Removal of Microplastics in a Hybrid Treatment Process of Ceramic Microfiltration and Photocatalyst-Mounted PES Spheres with Air Backwashing
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Removal of Microplastics in Ceramic Microfiltration Water Treatment Process
3.1.1. Impact of Organic Matter Concentration (Humic Acid, HA)
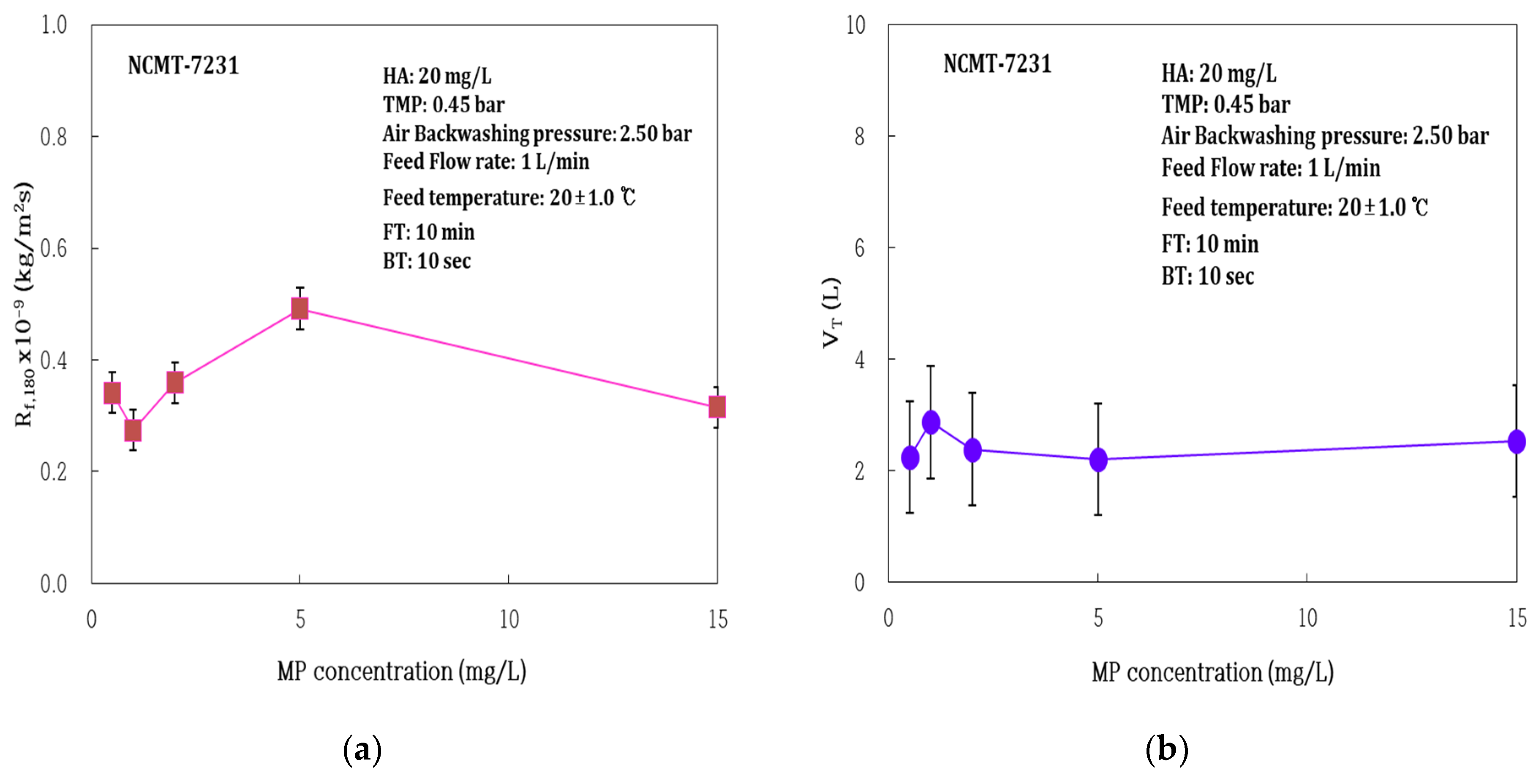
3.1.2. Impact of Microplastic (MP) Concentrations
3.2. Removal of Microplastics in Hybrid Water Treatment Processes of Ceramic Microfiltration (MF) and Photocatalyst (TiO2)-Mounted PES Spheres
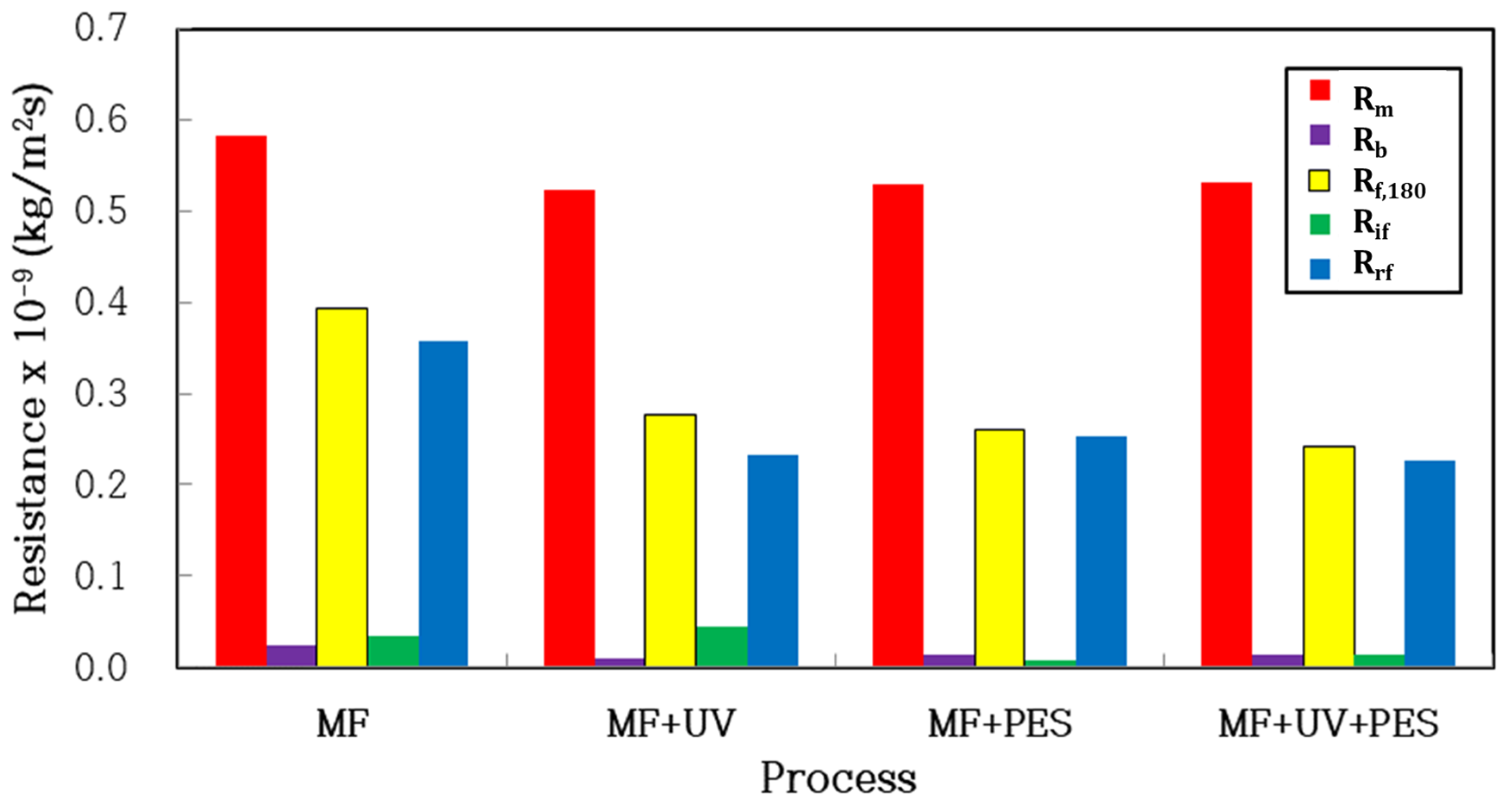
3.2.1. Roles of Microfiltration and Photocatalytic Adsorption, Photooxidation in Periodic Air Backwashing
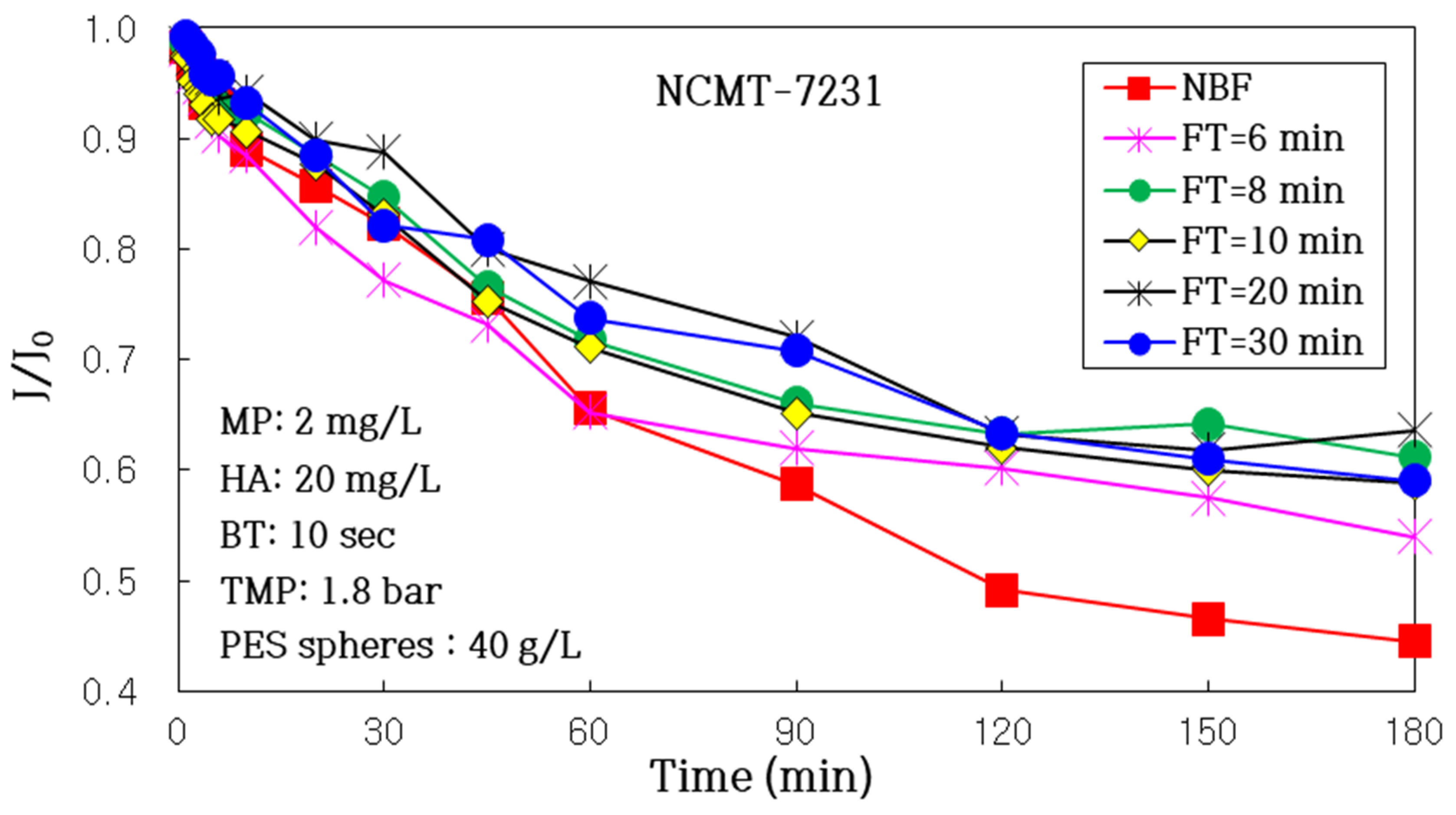
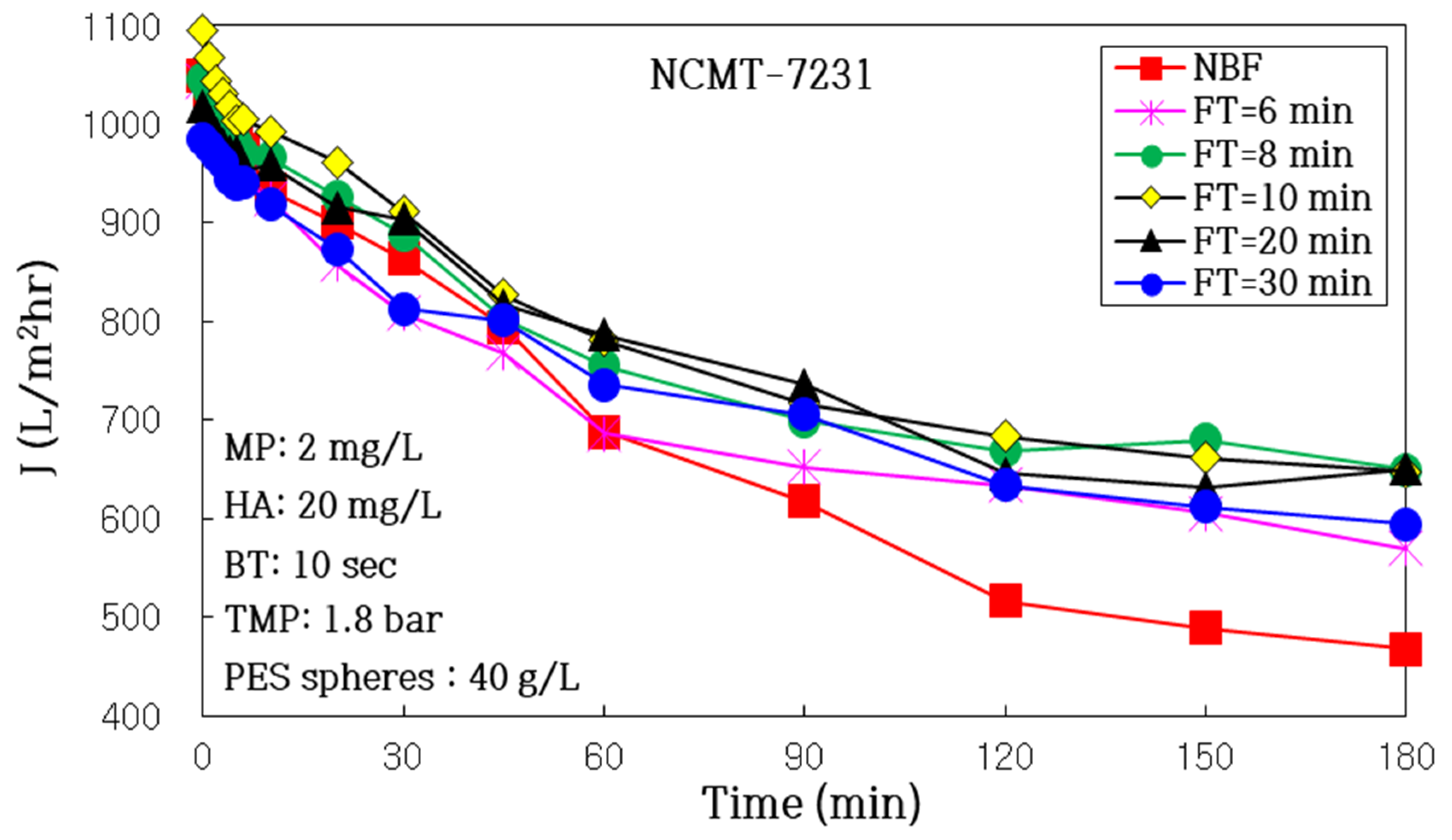
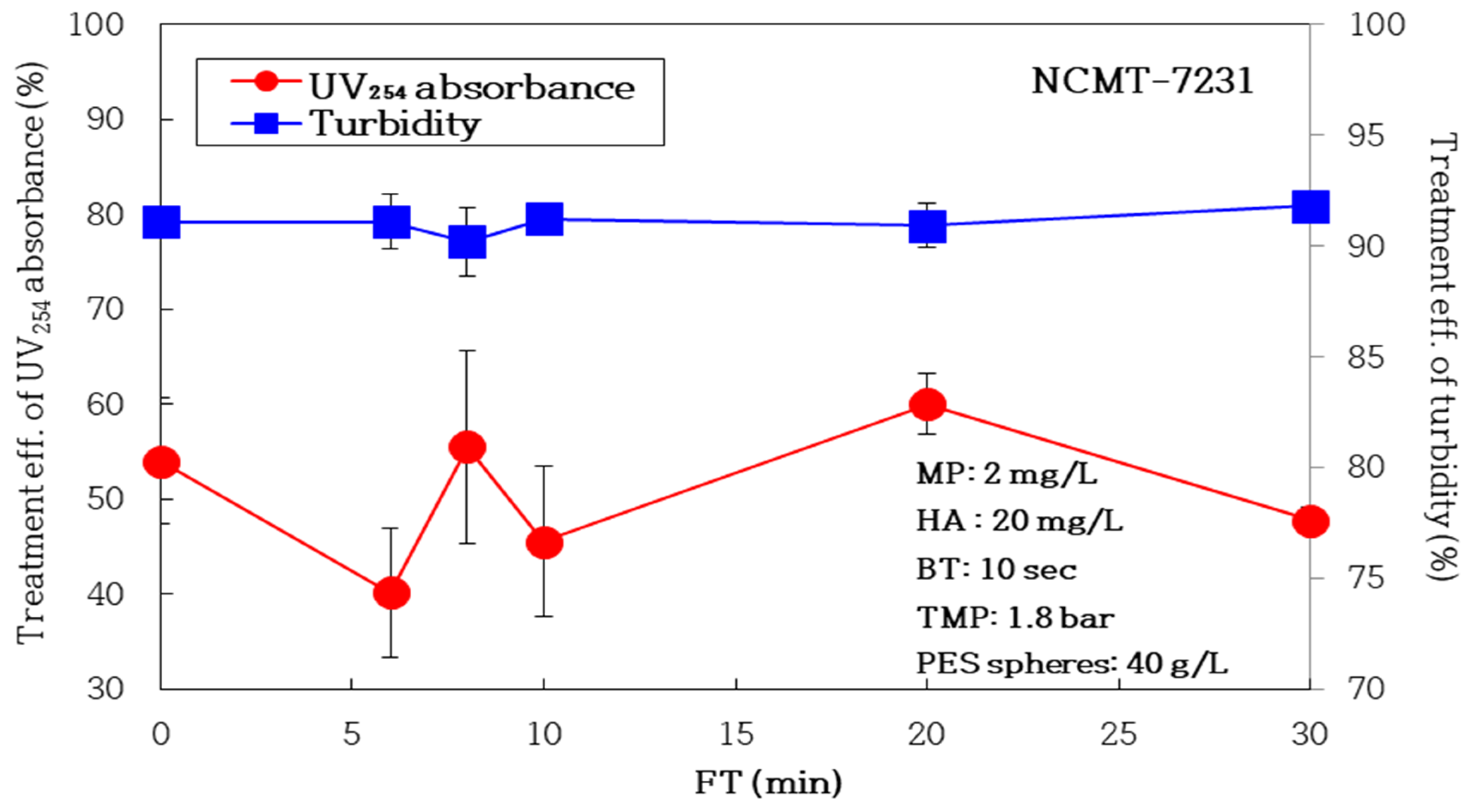
3.2.2. Impact of Air Backwashing Cycle (FT, Filtration Time)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.; Li, M.C.; Lee, J.; Liu, C.; Mei, C. Microplastic remediation technologies in water and wastewater treatment processes: Current status and future perspectives. Sci. Total Environ. 2023, 868, 161618. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Ning, R.; Deng, X. Feasibility, challenges, and future prospects of microalgae-based bioremediation technique for removing microplastics from wastewater. Front. Bioeng. Biotechnol. 2023, 11, 1288439. [Google Scholar] [CrossRef] [PubMed]
- Blair, R.M.; Waldron, S.; Phoenix, V.R.; Gauchotte-Lindsay, C. Microscopy and elemental analysis characterisation of microplastics in sediment of a freshwater urban river in Scotland, UK. Environ. Sci. Pollut. Res. 2019, 26, 12491–12504. [Google Scholar] [CrossRef] [PubMed]
- Cherian, A.G.; Liu, Z.; McKie, M.J.; Almuhtaram, H.; Andrews, R.C. Microplastic Removal from Drinking Water Using Point-of-Use Devices. Polymers 2023, 15, 1331. [Google Scholar] [CrossRef] [PubMed]
