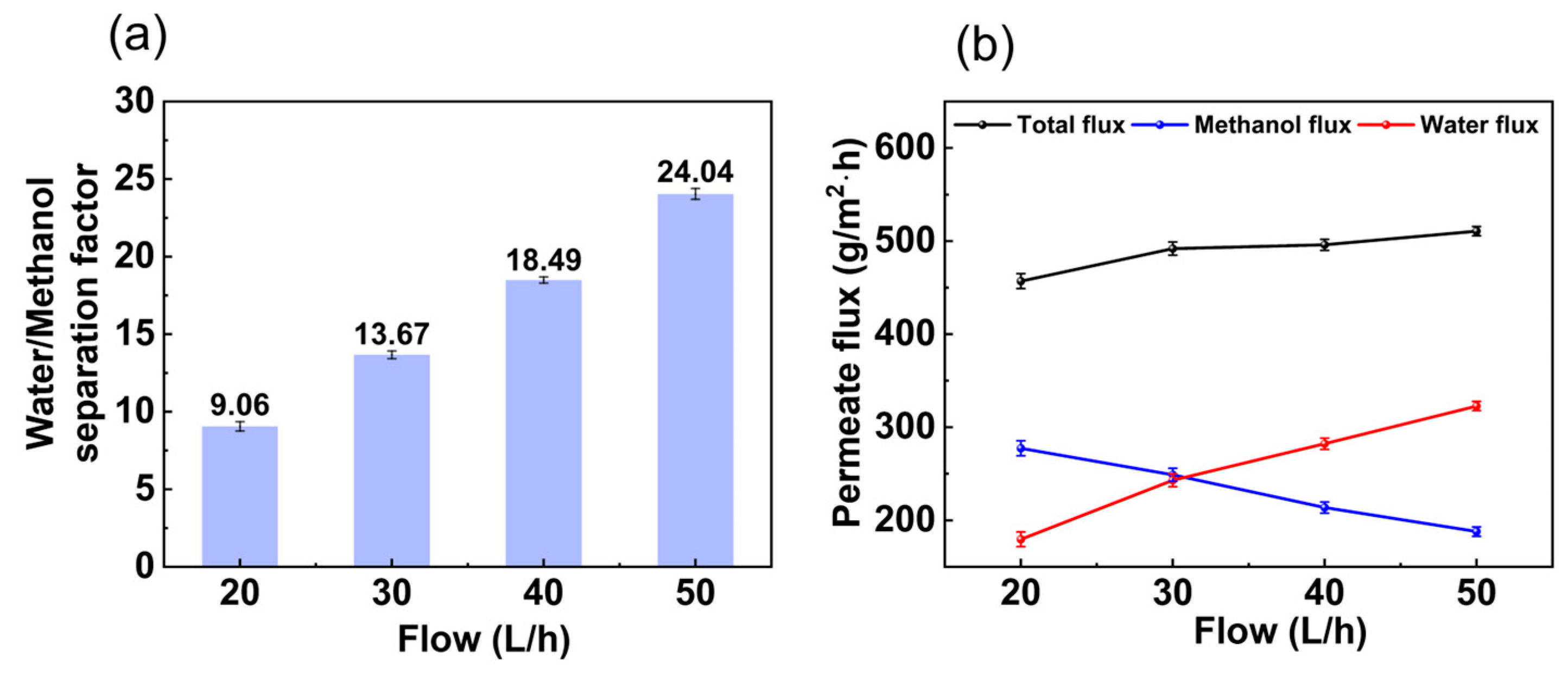
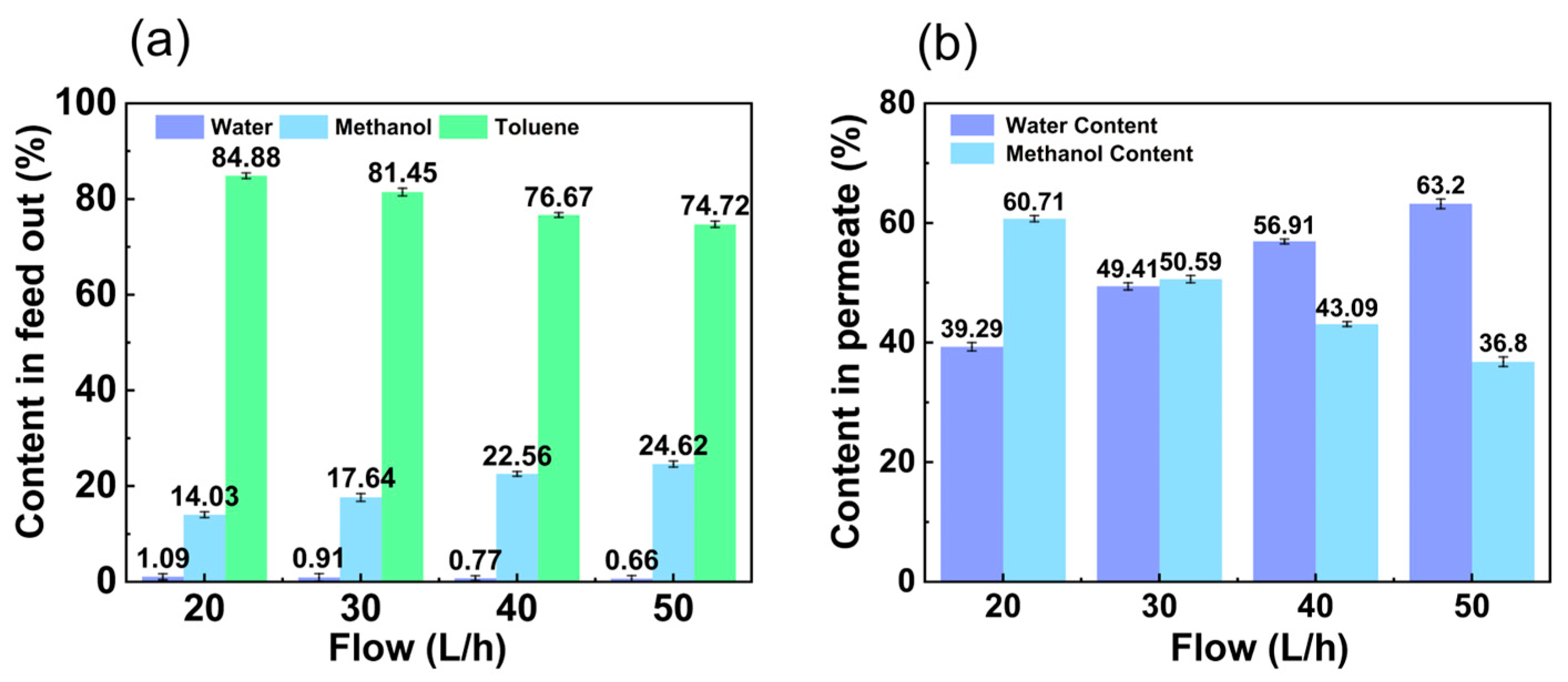
3.1.2. Influence of Feed Flow Rate
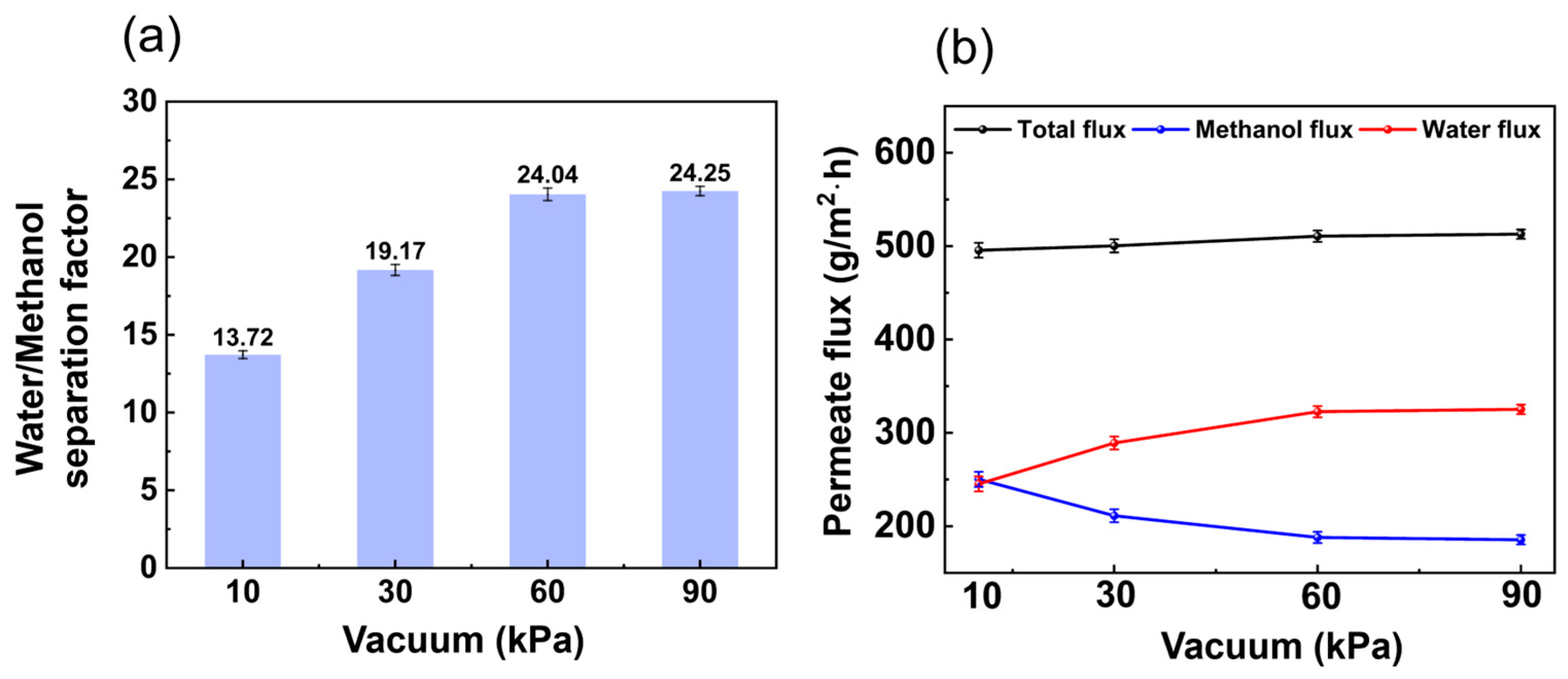
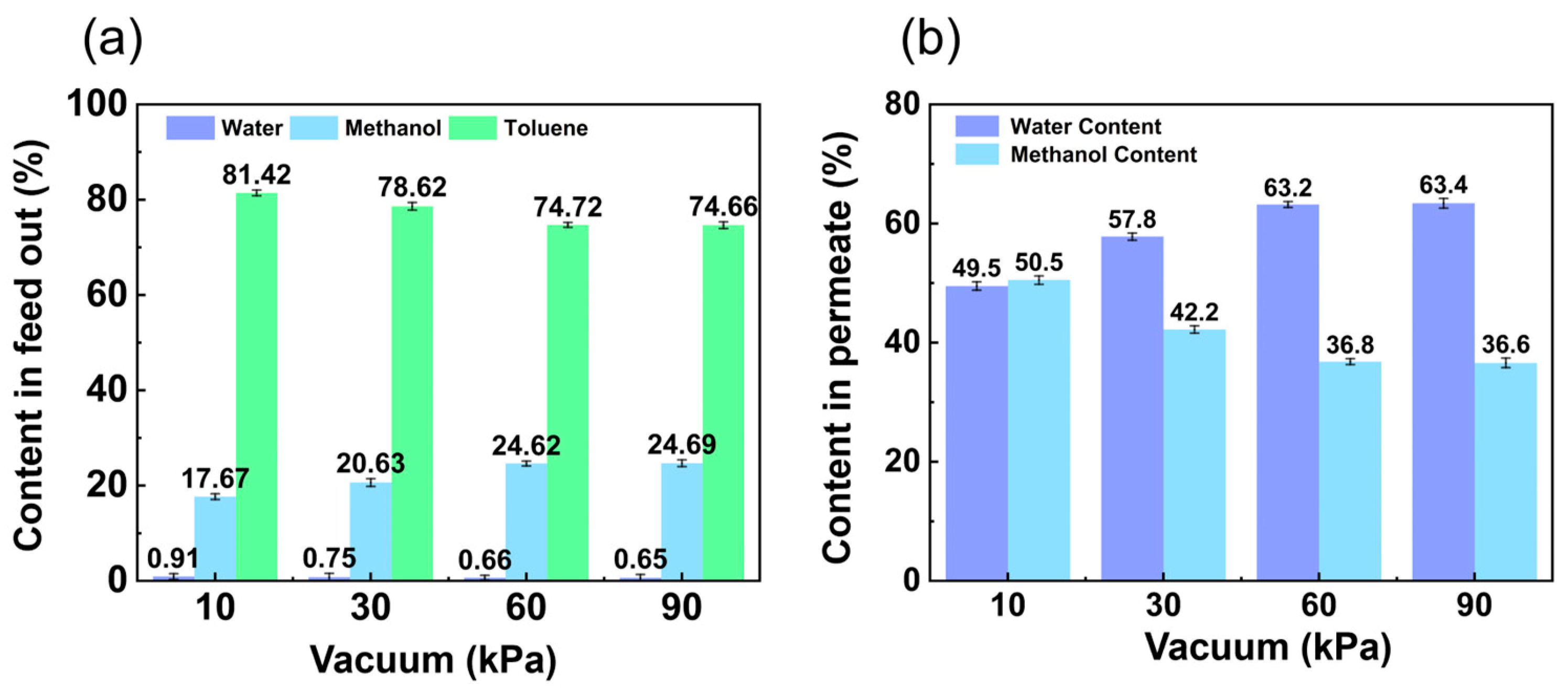
3.1.3. Influence of Vacuum
3.2. Operational Stability of Pervaporation
3.3. PVA Membrane Properties Analysis
3.3.1. SEM Analysis
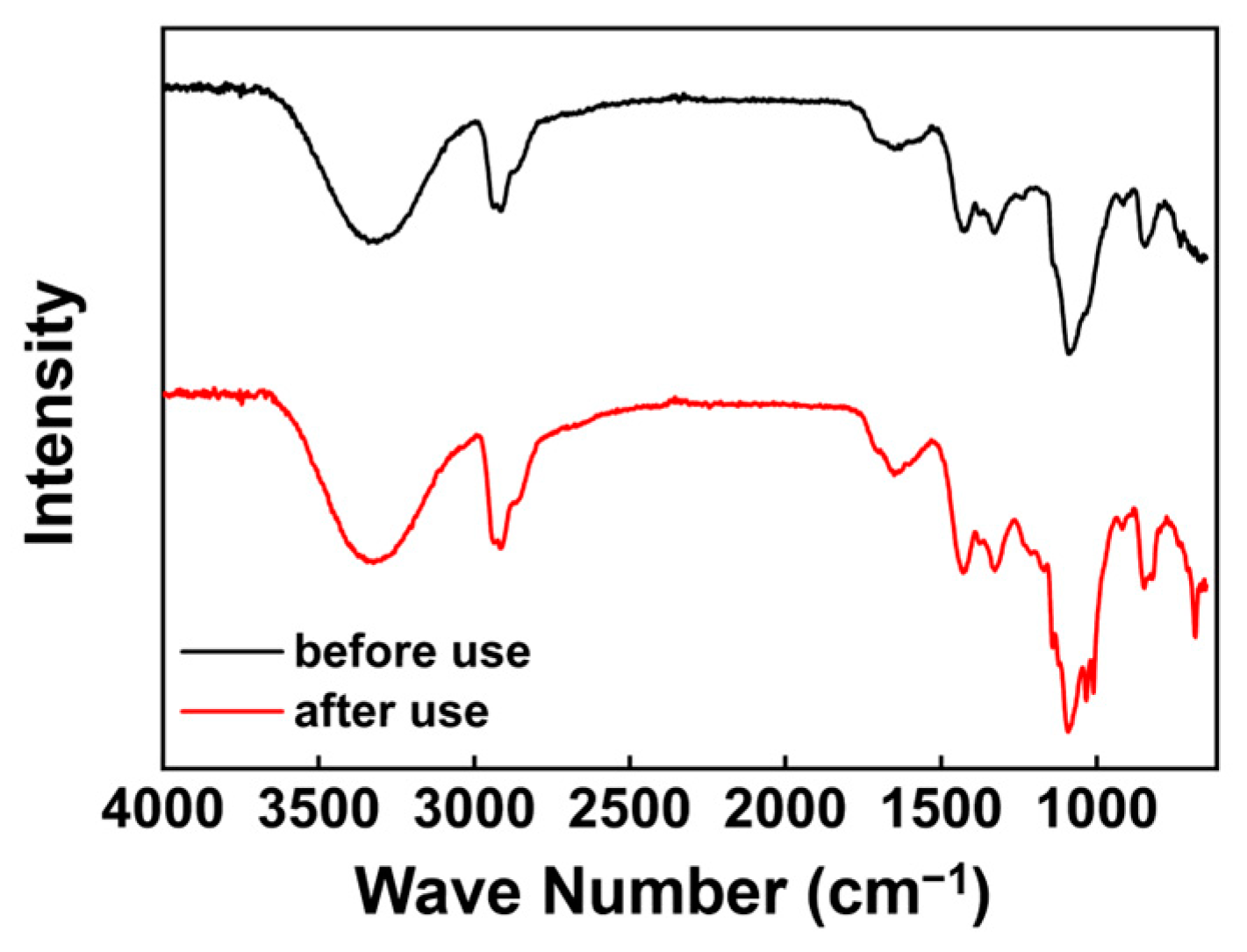
3.3.2. FT-IR Analysis
3.4. Energy Consumption
Pervaporation Energy Consumption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Wang, C.; Wang, B.; Li, K. Novel Organic-Dehydration Membranes Prepared from Zirconium Metal-Organic Frameworks. Adv. Funct. Mater. 2017, 27, 1604311. [Google Scholar] [CrossRef]
- Shan, B.; Wang, S.; Xu, Q.; Wang, Y.; Cui, P.; Zhang, F. Design and multi-objective optimization of hybrid extractive distillation process for separating the toluene-methanol-water ternary azeotrope. Sep. Purif. Technol. 2024, 336, 126335. [Google Scholar] [CrossRef]
- Yi, C.-C.; Shen, W.; Chien, I.L. Design and control of an energy-efficient alternative process for the separation of methanol/toluene/water ternary azeotropic mixture. Sep. Purif. Technol. 2018, 207, 489–497. