Elucidating the Mechanism of Electro-Adsorption on Electrically Conductive Ultrafiltration Membranes via Modified Poisson-Boltzmann Equation
Abstract
1. Introduction
2. Material and Methods
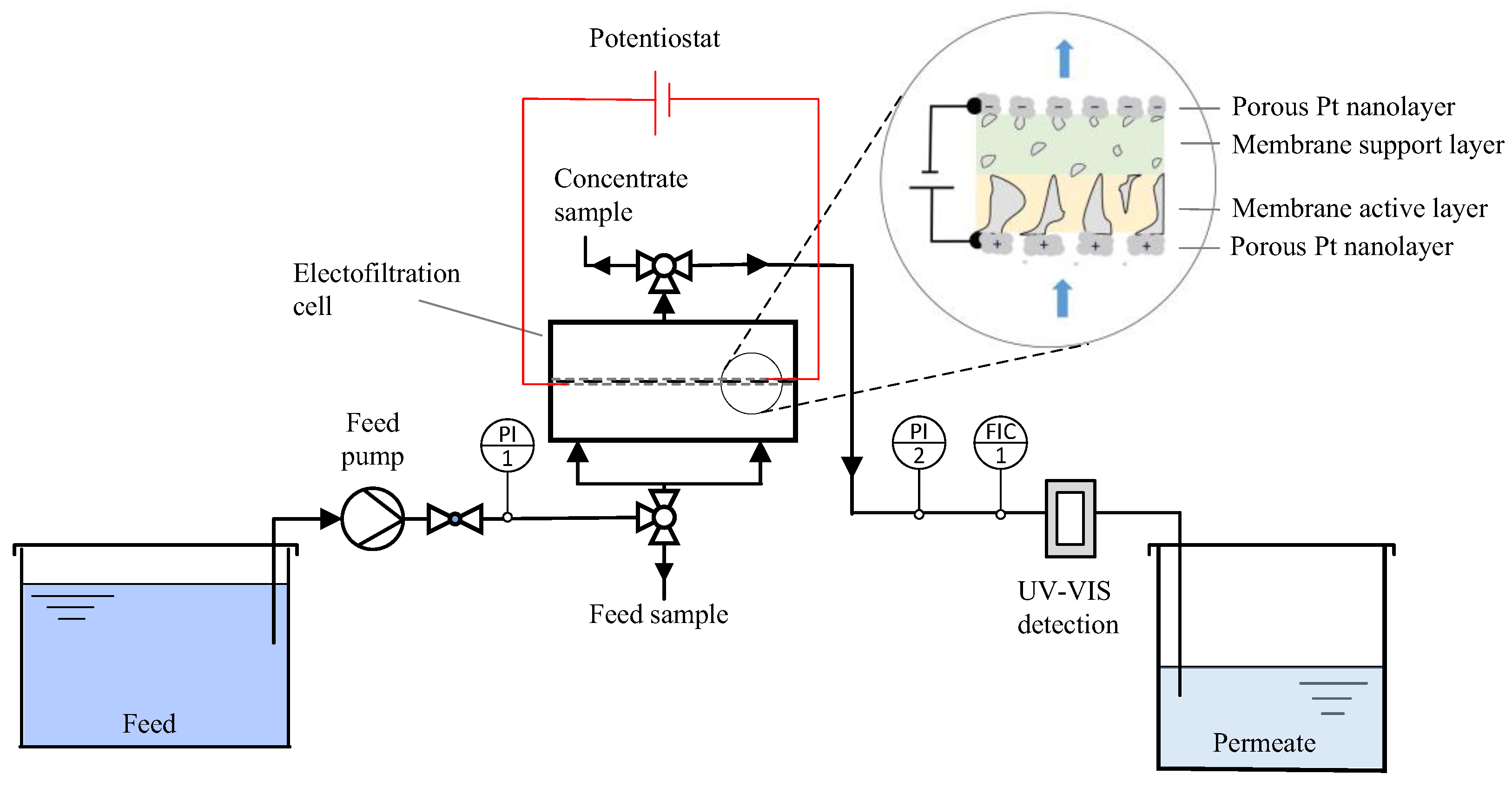
2.1. Electrically Conductive Ultrafiltration Membrane
2.2. Feed Solution
2.3. Dead-End Electrofiltration Experiments
| Tested Parameters | Cell Potential (V) | pH of Feed Solution | Ionic Strength (mmol·L−1) |
|---|---|---|---|
| Membrane intrinsic surface charge | 5, 6, 7, 8 | 1 | |