- Park, T.J.; Lee, S.H.; Lee, M.S.; Lee, J.K.; Lee, S.H.; Zoh, K.D. Occurrence of microplastics in the Han River and riverine fish in South Korea. Sci. Total Environ. 2020, 708, 134535. [Google Scholar] [CrossRef] [PubMed]
- Eo, S.; Hong, S.H.; Song, Y.K.; Han, G.M.; Shim, W.J. Spatiotemporal distribution and annual load of microplastics in the Nakdong River, South Korea. Water Res. 2019, 160, 228–237. [Google Scholar] [CrossRef] [PubMed]
- D’Avignon, G.; Gregory-Eaves, I.; Ricciardi, A. Microplastics in lakes and rivers: An issue of emerging significance to limnology. Environ. Rev. 2022, 30, 228–244. [Google Scholar] [CrossRef]
- Violetta, G.E.C. Microplastics in Food Commodities: A Food Safety Review on Human Exposure through Dietary Sources; Food Safety and Quality Series; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022; Volume 18, pp. 1–124. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). From Pollution to Solution; United Nations Environment Programme: Nairobi, Kenya, 2021. [Google Scholar]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 619–620, 1–8. [Google Scholar] [CrossRef]
- Acarer, S. Microplastics in wastewater treatment plants: Sources, properties, removal efficiency, removal mechanisms, and interactions with pollutants. Water Sci. Technol. 2023, 87, 685–710. [Google Scholar] [CrossRef] [PubMed]
- Acarer, S. A review of microplastic removal from water and wastewater by membrane technologies. Water Sci. Technol. 2023, 88, 199–219. [Google Scholar] [CrossRef]