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Z.; Shi, X.; Guang, C.; Gao, J. Triple-column side-stream extractive distillation optimization via simulated annealing for the benzene/isopropanol/water separation. Sep. Purif. Technol. 2020, 236, 116303. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Liu, X.; Bai, W.; Zhu, Z.; Wang, Y.; Gao, J. Control of extractive distillation process for separating heterogenerous ternary azeotropic mixture via adjusting the solvent content. Sep. Purif. Technol. 2018, 191, 8–26. [Google Scholar] [CrossRef]
- Jaime, J.A.; Rodríguez, G.; Gil, I.D. Control of an Optimal Extractive Distillation Process with Mixed-Solvents as Separating Agent. Ind. Eng. Chem. Res. 2018, 57, 9615–9626. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Wu, Y. Numerical Simulation of Spray Atomization in Supersonic Flows. Mod. Phys. Lett. B 2011, 24, 1299–1302. [Google Scholar] [CrossRef]
- Han, Y.-J.; Wang, K.-H.; Lai, J.-Y.; Liu, Y.-L. Hydrophilic chitosan-modified polybenzoimidazole membranes for pervaporation dehydration of isopropanol aqueous solutions. J. Membr. Sci. 2014, 463, 17–23. [Google Scholar] [CrossRef]
- Huang, Y.H.; Huang, S.H.; Chao, W.C.; Li, C.L.; Hsieh, Y.Y.; Hung, W.S.; Liaw, D.J.; Hu, C.C.; Lee, K.R.; Lai, J.Y. A Study on the Characteristics and Pervaporation Performance of Polyamide Thin-Film Composite Membranes with Modified Polyacrylonitrile as Substrate for Bioethanol Dehydration. Polym. Int. 2014, 63, 1478–1486. [Google Scholar] [CrossRef]
