Cationic Imidazolium-Urethane-Based Poly(Ionic Liquids) Membranes for Enhanced CO2/CH4 Separation: Synthesis, Characterization, and Performance Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
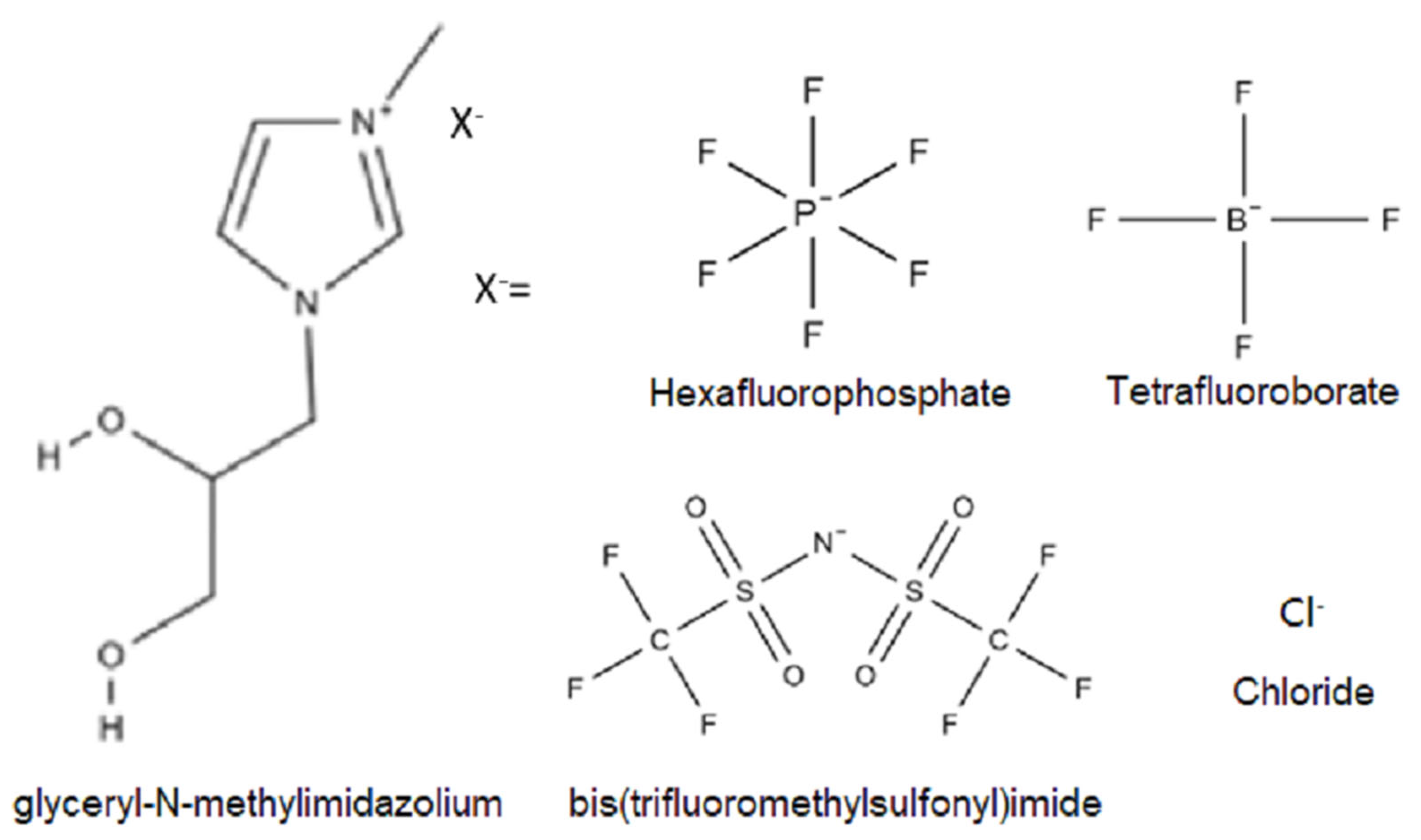
2.2. Synthesis of Hydroxyl-Functionalized Ionic Liquids
2.3. Cationic Poly(Ionic Liquids)
2.4. Syntheses of PU
2.5. Preparation of Dense Membranes
2.6. Characterization
2.7. CO2 Sorption Capacity
2.8. CO2 Permeability and Ideal CO2/CH4 Selectivity
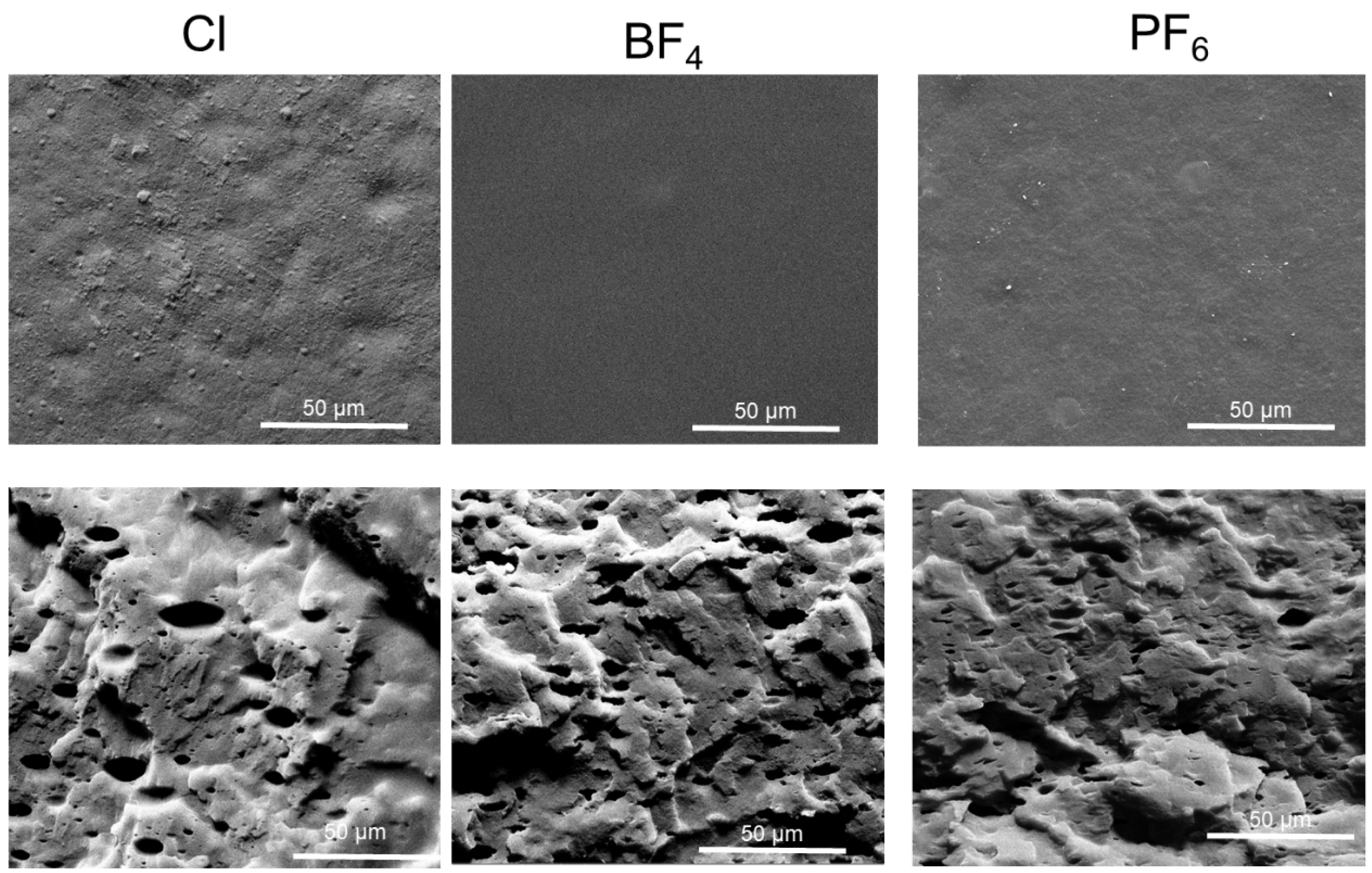
3. Results
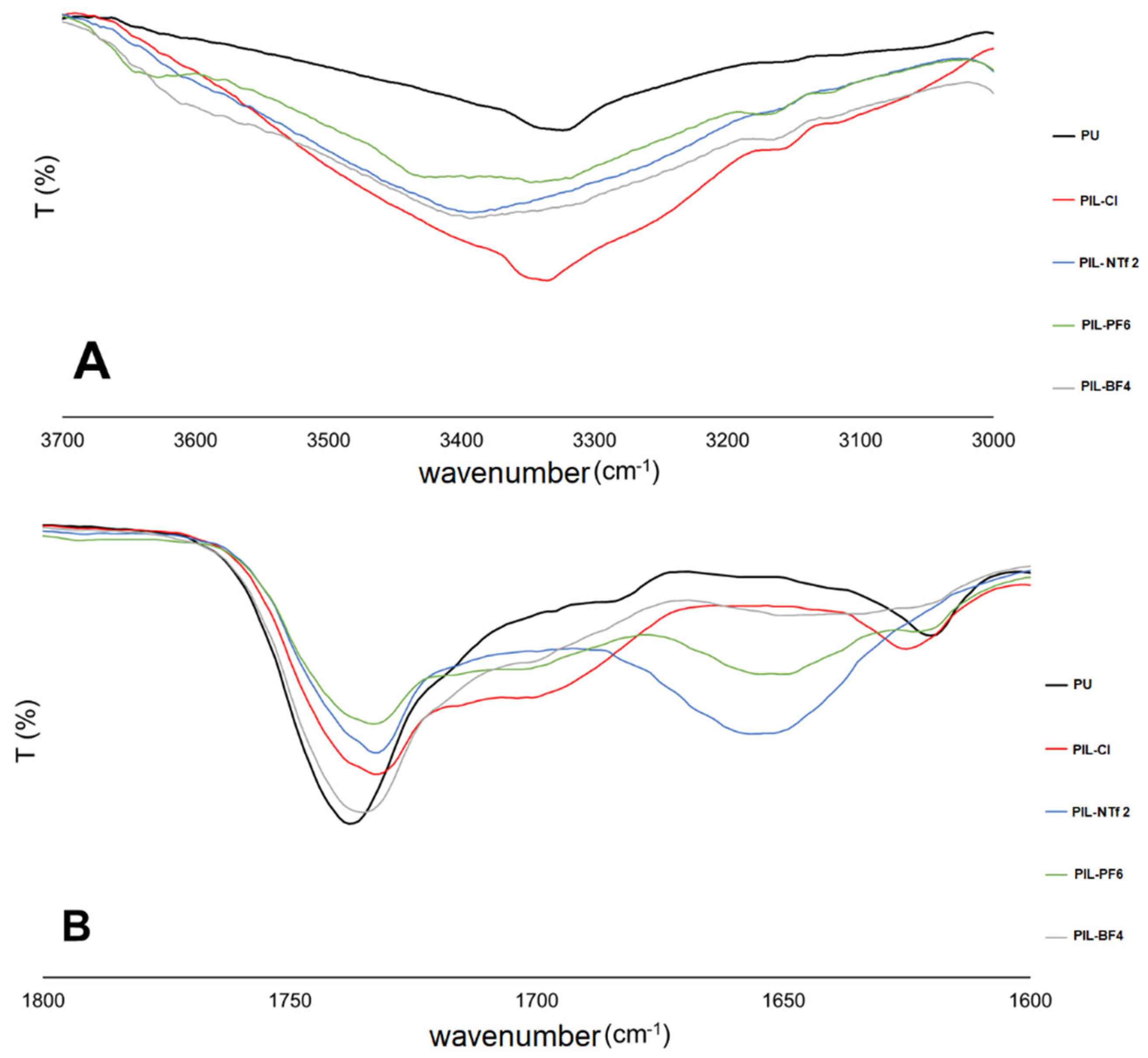
3.1. Characterization of the ILs
3.2. Characterization of Cationic Poly(Ionic Liquids)
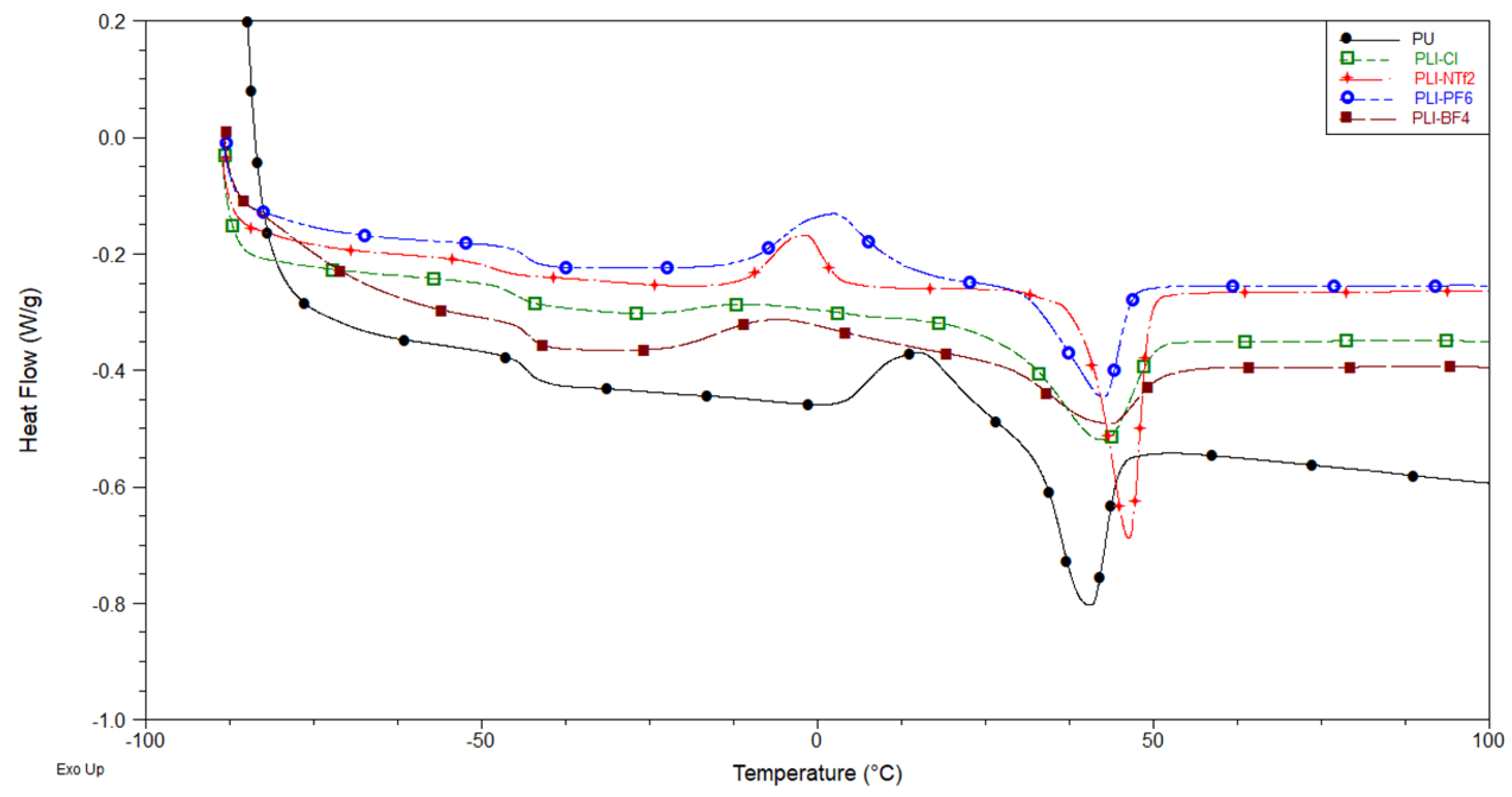
3.2.1. DSC
3.2.2. TGA
3.2.3. DMA
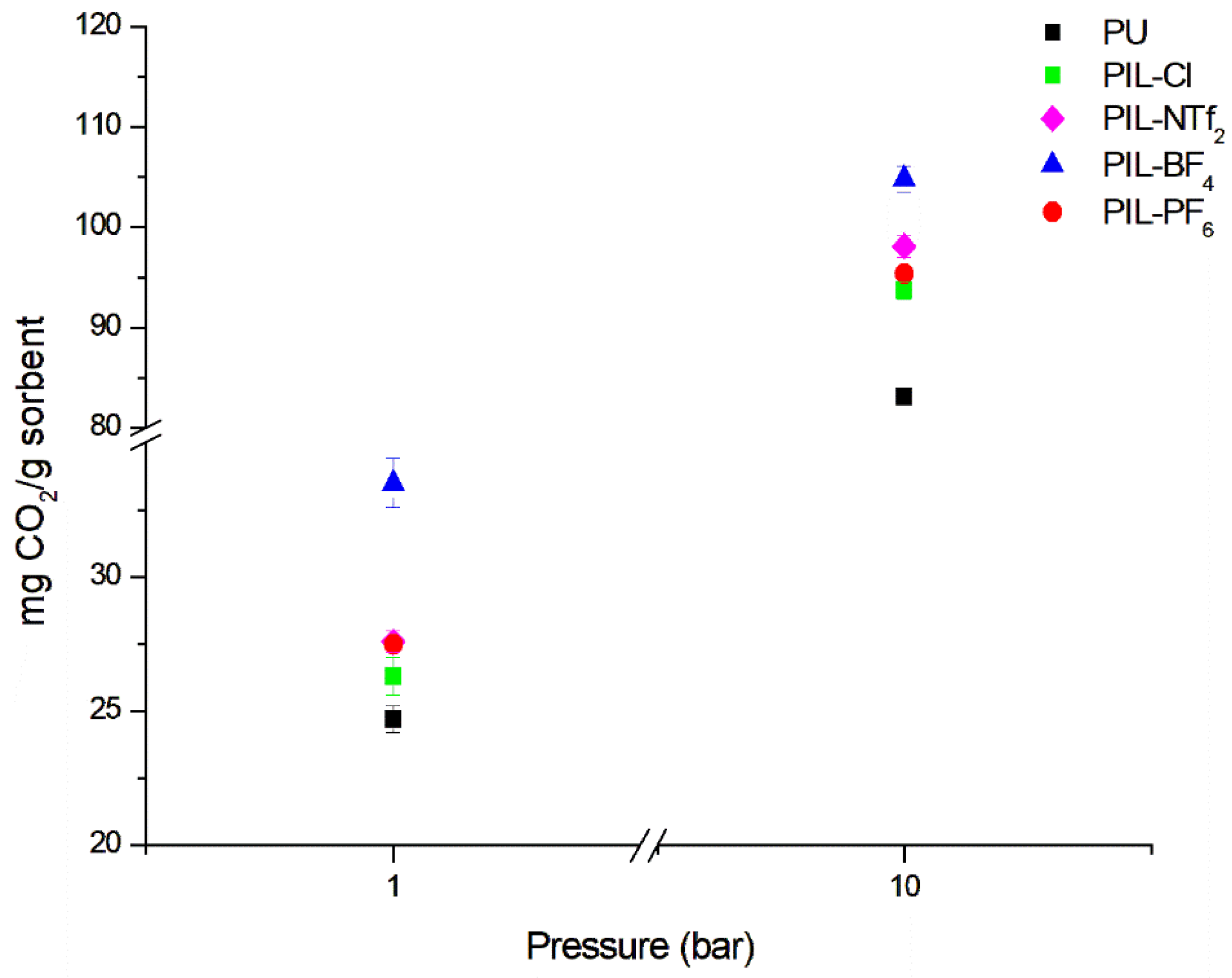
3.3. CO2 Sorption Capacity
3.4. Permeability and CO2 Selectivity
3.5. Comparison with Robeson Upper Bound
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| PILs | poly(ionic liquids) or polymerized ionic liquids |
| ILs | ionic liquids |
| GLYMIM | glyceryl-N-methylimidazolium |
| Cl− | chloride anion |
| NTf2− | bis(trifluoromethanesulfonyl)imide anion |
| PF6− | hexafluorophosphate |
| BF4− | tetrafluoroborate |
| PU | polyurethane |
| PTMG | polytetramethylene glycol |
| PCD | polycarbonate diol |
| PCL | polycaprolactone |
| HDI | hexamethylene diisocyanate |
| DBTDL | dibutyltin dilaurate |
| MEK | methyl ethylketone |
| PD | polydispersity |
| Mw | molecular weights |
| Tg | glass transition temperatures |
References
- Supasitmongkol, S.; Styring, P. High CO2 Solubility in Ionic Liquids and a Tetraalkylammonium-Based Poly(Ionic Liquid). Energy Environ. Sci. 2010, 3, 1961–1972. [Google Scholar] [CrossRef]
- Privalova, E.I.; Mäki-Arvela, P.; Murzin, D.Y.; Mikkhola, J.P. Capturing CO2: Conventional versus Ionic-Liquid Based Technologies. Russ. Chem. Rev. 2012, 81, 435–457. [Google Scholar] [CrossRef]
- Rockett, G.C. Associação de Fontes Emissoras e Reservatórios Potenciais para Armazenamento Geológico de CO2 na Bacia de Campos, Brasil. Bachelor’s Thesis, Pontifical Catholic University of Rio Grande do Sul, Porto Alegre, Brazil, 2010. [Google Scholar]