- Dalmau-Soler, J.; Ballesteros-Cano, R.; Boleda, M.R.; Paraira, M.; Ferrer, N.; Lacorte, S. Microplastics from headwaters to tap water: Occurrence and removal in a drinking water treatment plant in Barcelona Metropolitan area (Catalonia, NE Spain). Environ. Sci. Pollut. Res. 2021, 28, 59462–59472. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.U.; Ha, I.H.; Park, J.H.; Go, Y.J.; Yun, J.U.; Kim, H.S. Current status of research on ceramic membrane materials for water treatment. Ceramist 2012, 15, 14–27. [Google Scholar]
- Takeuchi, H.; Tanaka, S.; Koyuncu, C.Z.; Nakada, N. Removal of microplastics in wastewater by ceramic microfiltration. J. Water Process Eng. 2023, 54, 104010. [Google Scholar] [CrossRef]
- Ma, B.; Xue, W.; Ding, Y.; Hu, C.; Liu, H.; Qu, J. Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment. J. Environ. Sci. 2019, 78, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Monnot, M.; Sun, Y.; Asia, L.; Wong-Wah-Chung, P.; Doumeng, P.; Moulin, P. Microplastics in different water samples (seawater, freshwater, and wastewater): Removal efficiency of membrane treatment processes. Water Res. 2023, 232, 119673. [Google Scholar] [CrossRef] [PubMed]
- Mohana, A.A.; Rahman, M.; Sarker, S.K.; Haque, N.; Gao, L.; Pramanik, B.K. Nano/microplastics: Fragmentation, interaction with co-existing pollutants and their removal from wastewater using membrane processes. Chemosphere 2022, 309, 136682. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, G.; Yu, H.; Xing, J. Dynamic membrane for micro-particle removal in wastewater treatment: Performance and influencing factors. Sci. Total Environ. 2018, 627, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Ariza-Tarazona, M.C.; Villarreal-Chiu, J.F.; Barbieri, V.; Siligardi, C.; Cedillo-González, E.I. New strategy for microplastic degradation: Green photocatalysis using a protein-based porous N-TiO2 semiconductor. Ceram. Int. 2019, 45, 9618–9624. [Google Scholar] [CrossRef]