| External positive cell potential | +1.0, +1.5, +2.0, +2.5 | 5, 6, 7, 8 | 1 |
| External negative cell potential | −1.0, −1.5, −2.0, −2.5 | 5, 6, 7, 8 | 1 |
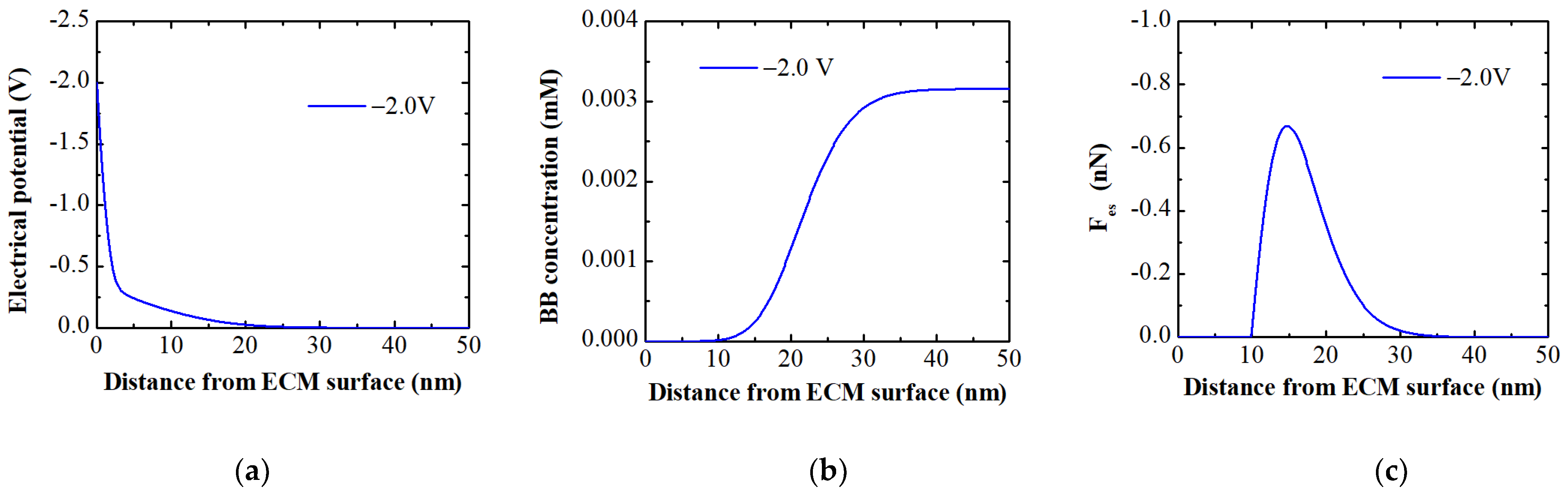
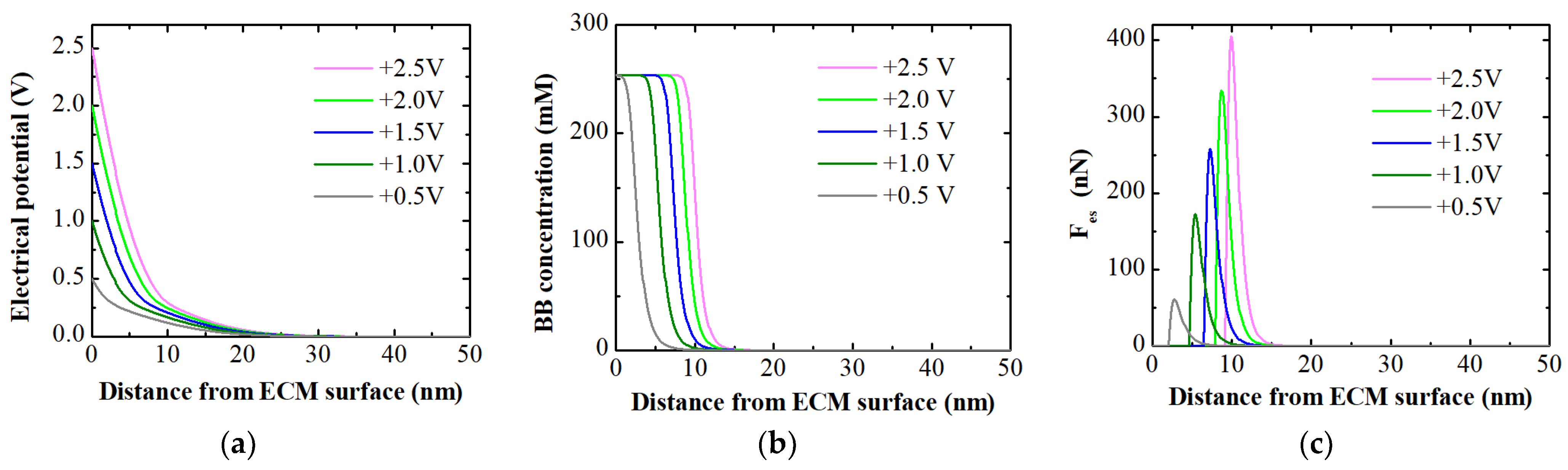
2.4. Modelling Ion Concentration and Electrostatic Force
3. Results and Discussion
3.1. Intrinsic Adsorption and Membrane Regeneration Using External Potential
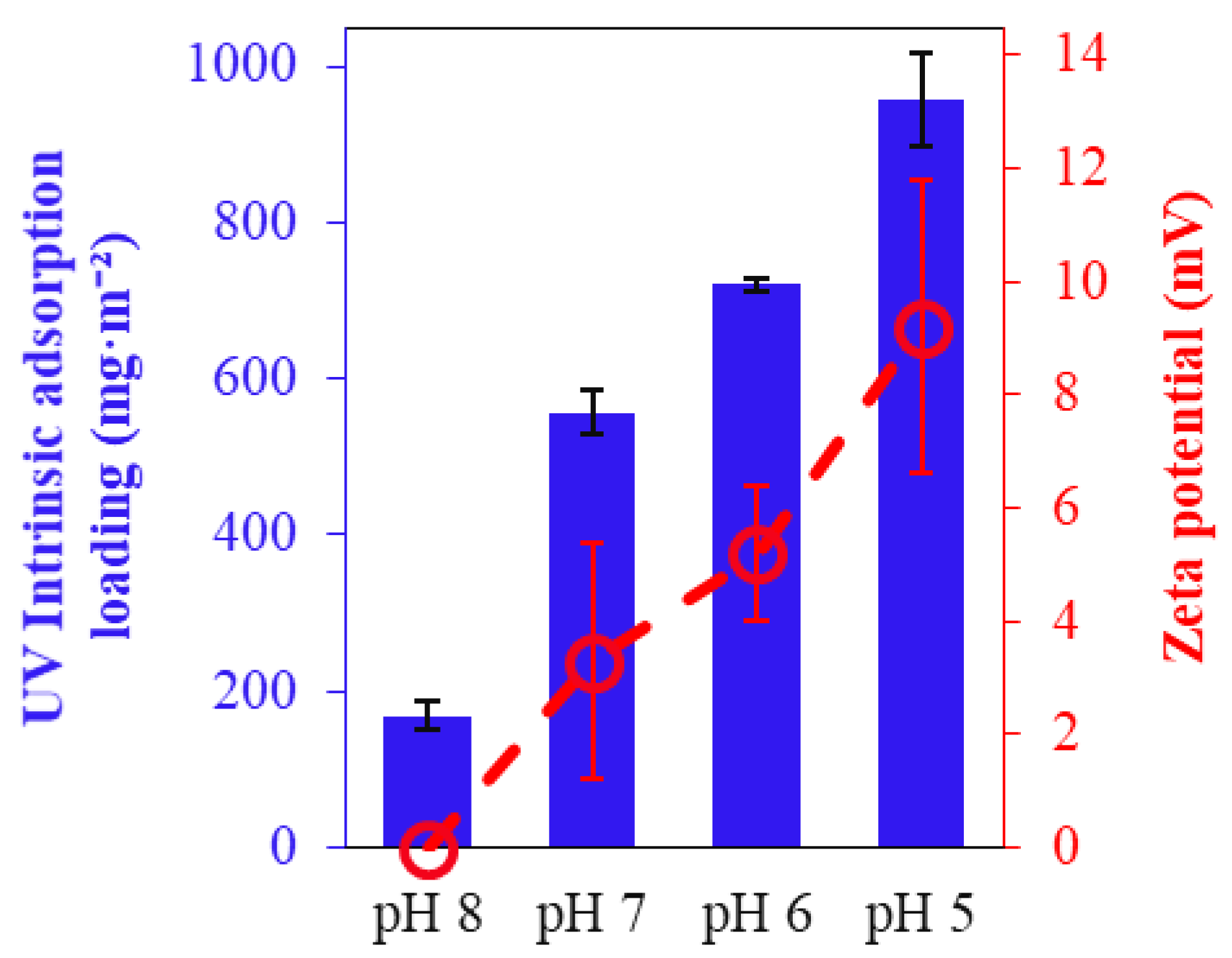
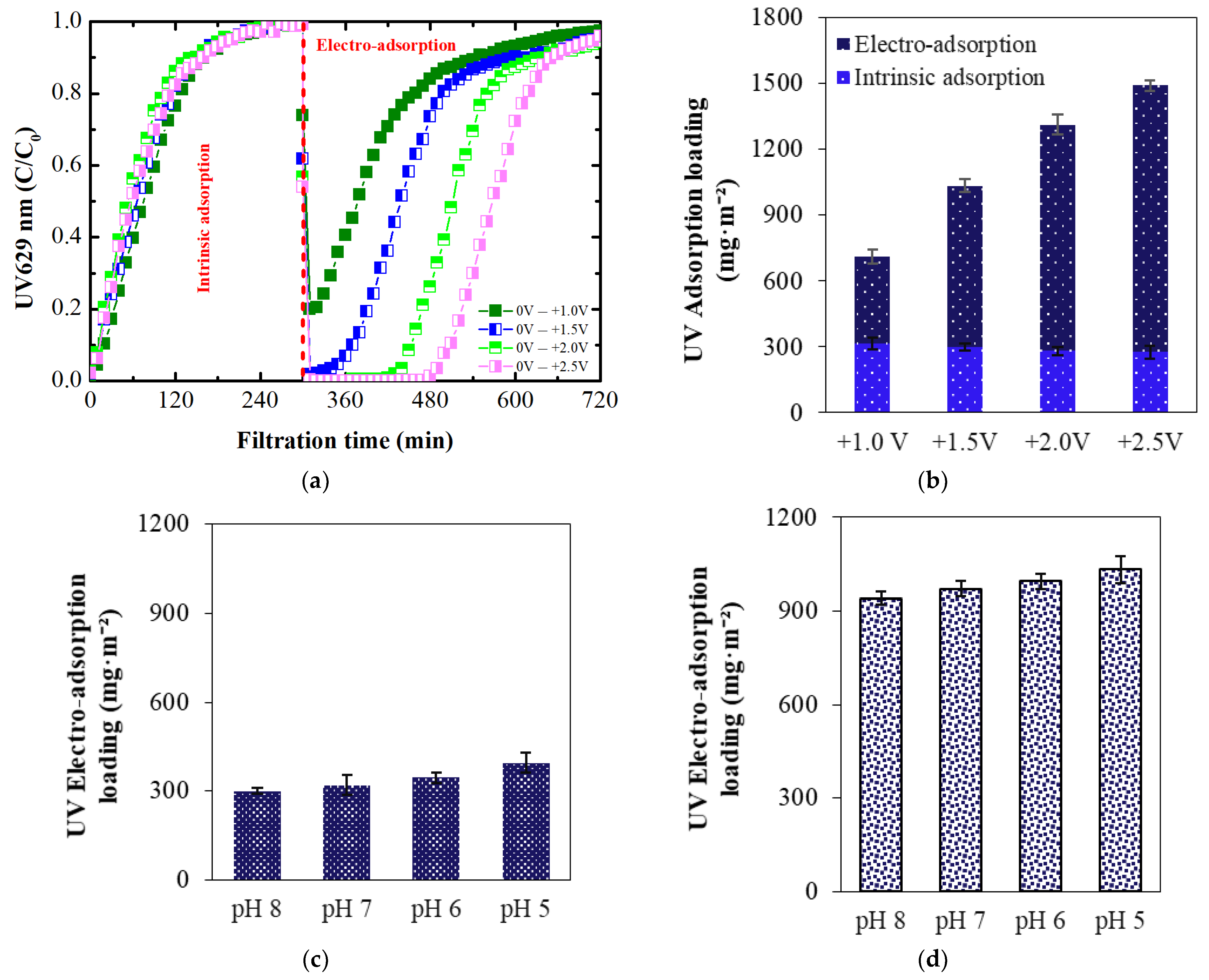
3.1.1. Dye Intrinsic Adsorption
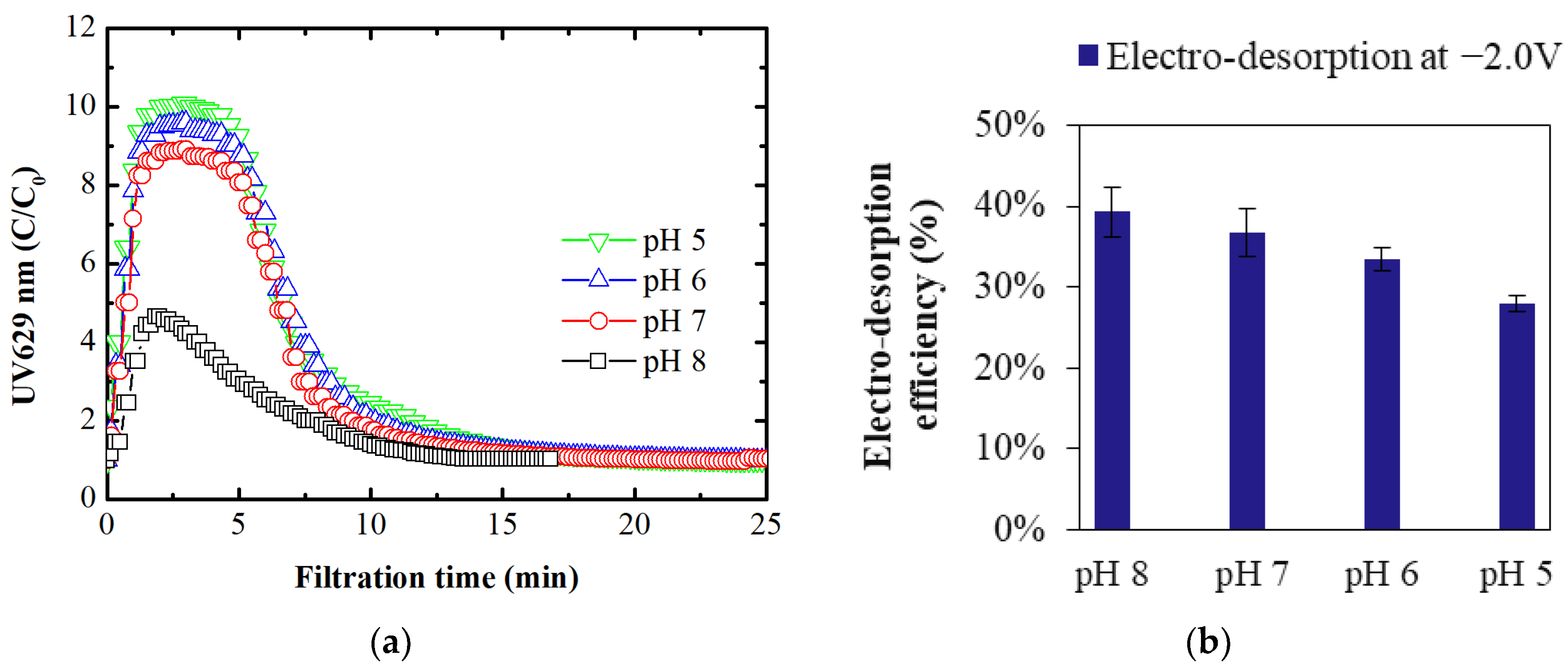