- Dunn, C.A.; Denning, S.; Crawford, J.M.; Zhou, R.; Dwulet, G.E.; Carreon, M.A.; Gin, D.L.; Noble, R.D. CO2/CH4 Separation Characteristics of Poly(RTIL)-RTIL-Zeolite Mixed-Matrix Membranes Evaluated under Binary Feeds up to 40 Bar and 50 °C. J. Memb. Sci. 2021, 621, 118979. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A Systematic Review on CO2 Capture with Ionic Liquids: Current Status and Future Prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Privalova, E.I.; Karjalainen, E.; Nurmi, M.; Mäki-Arvela, P.; Eränen, K.; Tenhu, H.; Murzin, D.Y.; Mikkola, J.P. Imidazolium-Based Poly(Ionic Liquid)s as New Alternatives for CO2 Capture. ChemSusChem 2013, 6, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Luis, P. Use of Monoethanolamine (MEA) for CO2 Capture in a Global Scenario: Consequences and Alternatives. Desalination 2016, 380, 93–99. [Google Scholar] [CrossRef]
- Kwak, N.S.; Lee, J.H.; Lee, I.Y.; Jang, K.R.; Shim, J.G. A Study of the CO2 Capture Pilot Plant by Amine Absorption. Energy 2012, 47, 41–46. [Google Scholar] [CrossRef]
- Panahi, M.; Skogestad, S. Economically Efficient Operation of CO2 Capturing Process Part I: Self-Optimizing Procedure for Selecting the Best Controlled Variables. Chem. Eng. Process. Process Intensif. 2011, 50, 247–253. [Google Scholar] [CrossRef]
- Kausar, A.; Nulwala, H.; Mirjafari, A.; Zhou, X.; Duan, N.; Sun, Z.; Ren, Y.; Liu, Z.; Liu, L.; Yan, F.; et al. Enhanced CO2 Absorption of Poly(Ionic Liquid)s. Macromolecules 2021, 38, 5477–5489. [Google Scholar] [CrossRef]
- Idem, R.; Wilson, M.; Tontiwachwuthikul, P.; Chakma, A.; Veawab, A.; Aroonwilas, A.; Gelowitz, D. Pilot Plant Studies of the CO2 Capture Performance of Aqueous MEA and Mixed MEA/MDEA Solvents at the University of Regina CO2 Capture Technology Development Plant and the Boundary Dam CO2 Capture Demonstration Plant. Ind. Eng. Chem. Res. 2006, 45, 2414–2420. [Google Scholar] [CrossRef]
- Liang, Z.; Rongwong, W.; Liu, H.; Fu, K.; Gao, H.; Cao, F.; Zhang, R.; Sema, T.; Henni, A.; Sumon, K.; et al. Recent Progress and New Developments in Post-Combustion Carbon-Capture Technology with Amine Based Solvents. Int. J. Greenh. Gas Control. 2015, 40, 26–54. [Google Scholar] [CrossRef]
- Lei, Z.; Dai, C.; Chen, B. Gas Solubility in Ionic Liquids. Chem. Rev. 2014, 114, 1289–1326. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, J.F.; Gurkan, B.E. Ionic Liquids for CO2 Capture and Emission Reduction. J. Phys. Chem. Lett. 2010, 1, 3459–3464. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Magna, L.; Morvan, D. Ionic Liquids and Catalysis: Recent Progress from Knowledge to Applications. Appl. Catal. A Gen. 2010, 373, 1–56. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Dong, H.; Zhao, Z.; Zhang, S.; Huang, Y. Carbon Capture with Ionic Liquids: Overview and Progress. Energy Environ. Sci. 2012, 5, 6668–6681. [Google Scholar] [CrossRef]