- Llorente-García, B.E.; Hernández-López, J.M.; Zaldívar-Cadena, A.A.; Siligardi, C.; Cedillo-González, E.I. First Insights into Photocatalytic Degradation of HDPE and LDPE Microplastics by a Mesoporous N–TiO2 Coating: Effect of Size and Shape of Microplastics. Coatings 2020, 10, 658. [Google Scholar] [CrossRef]
- Nabi, I.; Bacha, A.; Li, K.; Cheng, H.; Wang, T.; Liu, Y.; Ajmal, S.; Yang, Y.; Feng, Y.; Zhang, L. Complete Photocatalytic Mineralization of Microplastic on TiO2 Nanoparticle Film. iScience 2020, 23, 101326. [Google Scholar] [CrossRef]
- Ariza-Tarazona, M.C.; Villarreal-Chiu, J.F.; Hernández-López, J.M.; Rosa, J.R.D.I.; Barbieri, V.; Siligardi, C.; Cedillo-González, E.I. Microplastic pollution reduction by a carbon and nitrogen-doped TiO2: Effect of pH and temperature in the photocatalytic degradation process. J. Hazard. Mater. 2020, 395, 122632. [Google Scholar] [CrossRef]
- Lee, J.M.; Busquets, R.; Choi, I.C.; Lee, S.H.; Kim, J.K.; Campos, L.C. Photocatalytic Degradation of Polyamide 66; Evaluating the Feasibility of Photocatalysis as a Microfibre-Targeting Technology. Water 2020, 12, 3551. [Google Scholar] [CrossRef]
- Domínguez-Jaimes, L.P.; Cedillo-González, E.I.; Luévano-Hipólito, E.; Acuña-Bedoya, J.D.; Hernández-López, J.M. Degradation of primary nanoplastics by photocatalysis using different anodized TiO2 structures. J. Hazard. Mater. 2021, 413, 125452. [Google Scholar] [CrossRef]
- Fadli, M.H.; Ibadurrohman, M.; Slamet, S. Microplastic Pollutant Degradation in Water Using Modified TiO2 Photocatalyst Under UV-Irradiation. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1011, 012055. [Google Scholar] [CrossRef]
- Maulana, D.A.; Ibadurrohman, M. Slamet, Synthesis of Nano-Composite Ag/TiO2 for Polyethylene Microplastic Degradation Applications. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1011, 012054. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, F.; Zhang, J.; Zou, K.; Zhang, J.; Chen, C.; Long, M.; Zhang, Q.; Wang, J.; Zheng, C.; et al. Preparation of composite photocatalyst with tunable and self-indicating delayed onset of performance and its application in polyethylene degradation. Appl. Catal. B Environ. 2021, 286, 119918. [Google Scholar] [CrossRef]