- Moulai, S.; Ghezini, R.; Hasnaoui, A.; Bengueddach, A.; Weidler, P.G. Adsorption behavior of water and xylene isomers on AlPO-5 zeolite modified by different transition metals. Res. Chem. Intermed. 2019, 45, 1653–1668. [Google Scholar] [CrossRef]
- Truong, B.N.; Park, J.; Kwon, O.K.; Park, I. Water adsorption capacity enhancement of ferroaluminophosphate (FAPO4-5) by impregnation of CaCl2. Mater. Lett. 2018, 215, 137–139. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Msahel, A.; Galiano, F.; Serocki, M.; Ryl, J.; Hamouda, S.B.; Hafiane, A.; Boczkaj, G.; Figoli, A. Towards azeotropic MeOH-MTBE separation using pervaporation chitosan-based deep eutectic solvent membranes. Sep. Purif. Technol. 2022, 281, 119979. [Google Scholar] [CrossRef]
- Dong, G.; Nagasawa, H.; Yu, L.; Wang, Q.; Yamamoto, K.; Ohshita, J.; Kanezashi, M.; Tsuru, T. Pervaporation removal of methanol from methanol/organic azeotropes using organosilica membranes: Experimental and modeling. J. Membr. Sci. 2020, 610, 118284. [Google Scholar] [CrossRef]
- Xu, X.; Nikolaeva, D.; Hartanto, Y.; Luis, P. MOF-based membranes for pervaporation. Sep. Purif. Technol. 2021, 278, 119233. [Google Scholar] [CrossRef]