- Hasib-ur-Rahman, M.; Siaj, M.; Larachi, F. Ionic Liquids for CO2 Capture-Development and Progress. Chem. Eng. Process. Process Intensif. 2010, 49, 313–322. [Google Scholar] [CrossRef]
- Li, X.; Ding, S.; Zhang, J.; Wei, Z. Optimizing Microstructure of Polymer Composite Membranes by Tailoring Different Ionic Liquids to Accelerate CO2 Transport. Int. J. Greenh. Gas Control. 2020, 101, 103136. [Google Scholar] [CrossRef]
- Sodeifian, G.; Raji, M.; Asghari, M.; Rezakazemi, M.; Dashti, A. Polyurethane-SAPO-34 Mixed Matrix Membrane for CO2/CH4 and CO2/N2 Separation. Chin. J. Chem. Eng. 2019, 27, 322–334. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, J.; Lu, C.; Wang, F.; Ma, C.; Chen, Z.; Yang, G. Imidazolium-Based Polymeric Ionic Liquids for Heterogeneous Catalytic Conversion of CO2 into Cyclic Carbonates. Microporous Mesoporous Mater. 2020, 292, 109751. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas Separation Membrane Materials: A Perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Favre, E. Membrane Processes and Postcombustion Carbon Dioxide Capture: Challenges and Prospects. Chem. Eng. J. 2011, 171, 782–793. [Google Scholar] [CrossRef]
- Lian, S.; Song, C.; Liu, Q.; Duan, E.; Ren, H.; Kitamura, Y. Recent Advances in Ionic Liquids-Based Hybrid Processes for CO2 Capture and Utilization. J. Environ. Sci. 2021, 99, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chang, M.; Zang, X.; Zheng, L.; Wang, Y.; Wu, L.; Han, X.; Chen, Y.; Yu, Y.; Zhang, Z. The polymeric ionic liquids/mesoporous alumina composites: Synthesis, characterization and CO2 capture performance test. Polym. Test. 2020, 81, 106109. [Google Scholar] [CrossRef]
- Bellina, F.; Bertoli, A.; Melai, B.; Scalesse, F.; Signori, F.; Chiappe, C. Synthesis and Properties of Glycerylimidazolium Based Ionic Liquids: A Promising Class of Task-Specific Ionic Liquids. Green Chem. 2009, 11, 622–662. [Google Scholar] [CrossRef]
- Bernard, F.L.; dos Santos, L.M.; Schwab, M.B.; Polesso, B.B.; do Nascimento, J.F.; Einloft, S. Polyurethane-Based Poly(Ionic Liquid)s for CO2 Removal from Natural Gas. J. Appl. Polym. Sci. 2019, 136, 4–11. [Google Scholar] [CrossRef]