- Ariza-Tarazona, M.C.; Siligardi, C.; Carreón-López, H.A.; Valdéz-Cerda, J.E.; Pozzi, P.; Kaushik, G.; Villarreal-Chiu, J.F.; Cedillo-González, E.I. Low environmental impact remediation of microplastics: Visible-light photocatalytic degradation of PET microplastics using bio-inspired C,N-TiO2/SiO2 photocatalysts. Mar. Pollut. Bull. 2023, 193, 115206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Luo, H.; Zhang, F.; Wu, J.; Yang, J.; Wang, H. Efficient Photocatalytic Degradation of the Persistent PET Fiber-Based Microplastics over Pt Nanoparticles Decorated N-Doped TiO2 Nanoflowers. Adv. Fiber Mater. 2022, 4, 1094–1107. [Google Scholar] [CrossRef]
- Chattopadhyay, P.; Ariza-Tarazona, M.C.; Cedillo-González, E.I.; Siligardi, C.; Simmchen, J. Combining photocatalytic collection and degradation of microplastics using self-asymmetric Pac-Man TiO2. Nanoscale 2023, 15, 14774–14781. [Google Scholar] [CrossRef]
- Amato, P.; Fantauzzi, M.; Sannino, F.; Ritacco, I.; Santoriello, G.; Camellone, M.F.; Imparato, C.; Bifulco, A.; Vitiello, G.; Caporaso, L.; et al. Indirect daylight oxidative degradation of polyethylene microplastics by a bio-waste modified TiO2-based material. J. Hazard. Mater. 2024, 463, 132907. [Google Scholar] [CrossRef]
- Buonomenna, M.G.; Figoli, A.; Spezzano, I.; Davoli, M.; Drioli, E. New PVDF microcapsules for application in catalysis. Appl. Catal. B Environ. 2008, 80, 185–194. [Google Scholar] [CrossRef]
