Application of Recycled Ultrafiltration Membranes in an Aerobic Membrane Bioreactor (aMBR): A Validation Study
Abstract
1. Introduction
2. Materials and Methods
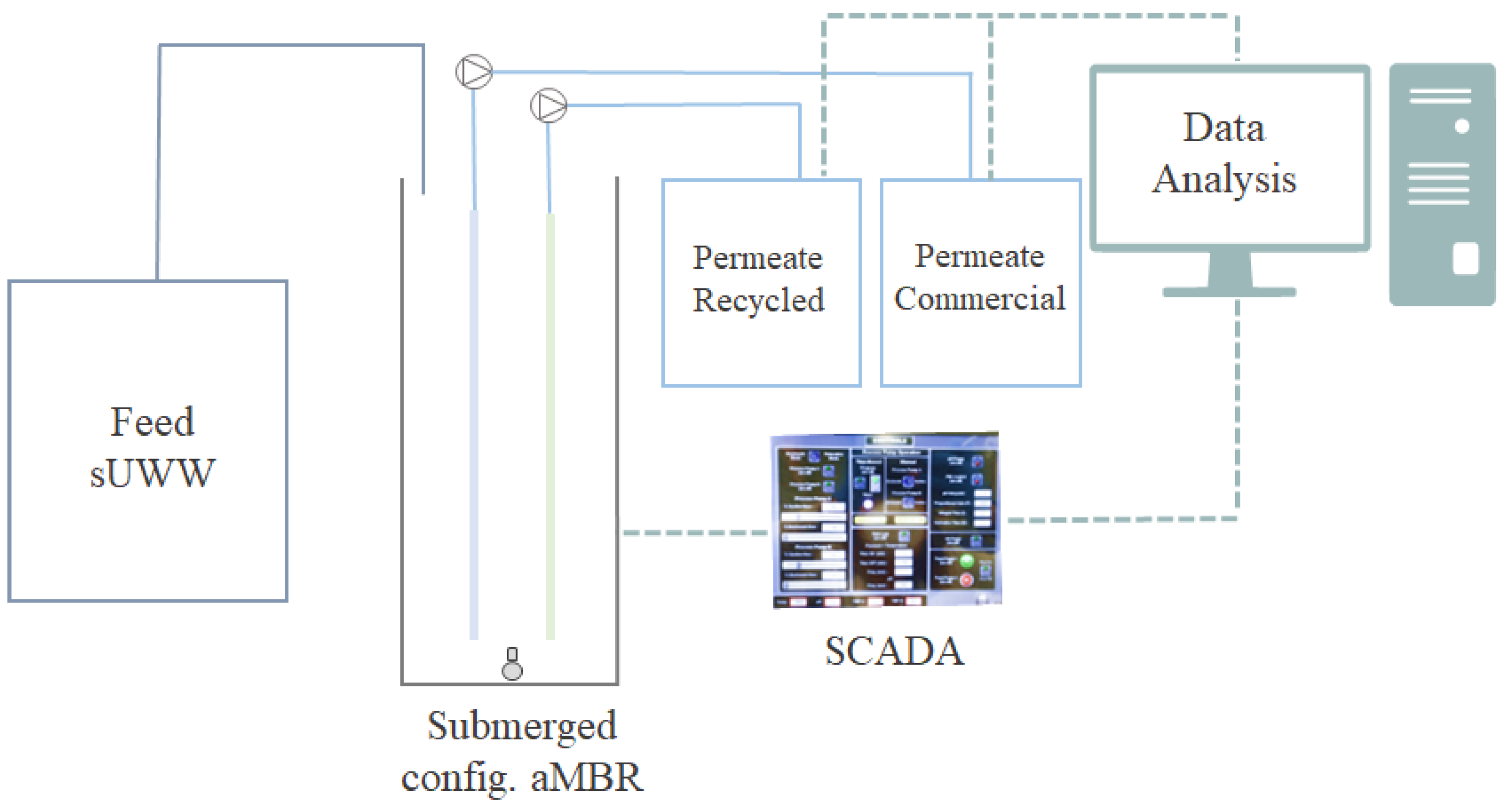
2.1. Experimental Set-Up
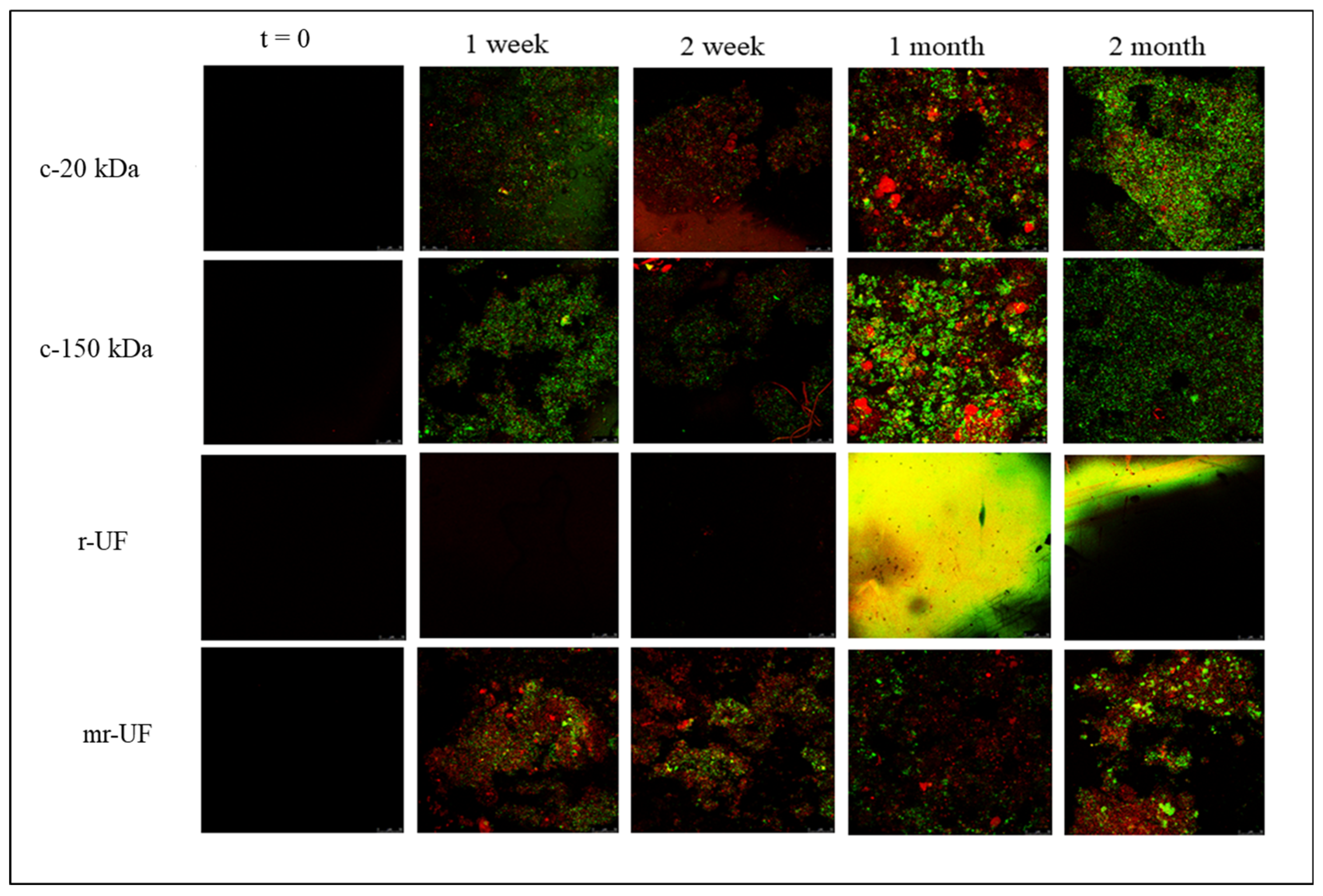
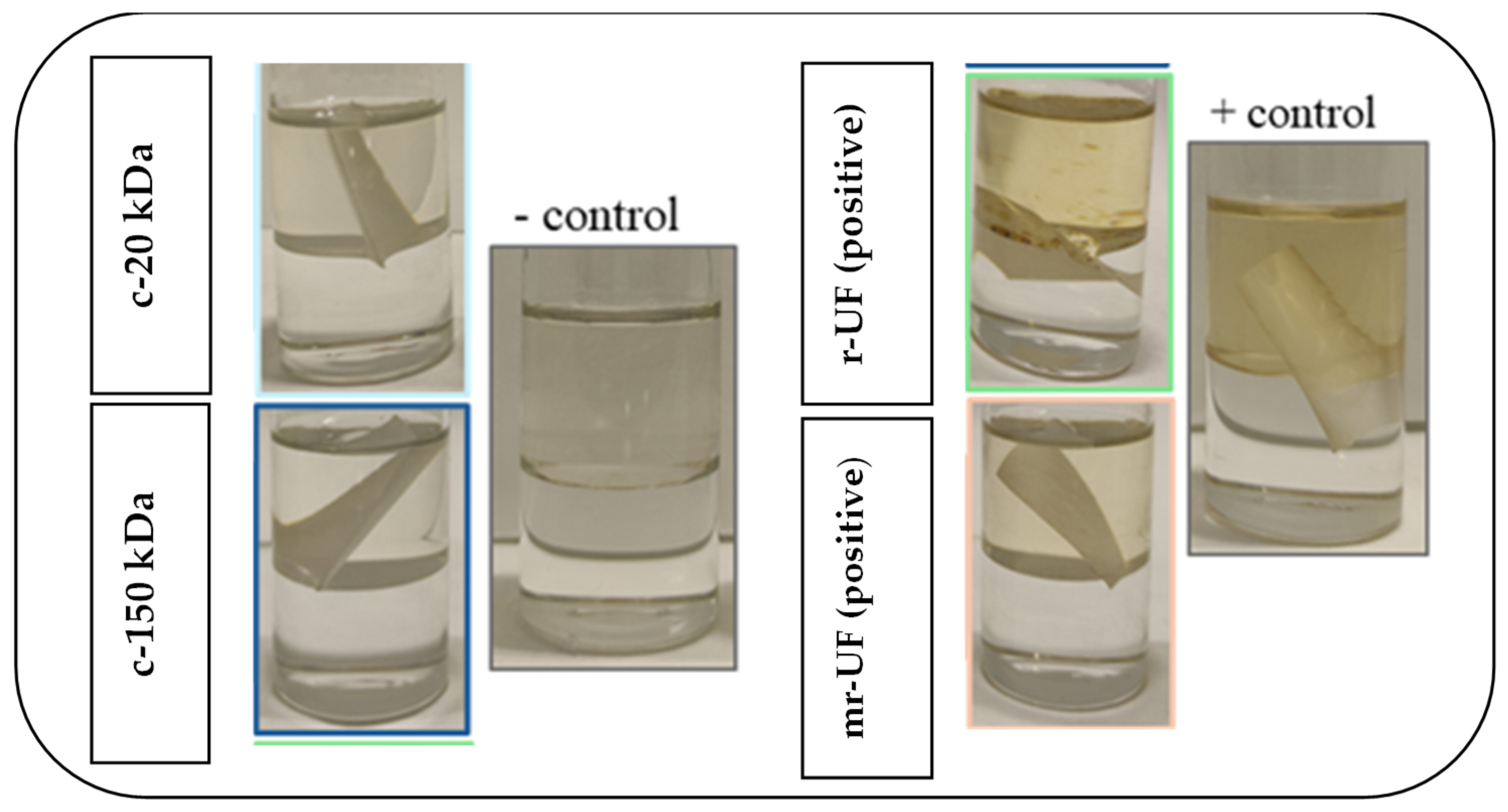