- da Luz, M.; Dias, G.; Zimmer, H.; Bernard, F.L.; do Nascimento, J.F.; Einloft, S. Poly(Ionic Liquid)s-Based Polyurethane Blends: Effect of Polyols Structure and ILs Counter Cations in CO2 Sorption Performance of PILs Physical Blends. Polym. Bull. 2022, 79, 6123–6139. [Google Scholar] [CrossRef]
- Morozova, S.M.; Lozinskaya, E.I.; Sardon, H.; Su, F.; Vlasov, P.S.; Vaudemont, R.; Vygodskii, Y.S.; Shaplov, A.S. Ionic Polyureas—A Novel Subclass of Poly(Ionic Liquid) s for CO2 Capture. Membranes 2020, 10, 240. [Google Scholar] [CrossRef]
- Morozova, S.M.; Shaplov, A.S.; Lozinskaya, E.I.; Mecerreyes, D.; Sardon, H.; Zulfiqar, S.; Suárez-García, F.; Vygodskii, Y.S. Ionic Polyurethanes as a New Family of Poly(Ionic Liquid)s for Efficient CO2 Capture. Macromolecules 2017, 50, 2814–2824. [Google Scholar] [CrossRef]
- Morozova, S.M.; Shaplov, A.S.; Lozinskaya, E.I.; Vlasov, P.S.; Sardon, H.; Mecerreyes, D.; Vygodskii, Y.S. Poly(Ionic Liquid)-Based Polyurethanes Having Imidazolium, Ammonium, Morpholinium or Pyrrolidinium Cations. High Perform. Polym. 2017, 29, 691–703. [Google Scholar] [CrossRef]
- Dong, Y.; Holm, J.; Kärkkäinen, J.; Nowicki, J.; Lassi, U. Dissolution and Hydrolysis of Fibre Sludge Using Hydroxyalkylimidazolium Hydrogensulphate Ionic Liquids. Biomass Bioenergy 2014, 70, 461–467. [Google Scholar] [CrossRef]
- Duan, N.; Sun, Z.; Ren, Y.; Liu, Z.; Liu, L.; Yan, F. Imidazolium-Based Ionic Polyurethanes with High Toughness, Tunable Healing Efficiency and Antibacterial Activities. Polym. Chem. 2020, 11, 867–875. [Google Scholar] [CrossRef]
- Sonnenschein, M.F.; Lysenko, Z.; Brune, D.A.; Wendt, B.L.; Schrock, A.K. Enhancing Polyurethane Properties via Soft Segment Crystallization. Polymer 2005, 46, 10158–10166. [Google Scholar] [CrossRef]
- Tsai, T.H.; Maes, A.M.; Vandiver, M.A.; Versek, C.; Seifert, S.; Tuominen, M.; Liberatore, M.W.; Herring, A.M.; Coughlin, E.B. Synthesis and Structure-Conductivity Relationship of Polystyrene-Block- Poly(Vinyl Benzyl Trimethylammonium) for Alkaline Anion Exchange Membrane Fuel Cells. J. Polym. Sci. B Polym. Phys. 2013, 51, 1751–1760. [Google Scholar] [CrossRef]
- ASTM D822 Standard. Available online: https://industrialphysics.com/standards/astm-d822/ (accessed on 2 July 2024).