- Amarsanaa, B.; Park, J.Y. Effect of water backwashing and PP beads in hybrid water treatment of multi-channel alumina MF and photocatalyst-coated PP beads. Desalin. Water Treat. 2015, 54, 1457–1469. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.; Park, J.Y. The role of adsorption, photo-oxidation, and PP spheres with air backwashing in hybrid water treatment of multichannel alumina MF and PP spheres. Membranes 2020, 10, 28. [Google Scholar] [CrossRef] [PubMed]

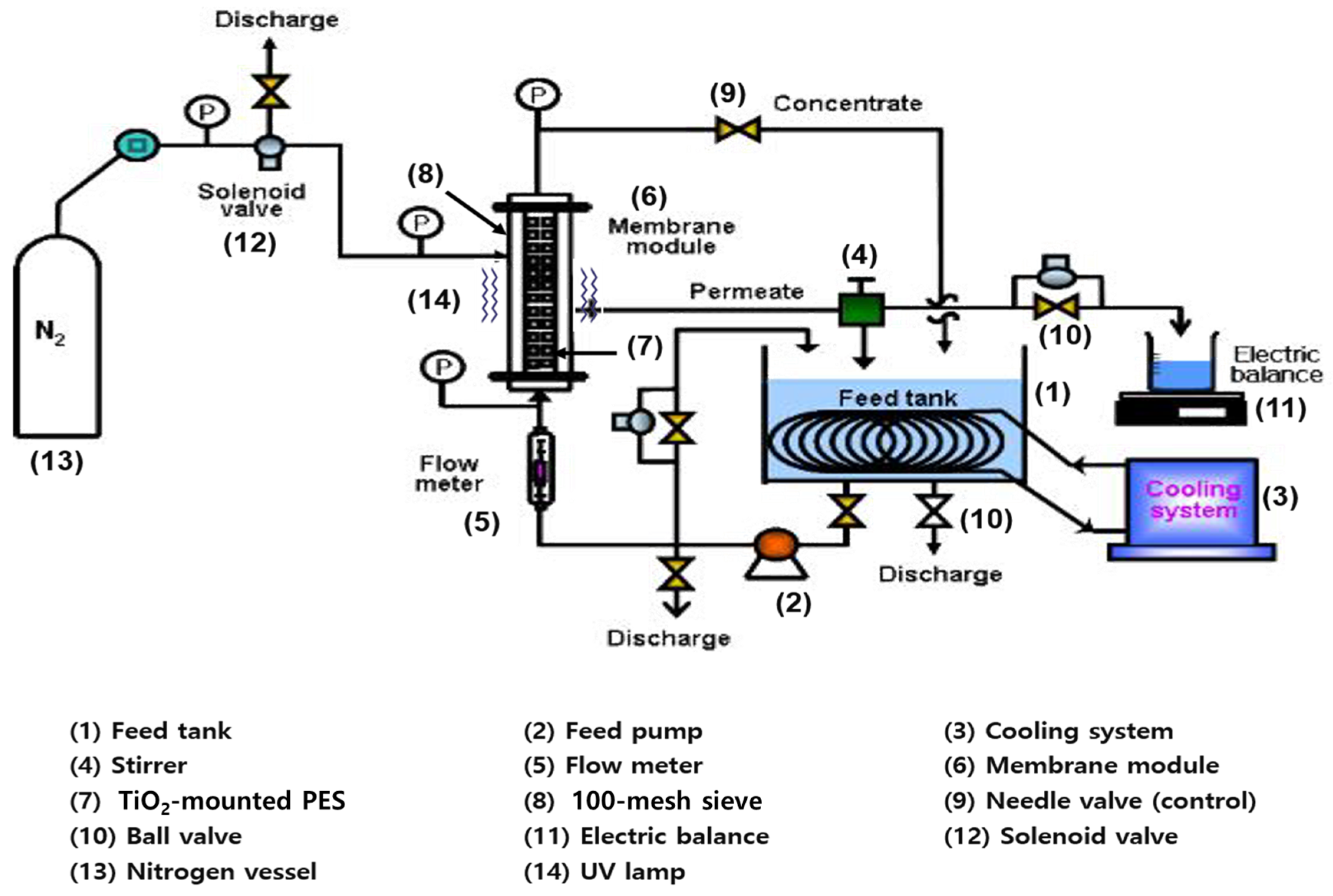
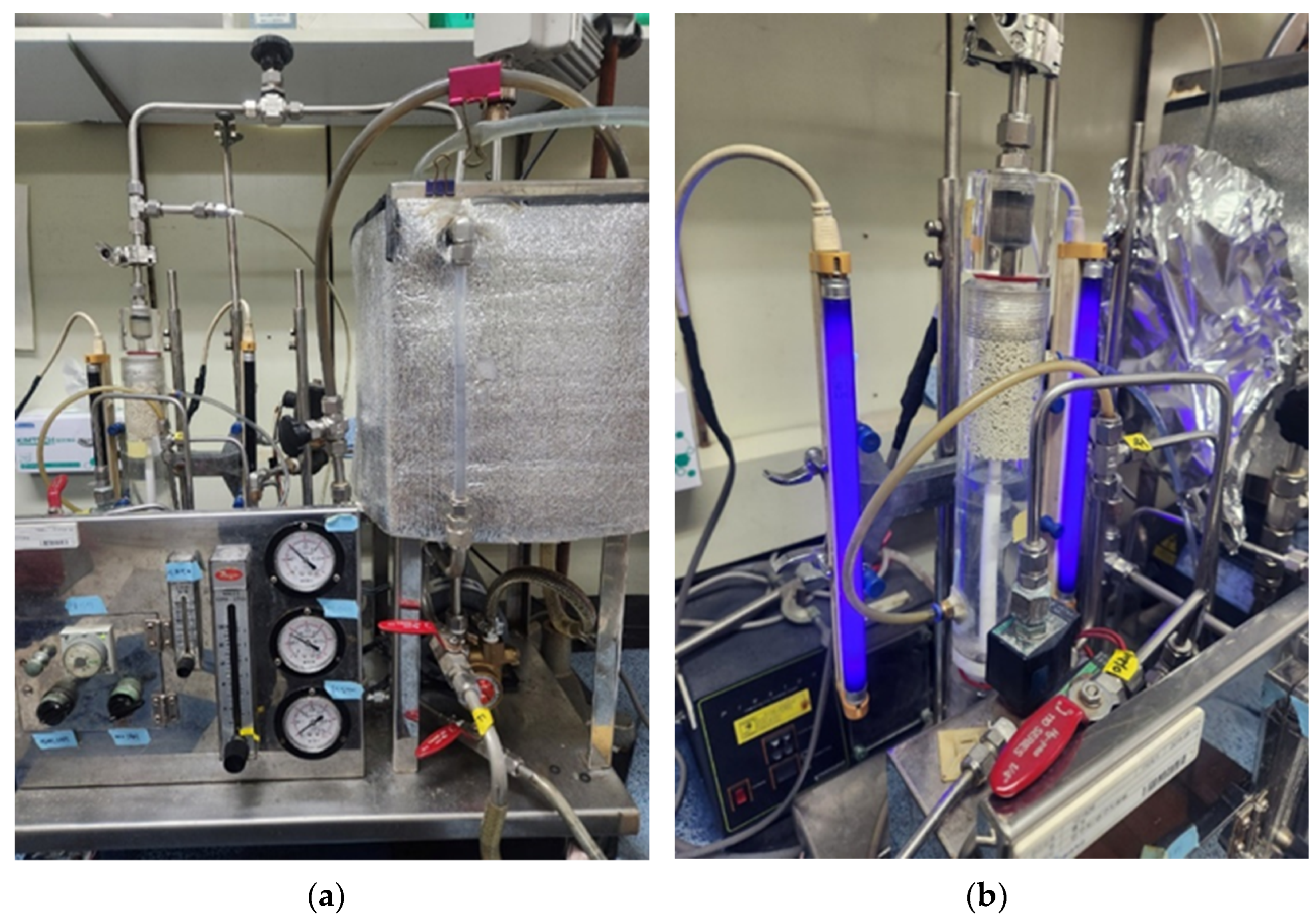
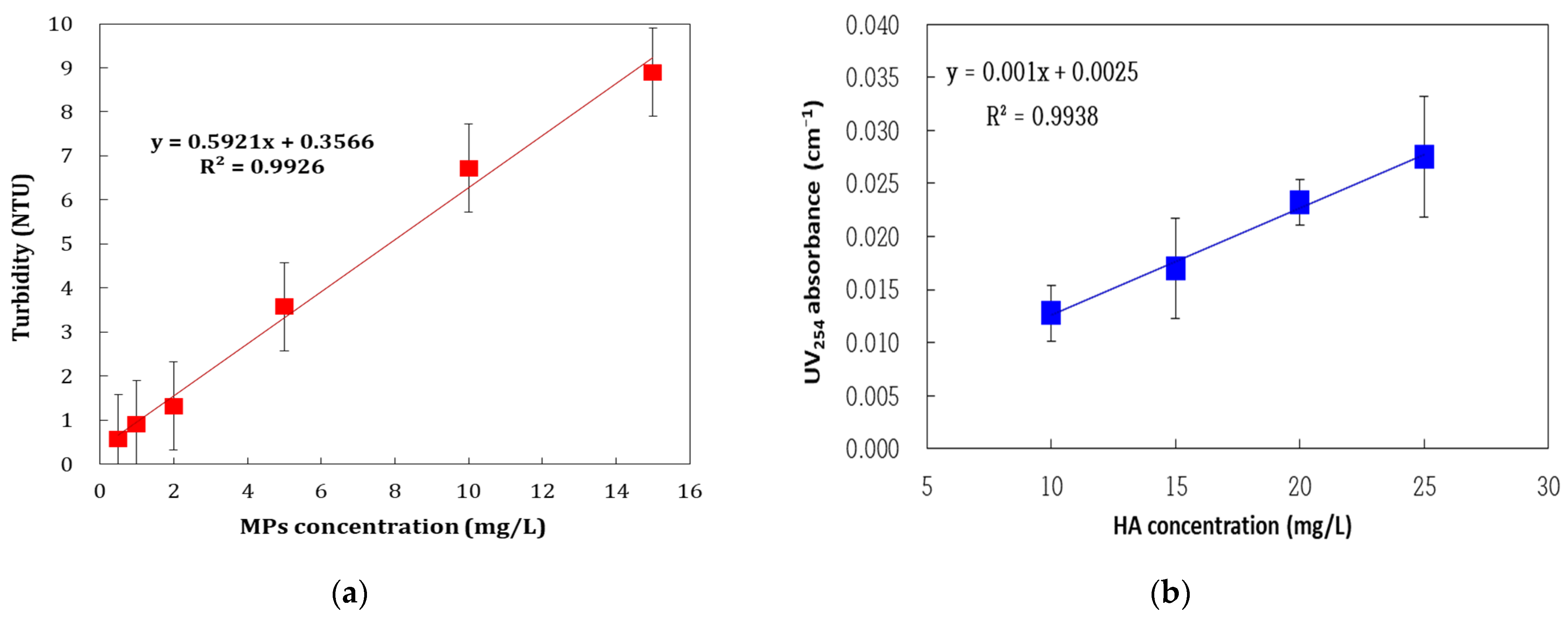

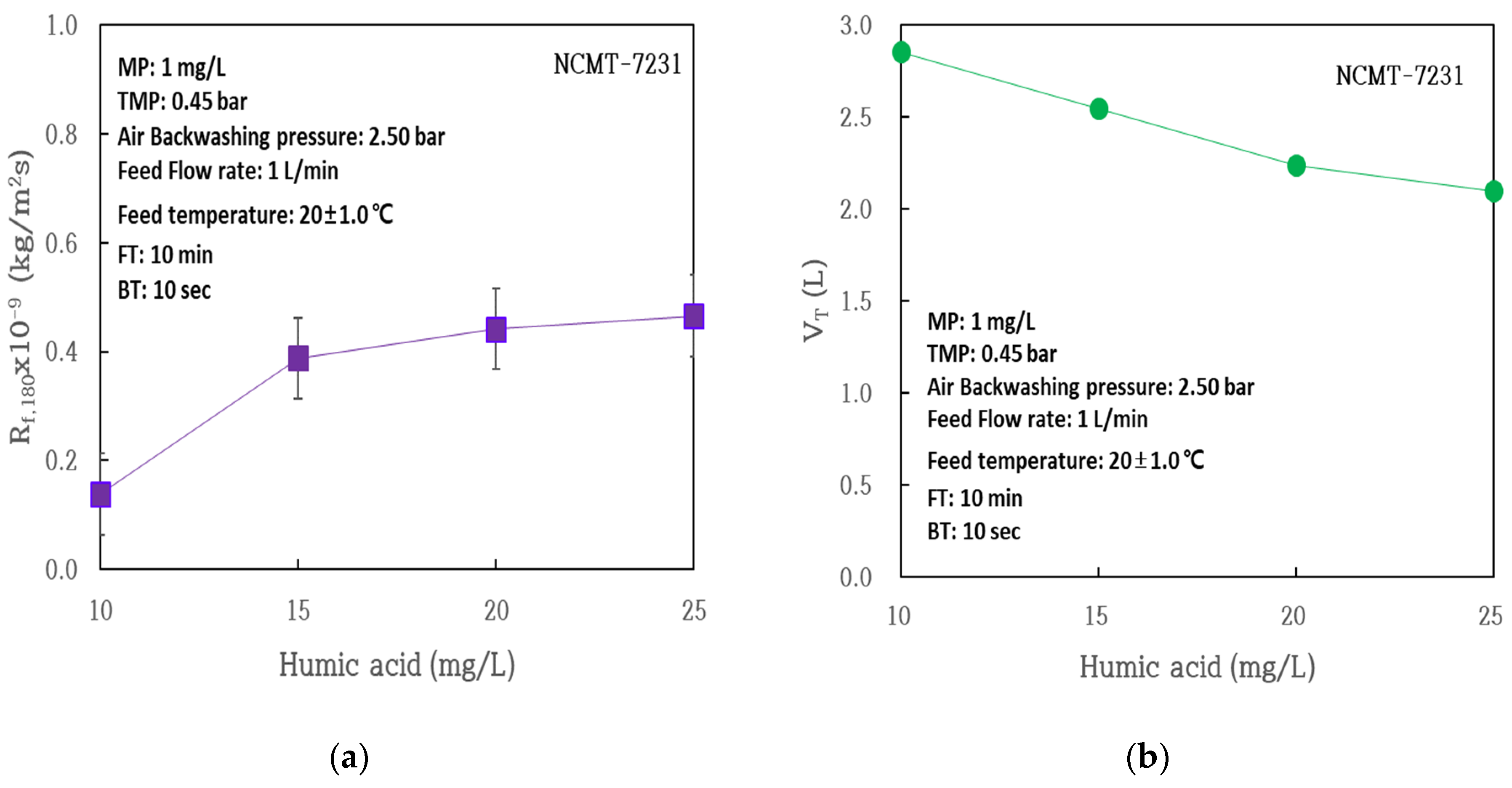
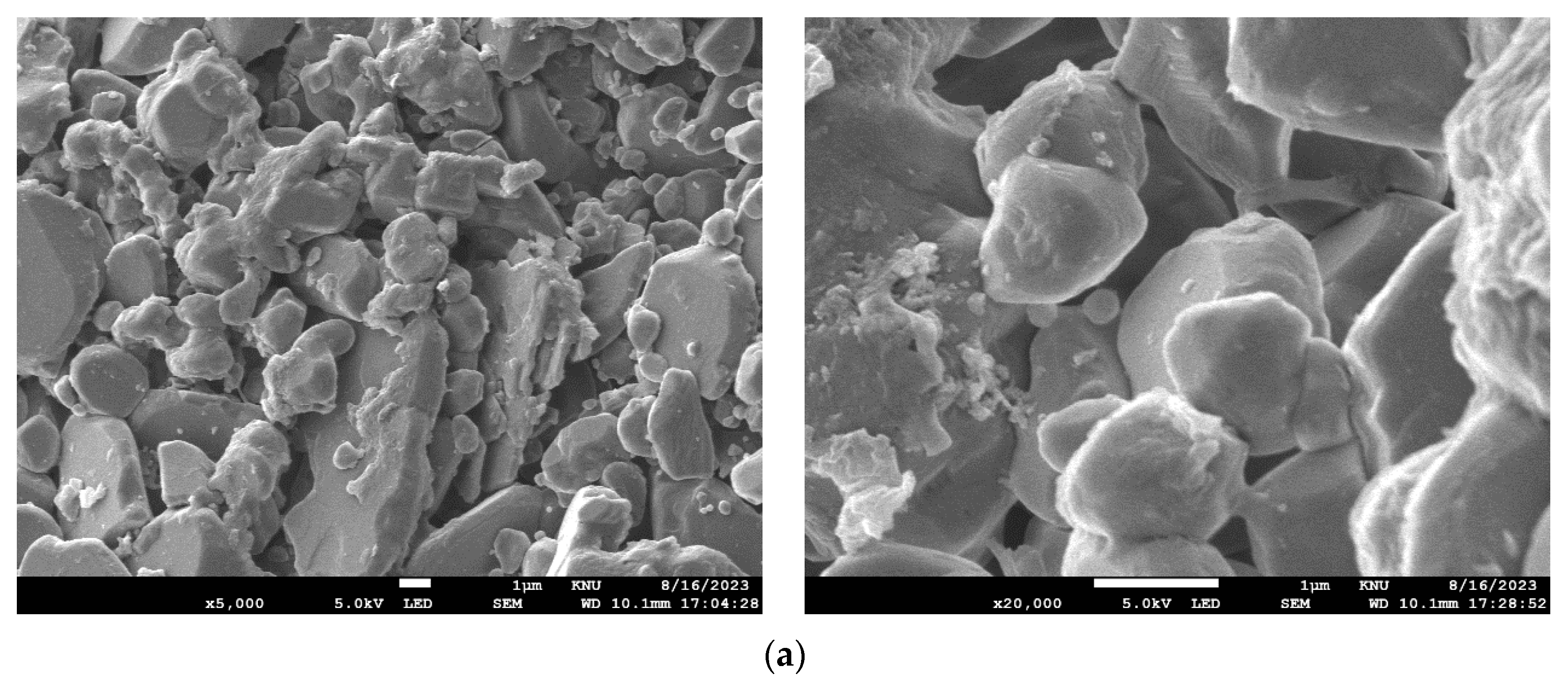
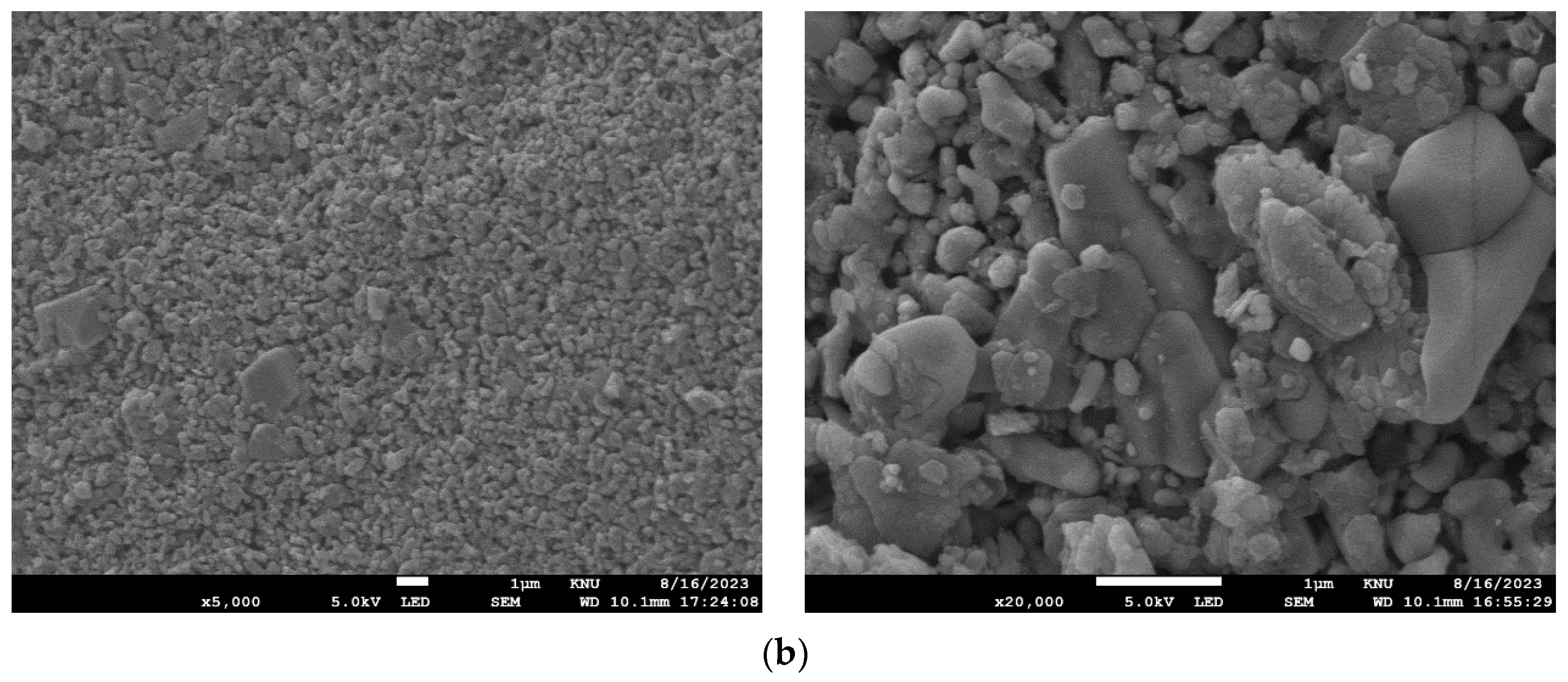






| Membrane Model | NCMT-7231 |
|---|---|
| Pore size (μm) | 0.1 |
| No. of channels | 1 |
| Outer diameter (mm) | 8 |
| Inner diameter (mm) | 6 |
| Length (mm) | 270 |
| Surface area (cm2) | 50.9 |
| Material | α-alumina |
| Company | Nano Pore Materials (Seoul, Korea) |
| HA (mg/L) | 10 | 15 | 20 | 25 |
|---|---|---|---|---|
| Rm × 10−9 (kg/m2s) | 0.479 | 0.667 | 0.579 | 0.624 |
| Rb × 10−9 (kg/m2s) | 0.021 | 0.080 | 0.046 | 0.217 |
| Rf,180 × 10−9 (kg/m2s) | 0.139 | 0.388 | 0.422 | 0.465 |
| Rif × 10−9 (kg/m2s) | 0.247 | 0.032 | 0.108 | 0.154 |
| Rrf × 10−9 (kg/m2s) | 0.123 | 0.356 | 0.334 | 0.311 |
| J0 (L/m2h) | 145 | 163 | 183 | 146 |
| J180 (L/m2h) | 247 | 213 | 175 | 189 |
| J180/J0 | 0.589 | 0.754 | 0.739 | 0.772 |
| Vt (L) | 2.85 | 2.54 | 2.24 | 2.10 |
| HA (mg/L) | 20 | ||||
|---|---|---|---|---|---|
| MP (mg/L) | 0.5 | 1 | 2 | 5 | 15 |
| Rm × 10−9 (kg/m2s) | 0.619 | 0.579 | 0.585 | 0.632 | 0.594 |
| Rb × 10−9 (kg/m2s) | 0.032 | 0.046 | 0.117 | 0.149 | 0.098 |
| Rf,180 × 10−9 (kg/m2s) | 0.341 | 0.274 | 0.359 | 0.492 | 0.315 |
| Rif × 10−9 (kg/m2s) | 0.153 | 0.163 | 0.013 | 0.046 | 0.032 |
| Rrf × 10−9 (kg/m2s) | 0.188 | 0.111 | 0.347 | 0.446 | 0.283 |
| J0 (L/m2h) | 165 | 186 | 168 | 142 | 178 |