2.2. Membrane Fouling Analysis
2.3. Chemicals
2.4. Membrane Characterization
2.5. Membranes
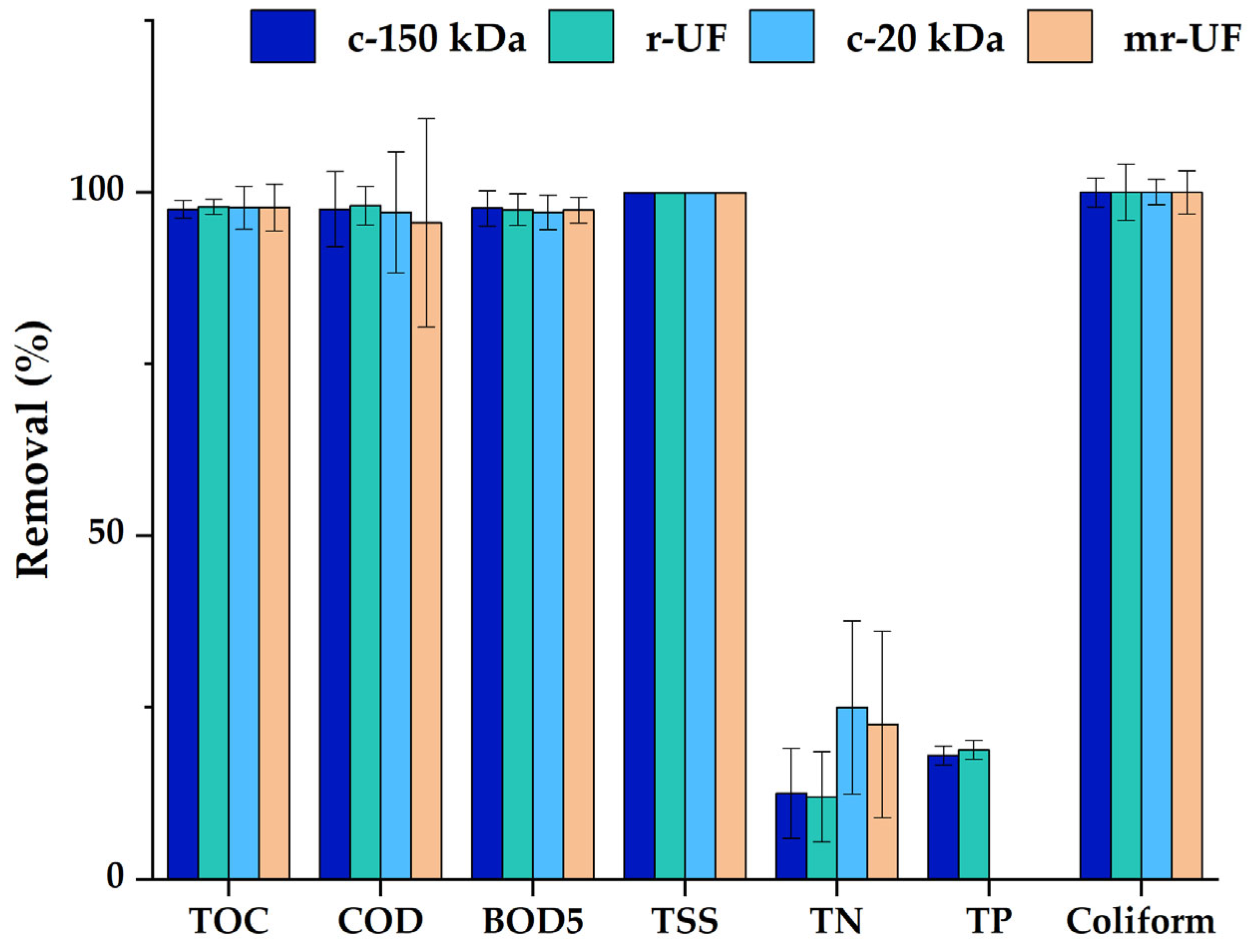
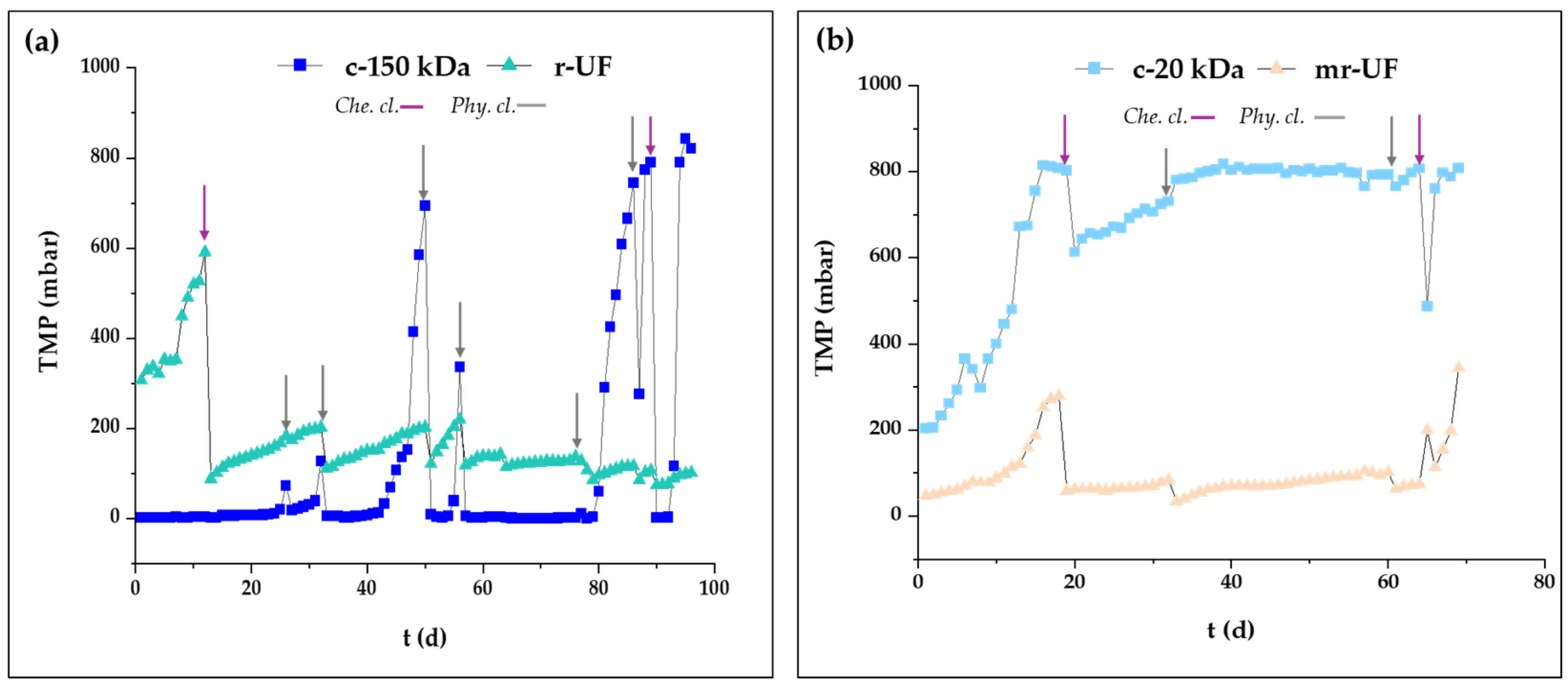