3.1.2. Membrane Regeneration Using External Potential
3.2. Electro-Adsorption at Varying Potential and pH
3.2.1. Electro-Adsorption at Varying Positive Potential
3.2.2. Electro-Adsorption at Varying pH
3.3. Membrane Regeneration Using Electro-Desorption at Varying Potential and pH
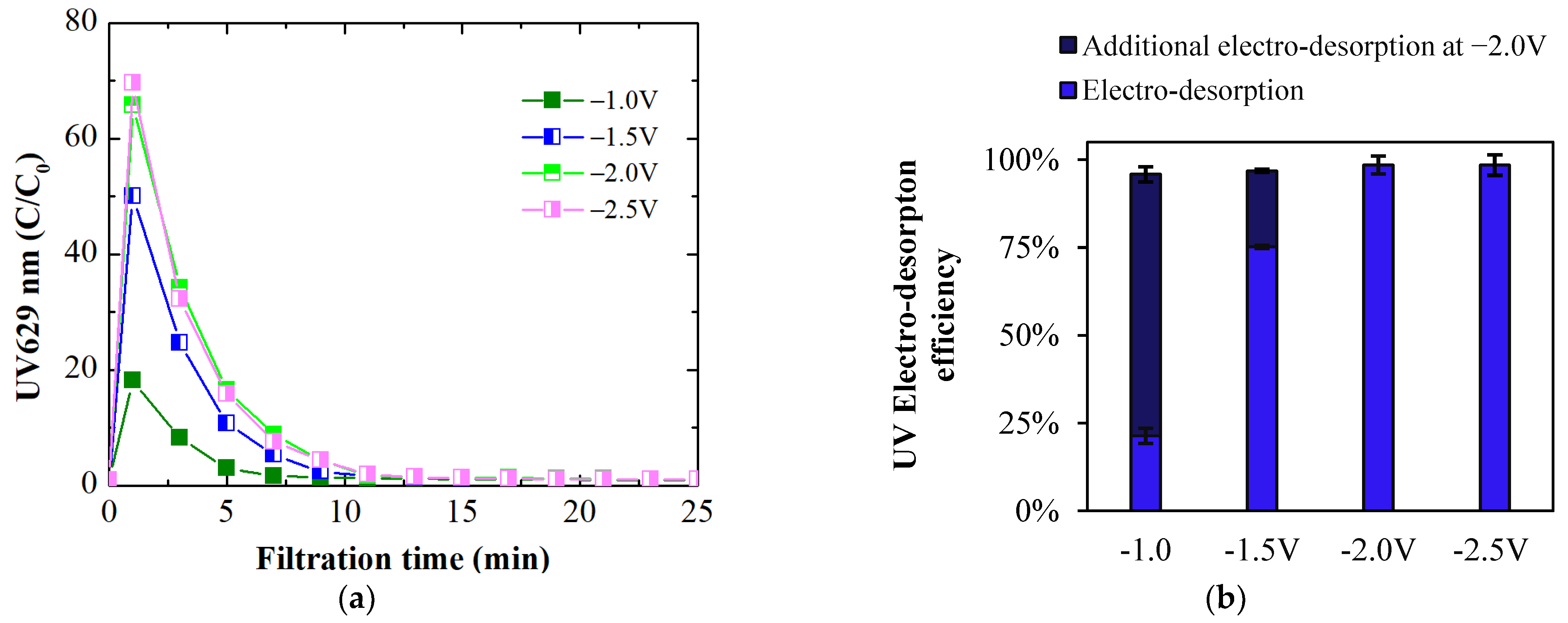
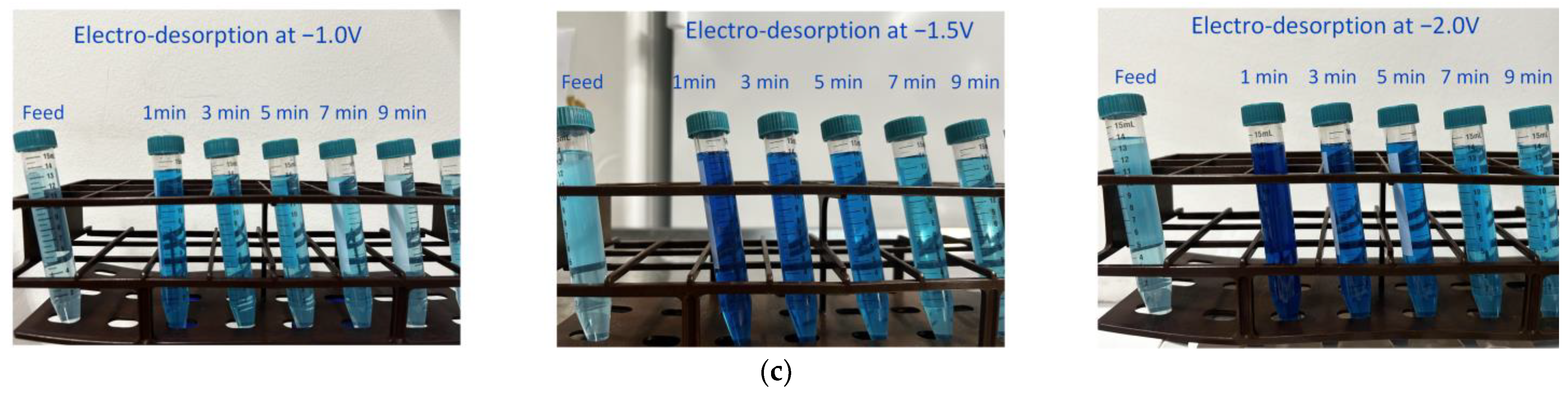
3.3.1. Membrane Regeneration Using Electro-Desorption at Varying Potential
3.3.2. Membrane Regeneration Using Electro-Desorption at Varying pH