- Barboza, E.M.; Delpech, M.C.; Garcia, M.E.F.; Pimenta, F.D. Avaliação Das Propriedades de Barreira de Membranas Obtidas a Partir de Dispersões Aquosas à Base de Poliuretanos e Argila. Polimeros 2014, 24, 94–100. [Google Scholar] [CrossRef]
- Ferrari, H.Z.; Rodrigues, D.M.; Bernard, F.L.; dos Santos, L.M.; Le Roux, C.; Micoud, P.; Martin, F.; Einloft, S. A New Class of Fillers in Mixed Matrix Membranes: Use of Synthetic Silico-Metallic Mineral Particles (SSMMP) as a Highly Selective Component for CO2/N2 Separation. Chem. Eng. J. Adv. 2023, 14, 100488. [Google Scholar] [CrossRef]
- Waheed, N.; Mushtaq, A.; Tabassum, S.; Gilani, M.A.; Ilyas, A.; Ashraf, F.; Jamal, Y.; Bilad, M.R.; Khan, A.U.; Khan, A.L. Mixed Matrix Membranes Based on Polysulfone and Rice Husk Extracted Silica for CO2 Separation. Sep. Purif. Technol. 2016, 170, 122–129. [Google Scholar] [CrossRef]
- Wu, H.; Thibault, J.; Kruczek, B. The Validity of the Time-Lag Method for the Characterization of Mixed-Matrix Membranes. J. Memb. Sci. 2021, 618, 118715. [Google Scholar] [CrossRef]
- Jacoby, C.G. Síntese de Compostos Imidazol-Tiazolidina e Sua Aplicação como Organocatalisadores em Reações Aldólicas Estereosseletivas. Master’s Thesis, The Federal University of Rio Grande do Sul, Porto Alegre, Brazil, 2016. [Google Scholar]
- Vollas, A.; Chouliaras, T.; Deimede, V.; Ioannides, T. New Pyridinium Type Poly(Ionic Liquids) as Membranes for CO2 Separation. Polymers 2018, 10, 912. [Google Scholar] [CrossRef]
- Yu, G.; Man, Z.; Li, Q.; Li, N.; Wu, X.; Asumana, C.; Chen, X. New Crosslinked-Porous Poly-Ammonium Microparticles as CO2 Adsorbents. React. Funct. Polym. 2013, 73, 1058–1064. [Google Scholar] [CrossRef]
- Zhang, Z.; Gai, H.; Li, Q.; Feng, B.; Xiao, M.; Huang, T.; Song, H. Effect Anions on the Hydrogenation of Nitrobenzene over N-Rich Poly(Ionic Liquid) Supported Pd Catalyst. Chem. Eng. J. 2022, 429, 132224. [Google Scholar] [CrossRef]
- Chiappe, C.; Pomelli, C.S.; Rajamani, S. Influence of Structural Variations in Cationic and Anionic Moieties on the Polarity of Ionic Liquids. J. Phys. Chem. B 2011, 115, 9653–9661. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhao, M.; Ti, Y.; Wang, B. Study on Structure and Performance of Polycarbonate Urethane Synthesized via Different Copolymerization Methods. J. Mater. Sci. 2007, 42, 5508–5515. [Google Scholar] [CrossRef]
- Rogulska, M. Polycarbonate-Based Thermoplastic Polyurethane Elastomers Modified by DMPA. Polym. Bull. 2019, 76, 4719–4733. [Google Scholar] [CrossRef]