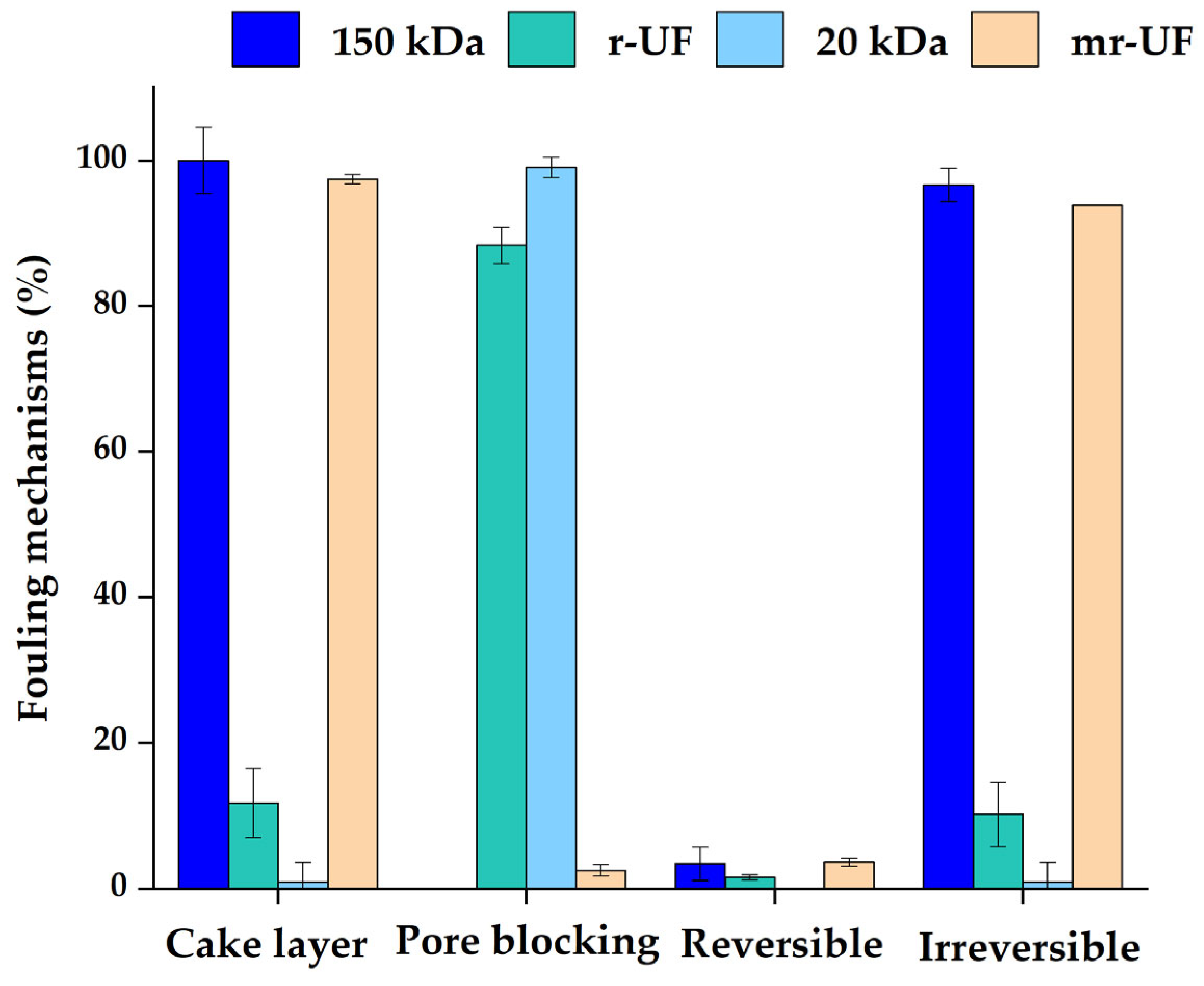
3. Results and Discussion
3.1. Membrane Performance
3.2. Membrane Resistance in Series (RIS) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MBR Global Capacity. 2019. Available online: https://www.thembrsite.com/membrane-bioreactor-global-capacity/ (accessed on 8 November 2021).