4. Conclusions
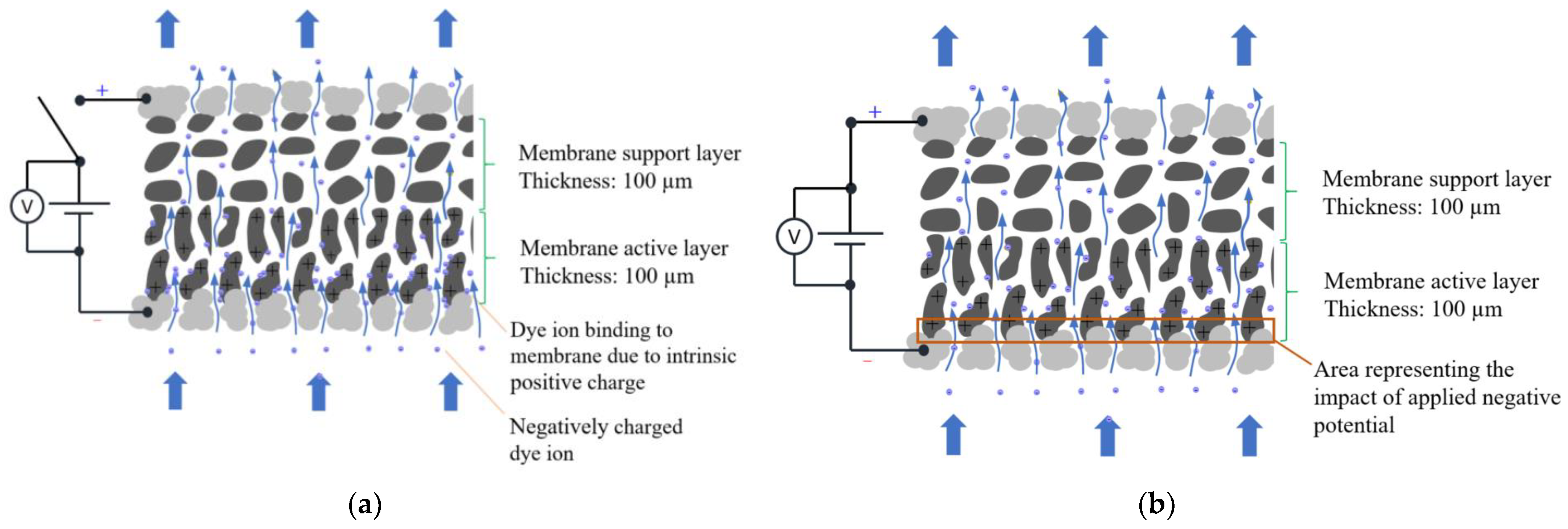
- When no electrical potential was applied to the ECM surface, adsorption was due to the membrane’s intrinsic positive charge from amine groups. This intrinsic adsorption increased with decreasing pH due to the enhanced protonation of the amine groups.
- Applying a negative potential of −2.0 V led to partial electro-desorption (up to 39%) of BB ions via electrostatic repulsion. The MPB model indicated that this repulsive force extends up to 24 nm from the membrane surface, enabling this partial regeneration. At a constant potential, electro-desorption efficiency decreased as pH fell below the pHIEP due to the membrane’s increased intrinsic positive charge, countering the negative potential’s repulsive force.
- Using the ECM as an anode and applying positive potentials resulted in electrostatic attraction, facilitating the electro-adsorption of aqueous counterions on the ECM surface. The extent of electro-adsorption increased with higher applied positive potentials. The MPB model provided a comprehensive explanation for the experimentally observed electro-adsorption results. The electrostatic attractive force predicted by the MPB model at different positive potentials qualitatively agreed with the determined electro-adsorption loadings.
- The adsorption of BB ions on the ECM involves distinct mechanisms depending on the conditions: intrinsic membrane charge facilitates binding within the structure, while external positive potential induces electro-adsorption via direct electrostatic attraction.