- Cakić, S.M.; Špírková, M.; Ristić, I.S.; B-Simendić, J.K.; M-Cincović, M.; Porȩba, R. The Waterborne Polyurethane Dispersions Based on Polycarbonate Diol: Effect of Ionic Content. Mater. Chem. Phys. 2013, 138, 277–285. [Google Scholar] [CrossRef]
- Zhang, Y.; Sunarso, J.; Liu, S.; Wang, R. Current Status and Development of Membranes for CO2/CH4 Separation: A Review. Int. J. Greenh. Gas Control. 2013, 12, 84–107. [Google Scholar] [CrossRef]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(Ionic Liquid)s: An Update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Zhang, M.; Hemp, S.T.; Zhang, M.; Allen, M.H.; Carmean, R.N.; Moore, R.B.; Long, T.E. Water-Dispersible Cationic Polyurethanes Containing Pendant Trialkylphosphoniums. Polym. Chem. 2014, 5, 3795–3803. [Google Scholar] [CrossRef]
- Behera, P.K.; Usha, K.M.; Guchhait, P.K.; Jehnichen, D.; Das, A.; Voit, B.; Singha, N.K. A Novel Ionomeric Polyurethane Elastomer Based on Ionic Liquid as Crosslinker. RSC Adv. 2016, 6, 99404–99413. [Google Scholar] [CrossRef]
- Vila, J.; Varela, L.M.; Cabeza, O. Cation and Anion Sizes Influence in the Temperature Dependence of the Electrical Conductivity in Nine Imidazolium Based Ionic Liquids. Electrochim. Acta 2007, 52, 7413–7417. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Sarwar, M.I.; Mecerreyes, D. Polymeric Ionic Liquids for CO2 Capture and Separation: Potential, Progress and Challenges. Polym. Chem. 2015, 6, 6435–6451. [Google Scholar] [CrossRef]
- Raza, A.; Farrukh, S.; Hussain, A.; Khan, I.; Othman, M.H.D.; Ahsan, M. Performance Analysis of Blended Membranes of Cellulose Acetate with Variable Degree of Acetylation for CO2/CH4 Separation. Membranes 2021, 11, 245. [Google Scholar] [CrossRef]
- Mubashir, M.; Yeong, Y.F.; Lau, K.K.; Chew, T.L.; Norwahyu, J. Efficient CO2/N2 and CO2/CH4 Separation Using NH2-MIL-53(Al)/Cellulose Acetate (CA) Mixed Matrix Membranes. Sep. Purif. Technol. 2018, 199, 140–151. [Google Scholar] [CrossRef]
- Farrokhara, M.; Dorosti, F. New High Permeable Polysulfone/Ionic Liquid Membrane for Gas Separation. Chin. J. Chem. Eng. 2020, 28, 2301–2311. [Google Scholar] [CrossRef]
- Sajjan, P.; Nayak, V.; Padaki, M.; Zadorozhnyy, V.Y.; Klyamkin, S.N.; Konik, P.A. Fabrication of Cellulose Acetate Film through Blending Technique with Palladium Acetate for Hydrogen Gas Separation. Energy Fuels 2020, 34, 11699–11707. [Google Scholar] [CrossRef]