- Markets and Markets, External Research. Membrane Bioreactor Market by Membrane Type, Region—Global Forecast to 2026, Illinois, USA. 2024. Available online: https://www.marketsandmarkets.com/ (accessed on 26 February 2024).
- Cote, P.; Alam, Z.; Penny, J. Hollow fiber membrane life in membrane bioreactors (MBR). Desalination 2012, 288, 145–151. [Google Scholar] [CrossRef]
- Lo, C.H.; McAdam, E.; Judd, S. The cost of a small membrane bioreactor. Water Sci. Technol. 2015, 72, 1739–1746. [Google Scholar] [CrossRef]
- Fenu, A.; Roels, J.; Wambecq, T.; De Gussem, K.; Thoeye, C.; De Gueldre, G.; Van De Steene, B. Energy audit of a full scale MBR system. Desalination 2010, 262, 121–128. [Google Scholar] [CrossRef]
- de Paula, E.C.; Amaral, M.C.S. Environmental and economic evaluation of end-of-life reverse osmosis membranes recycling by means of chemical conversion. J. Clean. Prod. 2018, 194, 85–93. [Google Scholar] [CrossRef]
- Lawler, W.; Alvarez-Gaitan, J.; Leslie, G.; Le-Clech, P. Comparative life cycle assessment of end-of-life options for reverse osmosis membranes. Desalination 2015, 357, 45–54. [Google Scholar] [CrossRef]
- Senán-Salinas, J.; García-Pacheco, R.; Landaburu-Aguirre, J.; García-Calvo, E. Recycling of end-of-life reverse osmosis membranes: Comparative LCA and cost-effectiveness analysis at pilot scale. Resour. Conserv. Recycl. 2019, 150, 104423. [Google Scholar] [CrossRef]
- A European Strategy for Plastics in a Circular Economy. Brussels Title, COM 28 Final. 2018. Available online: https://eur-lex.europa.eu/ (accessed on 7 June 2021).
- European Comission. First Circular Economy Action Plan. 2020. Available online: https://ec.europa.eu/environment/circular-economy/ (accessed on 7 June 2021).
- European Comission. Circular Economy Action Plan, (n.d.). Available online: https://environment.ec.europa.eu/strategy/circular-economy-action-plan_en (accessed on 7 June 2021).
- European Comission. Water Reuse, (n.d.). Available online: https://environment.ec.europa.eu/topics/water/water-reuse_en (accessed on 7 June 2021).
- European Comission. Waste and Recycling, (n.d.). Available online: https://environment.ec.europa.eu/topics/waste-and-recycling_en (accessed on 7 June 2021).
- Lejarazu-Larrañaga, A.; Landaburu-Aguirre, J.; Senán-Salinas, J.; Ortiz, J.M.; Molina, S. Thin Film Composite Polyamide Reverse Osmosis Membrane Technology towards a Circular Economy. Membranes 2022, 12, 864. [Google Scholar] [CrossRef] [PubMed]
- Lawler, W.; Bradford-Hartke, Z.; Cran, M.J.; Duke, M.; Leslie, G.; Ladewig, B.P.; Le-Clech, P. Towards new opportunities for reuse, recycling and disposal of used reverse osmosis membranes. Desalination 2012, 299, 103–112. [Google Scholar] [CrossRef]
- Rahimi, Z.; Zinatizadeh, A.; Zinadini, S. Milk processing wastewater treatment in an MBR: A comparative study on the use of two synthetic anti-fouling PES-UF membranes. J. Environ. Chem. Eng. 2019, 7, 103369. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, X.; Chen, J.; Wang, G.; Yang, F. Highly effective antifouling performance of PVDF/graphene oxide composite membrane in membrane bioreactor (MBR) system. Desalination 2014, 340, 59–66. [Google Scholar] [CrossRef]
- Rodríguez-Sáez, L.; Landaburu-Aguirre, J.; Molina, S.; García-Payo, M.; García-Calvo, E. Study of surface modification of recycled ultrafiltration membranes using statistical design of experiments. Surf. Interfaces 2021, 23, 100978. [Google Scholar] [CrossRef]
- Ahsani, M.; Hazrati, H.; Javadi, M.; Ulbricht, M.; Yegani, R. Preparation of antibiofouling nanocomposite PVDF/Ag-SiO2 membrane and long-term performance evaluation in the MBR system fed by real pharmaceutical wastewater. Sep. Purif. Technol. 2020, 249, 116938. [Google Scholar] [CrossRef]
- Rodríguez-Sáez, L.; Patsios, S.I.; Senán-Salinas, J.; Landaburu-Aguirre, J.; Molina, S.; García-Calvo, E. A Novel Application of Recycled Ultrafiltration Membranes in an Aerobic Membrane Bioreactor (aMBR): A Proof-of-Concept Study. Membranes 2022, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- AENOR, UNE-EN 872:2006; Water Quality—Determination of Suspended Solids—Method by Filtration through Glass Fibre Filters. ISO: Geneva, Switzerland, 2006.