| J180 (L/m2h) | 244 | 256 | 226 | 203 | 230 |
| J180/J0 | 0.678 | 0.732 | 0.743 | 0.697 | 0.768 |
| Vt (L) | 2.24 | 2.86 | 2.38 | 2.19 | 2.53 |
| Process | MF | MF+UV | MF+PES | MF+UV+PES |
|---|---|---|---|---|
| Rm × 10−9 (kg/m2s) | 0.583 | 0.524 | 0.530 | 0.532 |
| Rb × 10−9 (kg/m2s) | 0.025 | 0.010 | 0.013 | 0.013 |
| Rf,180 × 10−9 (kg/m2s) | 0.393 | 0.277 | 0.261 | 0.242 |
| Rif × 10−9 (kg/m2s) | 0.035 | 0.045 | 0.008 | 0.015 |
| Rrf × 10−9 (kg/m2s) | 0.358 | 0.232 | 0.253 | 0.227 |
| J180 (L/m2h) | 1072 | 1190 | 1171 | 1165 |
| J0 (L/m2h) | 676 | 817 | 810 | 811 |
| J180/J0 | 0.624 | 0.685 | 0.691 | 0.697 |
| VT (L) | 11.6 | 13.8 | 13.7 | 13.5 |
| Experimental Condition | Turbidity (NTU) | Average Treatment Efficiency (%) | ||||
|---|---|---|---|---|---|---|
| HA (mg/L) | Process | Feed Water | Treated Water | |||
| Range | Average | Range | Average | |||
| 20 | MF+PES+UV | 15.0–18.6 | 16.8 | 1.130–1.330 | 1.225 | 92.5 |
| MF+PES | 13.2–14.7 | 13.9 | 0.880–1.350 | 1.051 | 92.5 | |
| MF+UV | 8.1–16.0 | 13.5 | 1.130–1.270 | 1.180 | 90.7 | |
| MF | 11.3–13.9 | 13.0 | 1.120–1.580 | 1.255 | 90.2 | |
| Experimental Condition | UV254 Absorbance (cm−1) | Average Treatment Efficiency (%) | ||||
|---|---|---|---|---|---|---|
| HA (mg/L) | Process | Feed Water | Treated Water | |||
| Range | Average | Range | Average | |||
| 20 | MF+PES+UV | 0.023–0.045 | 0.034 | 0.001–0.010 | 0.007 | 79.7 |
| MF+PES | 0.023–0.045 | 0.033 | 0.017–0.027 | 0.023 | 32.8 | |
| MF+UV | 0.020–0.037 | 0.025 | 0.013–0.018 | 0.016 | 32.6 | |
| MF | 0.025–0.049 | 0.034 | 0.016–0.027 | 0.023 | 29.1 | |
| Fraction of Treatment Efficiency | MPs (Turbidity) | HA (UV254 Absorbance) |
|---|---|---|
| Membrane filtration (%) (MF) | 90.2 | 29.1 |
| Adsorption (%) (MF+PES)-(MF) | 2.3 | 3.7 |
| UV photooxidation (%) (MF+UV)-(MF) | 0.5 | 3.5 |