- Akbarzadeh, E.; Shockravi, A.; Vatanpour, V. High Performance Compatible Thiazole-Based Polymeric Blend Cellulose Acetate Membrane as Selective CO2 Absorbent and Molecular Sieve. Carbohydr. Polym. 2021, 252, 117215. [Google Scholar] [CrossRef]
- Bara, J.E.; Lessmann, S.; Gabriel, C.J.; Hatakeyama, E.S.; Noble, R.D.; Gin, D.L. Synthesis and Performance of Polymerizable Room-Temperature Ionic Liquids as Gas Separation Membranes. Ind. Eng. Chem. Res. 2007, 46, 5397–5404. [Google Scholar] [CrossRef]
- Bara, J.E.; Gabriel, C.J.; Hatakeyama, E.S.; Carlisle, T.K.; Lessmann, S.; Noble, R.D.; Gin, D.L. Improving CO2 Selectivity in Polymerized Room-Temperature Ionic Liquid Gas Separation Membranes through Incorporation of Polar Substituents. J. Memb. Sci. 2008, 321, 3–7. [Google Scholar] [CrossRef]
- Horne, W.J.; Andrews, M.A.; Shannon, M.S.; Terrill, K.L.; Moon, J.D.; Hayward, S.S.; Bara, J.E. Effect of Branched and Cycloalkyl Functionalities on CO2 Separation Performance of Poly(IL) Membranes. Sep. Purif. Technol. 2015, 155, 89–95. [Google Scholar] [CrossRef]
- Shaligram, S.V.; Rewar, A.S.; Wadgaonkar, P.P.; Kharul, U.K. Incorporation of Rigid Polyaromatic Groups in Polybenzimidazole-Based Polymeric Ionic Liquids: Assertive Effects on Gas Permeation Properties. Polymer 2016, 93, 30–36. [Google Scholar] [CrossRef]
- Fang, W.; Luo, Z.; Jiang, J. CO2 Capture in Poly(Ionic Liquid) Membranes: Atomistic Insight into the Role of Anions. Phys. Chem. Chem. Phys. 2013, 15, 651–658. [Google Scholar] [CrossRef]
- Robeson, L.M. The Upper Bound Revisited. J. Memb. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of Separation Factor versus Permeability for Polymeric Membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Zhang, M.; Semiat, R.; He, X. Recent Advances in Poly(Ionic Liquids) Membranes for CO2 Separation. Sep. Purif. Technol. 2022, 299, 121784. [Google Scholar] [CrossRef]




| Sample | Tonset1 (°C) | Tonset2 (°C) |
|---|---|---|
| PU | 276 | 428 |
| PIL-Cl | 279 | 469 |
| PIL-NTf2 | 318 | 462 |
| PIL-PF6 | 300 | 440 |
| PIL-BF4 | 325 | 445 |
| Membrane | Perm. CO2 (Barrer) | Perm. CH4 (Barrer) | Sel. Ideal CO2/CH4 |
|---|---|---|---|
| PU | 2.0 ± 0.1 | 0.78 ± 0.02 | 2.5 |
| PIL-Cl | 17 ± 2.2 | 0.6 ± 0.1 | 30 |
| PIL-NTf2 | - | - | - |
| PIL-PF6 | 9 ± 1.7 | 0.6 ± 0.2 | 14 |
| PIL-BF4 | 41 ± 3.2 | 0.9 ± 0.3 | 44 |