- AENOR, UNE-EN ISO 9408:2000; Water Quality. Evaluation of Ultimate Aerobic Biodegradability of Organic Compounds in Aqueous Medium by Determintation of Oxygen Demand in a Closed Respirometer. (ISO 9408:1999); ISO: Geneva, Switzerland, 2000.
- AENOR, UNE 77004:2002; Water Quality. Determination of the Chemical Oxygen Demand (COD). Dichromate Method. ISO: Geneva, Switzerland, 2002.
- AENOR, UNE-EN ISO 6878:2005; Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method. (ISO 6878:2004); ISO: Geneva, Switzerland, 2005.
- AENOR, UNE-EN 25663:1994; Water Quality. Determination of Kjeldahl Nitrogen. ISO: Geneva, Switzerland, 1994.
- DIN 38405-9:1979-05; German Standard Methods for Examination of Water, Wastewater and Sludge; Anions (Group D), Determination of Nitrate Ion (D9). Deutsches Institut Fur Normung: Berlin, Germany, 2011.
- AENOR, UNE-EN ISO 6878; Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method. (ISO 6878:2004); ISO: Geneva, Switzerland, 2015.
- AENOR, UNE-EN ISO 9308-1:2014/A1:2017; Water Quality—Enumeration of Escherichia coli and Coliform Bacteria—Part 1: Membrane Filtration Method for Waters with Low Bacterial Background Flora. (ISO 9308-1:2014/Amd 1:2016); ISO: Geneva, Switzerland, 2014.
- Di Bella, G.; Di Trapani, D. A brief review on the resistance-in-series model in membrane bioreactors (MBRs). Membranes 2019, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, G.; Durante, F.; Torregrossa, M.; Viviani, G.; Mercurio, P.; Cicala, A. The role of fouling mechanisms in a membrane bioreactor. Water Sci. Technol. 2007, 55, 455–464. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xu, P.; Li, C.; Zhang, B. High-concentration food wastewater treatment by an anaerobic membrane bioreactor. Water Res. 2005, 39, 4110–4118. [Google Scholar] [CrossRef] [PubMed]
- Weis, A.; Bird, M.R.; Nyström, M.; Wright, C. The influence of morphology, hydrophobicity and charge upon the long-term performance of ultrafiltration membranes fouled with spent sulphite liquor. Desalination 2005, 175, 73–85. [Google Scholar] [CrossRef]
- Feng, S.; Yu, G.; Cai, X.; Eulade, M.; Lin, H.; Chen, J.; Liu, Y.; Liao, B.-Q. Effects of fractal roughness of membrane surfaces on interfacial interactions associated with membrane fouling in a membrane bioreactor. Bioresour. Technol. 2017, 244, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Li, D.; Zhang, B.; Xing, W. Membrane surface roughness characterization and its influence on ultrafine particle adhesion. Sep. Purif. Technol. 2012, 90, 140–146. [Google Scholar] [CrossRef]
- Woo, S.H.; Min, B.R.; Lee, J.S. Change of surface morphology, permeate flux, surface roughness and water contact angle for membranes with similar physicochemical characteristics (except surface roughness) during microfiltration. Sep. Purif. Technol. 2017, 187, 274–284. [Google Scholar] [CrossRef]
- Le-Clech, P.; Chen, V.; Fane, T.A. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Liu, Q.; Ren, J.; Lu, Y.; Zhang, X.; Roddick, F.A.; Fan, L.; Wang, Y.; Yu, H.; Yao, P. A review of the current in-situ fouling control strategies in MBR: Biological versus physicochemical. J. Ind. Eng. Chem. 2021, 98, 42–59. [Google Scholar] [CrossRef]