- Applying a negative potential to the ECM when it serves as the cathode in electrofiltration utilizes electrostatic repulsion to detach negatively charged organic ions from the ECM surface. The magnitude of this negative potential, requiring a potential of at least −2.0 V for complete desorption, plays a crucial role in ensuring the membrane’s effective regeneration and sustained performance. Optimal electro-desorption occurred at pH levels equal to pHIEP, where the membrane’s neutral charge maximized electrostatic repulsion, enhancing counterions detachment during membrane regeneration.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Castilla-Rodriguez, E.; Zhou, H. Organic Compounds Responsible for the Fouling of Ultrafiltration Membrane Treating Algae-Laden Water. Membranes 2023, 13, 787. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Yang, Z.; Gao, S.; Yu, H.; He, J.; Rong, H.; Tian, J. Impacts of Natural Organic Matter Adhesion on Irreversible Membrane Fouling during Surface Water Treatment Using Ultrafiltration. Membranes 2020, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Kammakakam, I.; Lai, Z. Next-generation ultrafiltration membranes: A review of material design, properties, recent progress, and challenges. Chemosphere 2023, 316, 137669. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Ma, Y.; Min, G.; Zhang, W.; Lv, L.; Zhang, W. A mini review of multifunctional ultrafiltration membranes for wastewater decontamination: Additional functions of adsorption and catalytic oxidation. Sci. Total Environ. 2021, 762, 143083. [Google Scholar] [CrossRef] [PubMed]
- Othman, N.H.; Alias, N.H.; Fuzil, N.S.; Marpani, F.; Shahruddin, M.Z.; Chew, C.M.; David Ng, K.M.; Lau, W.J.; Ismail, A.F. A review on the use of membrane technology systems in developing countries. Membranes 2021, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Bray, R.T.; Jankowska, K.; Kulbat, E.; Łuczkiewicz, A.; Sokołowska, A. Ultrafiltration Process in Disinfection and Advanced Treatment of Tertiary Treated Wastewater. Membranes 2021, 11, 221. [Google Scholar] [CrossRef]
- Mallya, D.S.; Abdikheibari, S.; Dumée, L.F.; Muthukumaran, S.; Lei, W.; Baskaran, K. Removal of natural organic matter from surface water sources by nanofiltration and surface engineering membranes for fouling mitigation—A review. Chemosphere 2023, 321, 138070. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Barbeau, B.; Bérubé, P. Nanofiltration and Tight Ultrafiltration Membranes for Natural Organic Matter Removal-Contribution of Fouling and Concentration Polarization to Filtration Resistance. Membranes 2017, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Avlonitis, S.A.; Poulios, I.; Sotiriou, D.; Pappas, M.; Moutesidis, K. Simulated cotton dye effluents treatment and reuse by nanofiltration. Desalination 2008, 221, 259–267. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Moutinho, J.; Shao, J.; Zydney, A.L.; He, Y. Recovery of small dye molecules from aqueous solutions using charged ultrafiltration membranes. J. Hazard. Mater. 2015, 284, 58–64. [Google Scholar] [CrossRef]
- Mantel, T.; Benne, P.; Parsin, S.; Ernst, M. Electro-Conductive Composite Gold-Polyethersulfone-Ultrafiltration-Membrane: Characterization of Membrane and Natural Organic Matter (NOM) Filtration Performance at Different In-Situ Applied Surface Potentials. Membranes 2018, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yang, F.; Chen, X.; Khor, C.M.; Jung, B.; Iddya, A.; Sant, G.; Jassby, D. Removal of As(III) by Electrically Conducting Ultrafiltration Membranes. Water Res. 2021, 204, 117592. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Jassby, D. Electroactive Membranes for Water Treatment: Enhanced Treatment Functionalities, Energy Considerations, and Future Challenges. Acc. Chem. Res. 2019, 52, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Mantel, T.; Jacki, E.; Ernst, M. Electrosorptive removal of organic water constituents by positively charged electrically conductive UF membranes. Water Res. 2021, 201, 117318. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Glass, S.; Mantel, T.; Filiz, V.; Ernst, M. Electro-sorption and -desorption characteristics of electrically conductive polyacrylonitrile membranes to remove aqueous natural organic matter in dead-end ultrafiltration system. J. Water Process Eng. 2024, 58, 104733. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, M.; Winter, L.R.; Lin, S.; Wang, Z.; Crittenden, J.C.; Ma, J. Emerging Challenges and Opportunities for Electrified Membranes to Enhance Water Treatment. Environ. Sci. Technol. 2022, 56, 3832–3835. [Google Scholar] [CrossRef] [PubMed]
- Anis, S.F.; Lalia, B.S.; Lesimple, A.; Hashaikeh, R.; Hilal, N. Electrically conductive membranes for contemporaneous dye rejection and degradation. Chem. Eng. J. 2022, 428, 131184. [Google Scholar] [CrossRef]
- Mantel, T.; Benne, P.; Ernst, M. Electrically conducting duplex-coated gold-PES-UF membrane for capacitive organic fouling mitigation and rejection enhancement. J. Membr. Sci. 2021, 620, 118831. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, G.; Li, Y.; Chen, X.; Yao, Y.; Ren, S.; Li, M.; Wu, Y.; An, C. Electrically conductive inorganic membranes: A review on principles, characteristics and applications. Chem. Eng. J. 2022, 427, 131987. [Google Scholar] [CrossRef]