- Liu, P.; Chen, M.; Ma, Y.; Hu, C.; Zhang, Q.; Zhu, A.; Liu, Q. A hydrophobic pervaporation membrane with hierarchical microporosity for high-efficient dehydration of alcohols. Chem. Eng. Sci. 2019, 206, 489–498. [Google Scholar] [CrossRef]
- Roy, S.; Singha, N. Polymeric Nanocomposite Membranes for Next Generation Pervaporation Process: Strategies, Challenges and Future Prospects. Membranes 2017, 7, 53. [Google Scholar] [CrossRef]
- Baik, J.H.; Park, K.; Jeong, C.; Kim, J.; Koh, D.J.; Cho, J.H.; van Veen, H.; van Tuel, M.; Motelica, A.; Vente, J.F. Pilot-scale azeotrope purification of dimethyl carbonate by cost-efficient pervaporation-assisted distillation. J. Clean. Prod. 2024, 434, 139963. [Google Scholar] [CrossRef]
- Cheng, X.; Jiang, Z.; Cheng, X.; Yang, H.; Tang, L.; Liu, G.; Wang, M.; Wu, H.; Pan, F.; Cao, X. Water-selective permeation in hybrid membrane incorporating multi-functional hollow ZIF-8 nanospheres. J. Membr. Sci. 2018, 555, 146–156. [Google Scholar] [CrossRef]
- Sapegin, D.A.; Kononova, S.V.; Gubanova, G.N.; Kruchinina, E.V.; Saprykina, N.N.; Popova, E.N.; Vlasova, E.N. Selective facilitated fixed-site carrier transport of methanol through sulfonated copolyimide pervaporation membranes for the separation of methanol/toluene mixtures. Sep. Purif. Technol. 2020, 236, 116274. [Google Scholar] [CrossRef]