| 1—CTA (25 °C, 5 bar) | 18 | 1.98 | 9 |
| 2—CA (25 °C, 3 bar) | 15 | 1.45 | 10 |
| 3—CA (25 °C, 1 bar) | 4 | 0.2 | 17 |
| 4—CA (35° C, 3 bar) | 4.3 | 0.21 | 21 |
| 5—Styrene-Based Poly(RTILS) (2 atm; RT) | 9.2 ± 0.5 | 0.24 ± 0.01 | 39 |
| 5—Acrylate-Based Poly(RTILS) (2 atm; RT) | 7.0 ± 0.4 | 0.19 ± 0.02 | 37 |
| 6—PIL-TFSI (1 atm; RT) | 4.1 ± 0.1 | - | 41 |
| 7—OEG1 (2 atm; 295 K) | 16 ± 1 | 0.48 ± 0.01 | 33 |
| 7—OEG2 (2 atm; 295 K) | 22 ± 1 | 0.74 ± 0.02 | 29 |
| 8—PIL-i-propyl (3 atm; 20 °C) | 10.4 ± 0.2 | 0.35 ± 0.01 | 30 |
| 9—Poly[DPyDBzPBI-BuI][Tf2N] (20 atm; 35 °C) | 36.2 | 1.25 | 29 |
| Membrane | D (10−8 cm²/s) | S (10−2 cm³(STP)/(cm³ cmHg)) | DCO2/CH4 | SCO2/CH4 | ||
|---|---|---|---|---|---|---|
| CO2 | CH4 | CO2 | CH4 | |||
| PIL-Cl | 21.60 | 2.32 | 0.78 | 0.26 | 9.32 | 3.04 |
| PIL-NTf2 | - | - | - | - | - | - |
| PIL-PF6 | 22.04 | 1.61 | 0.41 | 0.37 | 13.68 | 1.10 |
| PIL-BF4 | 32.67 | 1.79 | 1.26 | 0.50 | 18.22 | 2.52 |
| CA (35 °C, 3 bar) [57] | 2.05 | 0.55 | 2.11 | 0.38 | 3.72 | 5.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, G.; Rocca, L.; Ferrari, H.Z.; Bernard, F.L.; Brandão, F.G.; Pereira, L.; Einloft, S. Cationic Imidazolium-Urethane-Based Poly(Ionic Liquids) Membranes for Enhanced CO2/CH4 Separation: Synthesis, Characterization, and Performance Evaluation. Membranes 2024, 14, 151. https://doi.org/10.3390/membranes14070151
Dias G, Rocca L, Ferrari HZ, Bernard FL, Brandão FG, Pereira L, Einloft S. Cationic Imidazolium-Urethane-Based Poly(Ionic Liquids) Membranes for Enhanced CO2/CH4 Separation: Synthesis, Characterization, and Performance Evaluation. Membranes. 2024; 14(7):151. https://doi.org/10.3390/membranes14070151
Chicago/Turabian StyleDias, Guilherme, Laura Rocca, Henrique Z. Ferrari, Franciele L. Bernard, Fernando G. Brandão, Leonardo Pereira, and Sandra Einloft. 2024. "Cationic Imidazolium-Urethane-Based Poly(Ionic Liquids) Membranes for Enhanced CO2/CH4 Separation: Synthesis, Characterization, and Performance Evaluation" Membranes 14, no. 7: 151. https://doi.org/10.3390/membranes14070151
APA StyleDias, G., Rocca, L., Ferrari, H. Z., Bernard, F. L., Brandão, F. G., Pereira, L., & Einloft, S. (2024). Cationic Imidazolium-Urethane-Based Poly(Ionic Liquids) Membranes for Enhanced CO2/CH4 Separation: Synthesis, Characterization, and Performance Evaluation. Membranes, 14(7), 151. https://doi.org/10.3390/membranes14070151