| Photooxidation with PES beads (%) (MF+PES+UV)-(MF+PES) | 0 | 46.9 |
| Total treatment efficiency (%) (MF+PES+UV) | 92.5 | 79.7 |
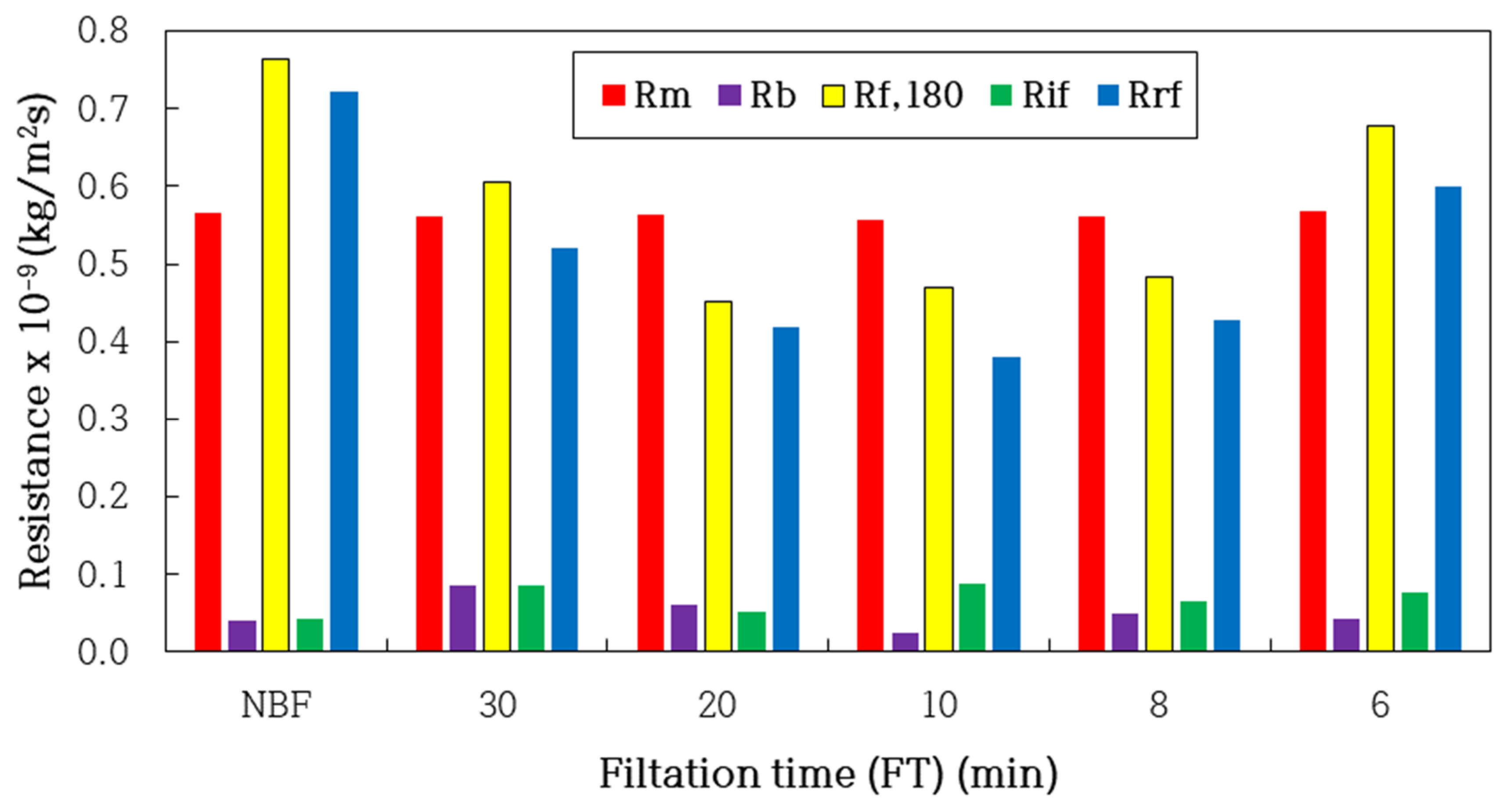
| FT (min) | NBF | 30 | 20 | 10 | 8 | 6 |
|---|---|---|---|---|---|---|
| Rm × 10−9 (kg/m2s) | 0.565 | 0.562 | 0.563 | 0.556 | 0.562 | 0.568 |
| Rb × 10−9 (kg/m2s) | 0.041 | 0.086 | 0.062 | 0.025 | 0.048 | 0.042 |
| Rf,180 × 10−9 (kg/m2s) | 0.764 | 0.606 | 0.451 | 0.470 | 0.483 | 0.677 |
| Rif × 10−9 (kg/m2s) | 0.043 | 0.086 | 0.052 | 0.089 | 0.065 | 0.077 |
| Rrf × 10−9 (kg/m2s) | 0.721 | 0.520 | 0.419 | 0.381 | 0.427 | 0.600 |
| J0 (L/m2h) | 1049 | 985 | 1018 | 1095 | 1046 | 1043 |
| J180 (L/m2h) | 467 | 594 | 650 | 647 | 649 | 570 |
| J180/J0 | 0.445 | 0.590 | 0.636 | 0.588 | 0.612 | 0.540 |
| Vt (L) | 9.96 | 10.88 | 11.37 | 11.51 | 11.23 | 10.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.; Park, J.Y. Removal of Microplastics in a Hybrid Treatment Process of Ceramic Microfiltration and Photocatalyst-Mounted PES Spheres with Air Backwashing. Membranes 2024, 14, 169. https://doi.org/10.3390/membranes14080169
Song M, Park JY. Removal of Microplastics in a Hybrid Treatment Process of Ceramic Microfiltration and Photocatalyst-Mounted PES Spheres with Air Backwashing. Membranes. 2024; 14(8):169. https://doi.org/10.3390/membranes14080169
Chicago/Turabian StyleSong, Minjoo, and Jin Yong Park. 2024. "Removal of Microplastics in a Hybrid Treatment Process of Ceramic Microfiltration and Photocatalyst-Mounted PES Spheres with Air Backwashing" Membranes 14, no. 8: 169. https://doi.org/10.3390/membranes14080169
APA StyleSong, M., & Park, J. Y. (2024). Removal of Microplastics in a Hybrid Treatment Process of Ceramic Microfiltration and Photocatalyst-Mounted PES Spheres with Air Backwashing. Membranes, 14(8), 169. https://doi.org/10.3390/membranes14080169