- Kachhadiya, D.D.; Murthy, Z.V.P. Microfluidic synthesized ZIF-67 decorated PVDF mixed matrix membranes for the pervaporation of toluene/water mixtures. J. Membr. Sci. 2023, 676, 121607. [Google Scholar] [CrossRef]
- Salehi Shahrabi, S.; Mortaheb, H.R.; Barzin, J.; Ehsani, M.R. Pervaporative performance of a PDMS/blended PES composite membrane for removal of toluene from water. Desalination 2012, 287, 281–289. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, M.; Ji, C.-H.; Xu, X.-R.; Ma, X.-H.; Xu, Z.-L. Multilayer assembled CS-PSS/ceramic hollow fiber membranes for pervaporation dehydration. Sep. Purif. Technol. 2018, 203, 84–92. [Google Scholar] [CrossRef]
- Chen, K.-F.; Zheng, P.-Y.; Wu, J.-K.; Wang, N.-X.; An, Q.-F.; Lee, K.-R. Polyelectrolyte complexes/silica hybrid hollow fiber membrane for fusel oils pervaporation dehydration processes. J. Membr. Sci. 2018, 545, 284–291. [Google Scholar] [CrossRef]
- Tong, Z.; Liu, X.; Zhang, B. Sulfonated graphene oxide based membranes with enhanced water transport capacity for isopropanol pervaporation dehydration. J. Membr. Sci. 2020, 612, 118446. [Google Scholar] [CrossRef]