- Kim, J.; Bae, E.; Park, H.; Park, H.-J.; Shah, S.S.A.; Lee, K.; Lee, J.; Oh, H.-S.; Park, P.-K.; Shin, Y.C.; et al. Membrane reciprocation and quorum quenching: An innovative combination for fouling control and energy saving in membrane bioreactors. Water Res. 2024, 250, 121035. [Google Scholar] [CrossRef]
- Hou, S.; Xing, J.; Dong, X.; Zheng, J.; Li, S. Integrated antimicrobial and antifouling ultrafiltration membrane by surface grafting PEO and N-chloramine functional groups. J. Colloid Interface Sci. 2017, 500, 333–340. [Google Scholar] [CrossRef]


| Average | Standard Deviation | |
|---|---|---|
| EC (mS/cm) | 2.9 | 0.4 |
| COD (mg/L) | 421.4 | 23.1 |
| BOD5 (mg/L) | 226.3 | 17.4 |
| TOC (mg/L) | 132.5 | 9.6 |
| TN (mg/L) | 31.4 | 2.0 |
| TP (mg/L) | 5.0 | 0.9 |
| c-150 kDa | r-UF | c-20 kDa | mr-UF | ||
|---|---|---|---|---|---|
| Material | Polyethersulfone (PES) | Polyethersulfone (PES) | Polyethersulfone (PES) | Polyethersulfone (PES) | |
| Backing material | Polypropylene (PP) | Polyethylene terephthalate (PET) | Polypropylene (PP) | Polyethylene terephthalate (PET) | |
| Permeability (L·m−2·h−1 bar−1) | 566.17 ± 9.39 | 39.03 ± 1.74 | 56.97 ± 1.55 | 192.68 ± 1.57 | |
| M.W.C.O (kDa) | 87.64 ± 0.65 | 33.76 ± 4.16 | 24.63 ± 3.82 | 85.04 ± 6.87 | |
| Contact angle | 58.00 ± 0.99 | 59.01 ± 1.25 | 57.05 ± 1.25 | 59.21 ± 1.76 | |
| Roughness | Ra (nm) | 8.4 ± 2.6 | 4.7 ± 0.6 | 11.7 ± 3.7 | 5.2 ± 0.9 |
| Rq (nm) | 6.6 ± 2.2 | 6.3 ± 1.2 | 8.9 ± 2.7 | 7.5 ± 1.3 | |
| c-150 kDa | r-UF [17] | c-20 kDa | mr-UF [17] | |||||
|---|---|---|---|---|---|---|---|---|
| % Weight | % Atomic | % Weight | % Atomic | % Weight | % Atomic | % Weight | % Atomic | |
| C | 59.12 | 68.69 | 69.75 | 77.51 | 59.74 | 69.25 | 64.25 | 71.94 |
| N | 2.91 | 2.90 | 1.32 | 1.26 | 2.84 | 2.82 | 3.57 | 3.34 |
| O | 27.18 | 23.70 | 21.94 | 18.30 | 26.79 | 23.31 | 26.47 | 22.25 |
| S | 10.48 | 4.56 | 5.97 | 2.49 | 10.52 | 4.57 | 5.23 | 2.19 |
| Cl | 0.13 | 0.05 | 0.42 | 0.16 | 0.11 | 0.04 | 0.49 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Sáez, L.; Landaburu-Aguirre, J.; García-Calvo, E.; Molina, S. Application of Recycled Ultrafiltration Membranes in an Aerobic Membrane Bioreactor (aMBR): A Validation Study. Membranes 2024, 14, 149. https://doi.org/10.3390/membranes14070149
Rodríguez-Sáez L, Landaburu-Aguirre J, García-Calvo E, Molina S. Application of Recycled Ultrafiltration Membranes in an Aerobic Membrane Bioreactor (aMBR): A Validation Study. Membranes. 2024; 14(7):149. https://doi.org/10.3390/membranes14070149
Chicago/Turabian StyleRodríguez-Sáez, Laura, Junkal Landaburu-Aguirre, Eloy García-Calvo, and Serena Molina. 2024. "Application of Recycled Ultrafiltration Membranes in an Aerobic Membrane Bioreactor (aMBR): A Validation Study" Membranes 14, no. 7: 149. https://doi.org/10.3390/membranes14070149
APA StyleRodríguez-Sáez, L., Landaburu-Aguirre, J., García-Calvo, E., & Molina, S. (2024). Application of Recycled Ultrafiltration Membranes in an Aerobic Membrane Bioreactor (aMBR): A Validation Study. Membranes, 14(7), 149. https://doi.org/10.3390/membranes14070149