- Mohammadi, F. Phosphoric Acid Industry Waste Valorization Through Fabrication of Alkali-Activated Phosphorus Slag-Based Ceramic Membranes: Synthesis and Optimization for Dehydration of Ethanol. Waste Biomass Valorization 2023. [Google Scholar] [CrossRef]
- Haaz, E.; Toth, A.J. Methanol dehydration with pervaporation: Experiments and modelling. Sep. Purif. Technol. 2018, 205, 121–129. [Google Scholar] [CrossRef]
- Toth, A.J.; Andre, A.; Haaz, E.; Mizsey, P. New horizon for the membrane separation: Combination of organophilic and hydrophilic pervaporations. Sep. Purif. Technol. 2015, 156, 432–443. [Google Scholar] [CrossRef]
- Criscuoli, A. Osmotic Distillation and vacuum membrane Distillation for juice concentration: A comparison in terms of energy consumption at the permeate side. Sep. Purif. Technol. 2021, 278, 119593. [Google Scholar] [CrossRef]
- Sawatdiruk, S.; Charoensuppanimit, P.; Faungnawakij, K.; Klaysom, C. POSS/PDMS composite pervaporation membranes for furfural recovery. Sep. Purif. Technol. 2021, 278, 119281. [Google Scholar] [CrossRef]
- Liu, J.; Bernstein, R. High-flux thin-film composite polyelectrolyte hydrogel membranes for ethanol dehydration by pervaporation. J. Membr. Sci. 2017, 534, 83–91. [Google Scholar] [CrossRef]






| Water | Methanol | Toluene | |
|---|---|---|---|
| Feed/kg | 25 | 350 | 625 |
| Feed out/kg | 6.6 | 246.2 | |
| Permeate/kg | 18.4 | 103.8 | |
| ΔHev/J/g | 2260 | 1109 | |
| Qpevap/kW·h | 18.4 × 2260 + 103.8 × 1109 = 156,698 kJ = 43.53 kW·h (1 kW·h = 3600 kJ) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Y.; Xu, S.; Wu, Y.; Zhang, L.; Xie, L. Dehydration of Organic Solvents from Ternary Mixtures Containing Toluene/Methanol/Water by Pervaporation. Membranes 2024, 14, 139. https://doi.org/10.3390/membranes14060139
Qiao Y, Xu S, Wu Y, Zhang L, Xie L. Dehydration of Organic Solvents from Ternary Mixtures Containing Toluene/Methanol/Water by Pervaporation. Membranes. 2024; 14(6):139. https://doi.org/10.3390/membranes14060139
Chicago/Turabian StyleQiao, Ying, Shichang Xu, Yixuan Wu, Long Zhang, and Lixin Xie. 2024. "Dehydration of Organic Solvents from Ternary Mixtures Containing Toluene/Methanol/Water by Pervaporation" Membranes 14, no. 6: 139. https://doi.org/10.3390/membranes14060139
APA StyleQiao, Y., Xu, S., Wu, Y., Zhang, L., & Xie, L. (2024). Dehydration of Organic Solvents from Ternary Mixtures Containing Toluene/Methanol/Water by Pervaporation. Membranes, 14(6), 139. https://doi.org/10.3390/membranes14060139