- Dudchenko, A.V.; Rolf, J.; Russell, K.; Duan, W.; Jassby, D. Organic fouling inhibition on electrically conducting carbon nanotube–polyvinyl alcohol composite ultrafiltration membranes. J. Membr. Sci. 2014, 468, 1–10. [Google Scholar] [CrossRef]
- Ronen, A.; Duan, W.; Wheeldon, I.; Walker, S.; Jassby, D. Microbial Attachment Inhibition through Low-Voltage Electrochemical Reactions on Electrically Conducting Membranes. Environ. Sci. Technol. 2015, 49, 12741–12750. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Chen, G.; Chen, C.; Sanghvi, R.; Iddya, A.; Walker, S.; Liu, H.; Ronen, A.; Jassby, D. Electrochemical removal of hexavalent chromium using electrically conducting carbon nanotube/polymer composite ultrafiltration membranes. J. Membr. Sci. 2017, 531, 160–171. [Google Scholar] [CrossRef]
- Duan, W.; Ronen, A.; Walker, S.; Jassby, D. Polyaniline-Coated Carbon Nanotube Ultrafiltration Membranes: Enhanced Anodic Stability for In Situ Cleaning and Electro-Oxidation Processes. ACS Appl. Mater. Interfaces 2016, 8, 22574–22584. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Choi, Y.J.; Fang, Y.; Pham, H.S.; Nou, A.T.; Lee, L.S.; Niu, J.; Warsinger, D.M. Electric Field-Assisted Nanofiltration for PFOA Removal with Exceptional Flux, Selectivity, and Destruction. Environ. Sci. Technol. 2023, 57, 18519–18528. [Google Scholar] [CrossRef] [PubMed]
- Vecitis, C.D.; Gao, G.; Liu, H. Electrochemical Carbon Nanotube Filter for Adsorption, Desorption, and Oxidation of Aqueous Dyes and Anions. J. Phys. Chem. C 2011, 115, 3621–3629. [Google Scholar] [CrossRef]
- Tsai, Y.-T.; Weng, Y.-H.; Lin, A.Y.-C.; Li, K.-C. Electro-microfiltration treatment of water containing natural organic matter and inorganic particles. Desalination 2011, 267, 133–138. [Google Scholar] [CrossRef]
- Halali, M.A.; Lannoy, C.-F.D. Quantifying the Impact of Electrically Conductive Membrane-Generated Hydrogen Peroxide and Extreme pH on the Viability of Escherichia coli Biofilms. Ind. Eng. Chem. Res. 2022, 61, 660–671. [Google Scholar] [CrossRef]
- Jung, B.; Kim, C.Y.; Jiao, S.; Rao, U.; Dudchenko, A.V.; Tester, J.; Jassby, D. Enhancing boron rejection on electrically conducting reverse osmosis membranes through local electrochemical pH modification. Desalination 2020, 476, 114212. [Google Scholar] [CrossRef]
- Sun, M.; Wang, X.; Winter, L.R.; Zhao, Y.; Ma, W.; Hedtke, T.; Kim, J.-H.; Elimelech, M. Electrified Membranes for Water Treatment Applications. ACS EST Eng. 2021, 1, 725–752. [Google Scholar] [CrossRef]
- Kishore Chand, A.A.; Bajer, B.; Schneider, E.S.; Mantel, T.; Ernst, M.; Filiz, V.; Glass, S. Modification of polyacrylonitrile ultrafiltration membranes to enhance the adsorption of cations and anions. Membranes 2022, 12, 580. [Google Scholar] [CrossRef]
- Glass, S.; Mantel, T.; Appold, M.; Sen, S.; Usman, M.; Ernst, M.; Filiz, V. Amine-terminated PAN membranes as anion-adsorber materials. Chem. Ing. Tech. 2021, 93, 1396–1400. [Google Scholar] [CrossRef]
- Jing, Y.; Guo, L.; Chaplin, B.P. Electrochemical impedance spectroscopy study of membrane fouling and electrochemical regeneration at a sub-stoichiometric TiO2 reactive electrochemical membrane. J. Membr. Sci. 2016, 510, 510–523. [Google Scholar] [CrossRef]
- Sun, L.; Park, S.S.; Sheberla, D.; Dincă, M. Measuring and reporting electrical conductivity in metal organic frameworks: Cd2(TTFTB) as a case study. J. Am. Chem. Soc. 2016, 138, 14772–14782. [Google Scholar] [CrossRef]
- Zarzycki, P.K.; Lewandowska, L.; Fenert, B.; Piaskowski, K.; Kobaka, J. Investigation of hybrid methods for elimination of brilliant blue dye from water phase using various nanomaterials combined with activated sludge and duckweed. Nanomaterials 2021, 11, 1747. [Google Scholar] [CrossRef]
- Chebotarev, A.N.; Bevziuk, K.V.; Snigur, D.V.; Bazel, Y.R. The brilliant blue FCF ion-molecular forms in solutions according to the spectrophotometry data. Russ. J. Phys. Chem. 2017, 91, 1907–1912. [Google Scholar] [CrossRef]
- Daubinger, P.; Kieninger, J.; Unmüssig, T.; Urban, G.A. Electrochemical characteristics of nanostructured platinum electrodes--a cyclic voltammetry study. Phys. Chem. Chem. Phys. 2014, 16, 8392–8399. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, Y.; Liu, R.; Wu, H.; Wang, G.; Cao, D. Poisoning of acetone to Pt and Au electrodes for electrooxidation of 2-propanol in alkaline medium. Electrochim. Acta 2012, 76, 174–178. [Google Scholar] [CrossRef]
- Kilic, M.S.; Bazant, M.Z.; Ajdari, A. Steric effects in the dynamics of electrolytes at large applied voltages. I. Double-layer charging. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2007, 75, 21502. [Google Scholar] [CrossRef] [PubMed]
- Kilic, M.S.; Bazant, M.Z.; Ajdari, A. Steric effects in the dynamics of electrolytes at large applied voltages. II. Modified Poisson-Nernst-Planck equations. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2007, 75, 21503. [Google Scholar] [CrossRef]
- Solomon, H. Random Packing Density; University of California Press: Berkeley, CA, USA, 1967; Volume 3, pp. 119–134. [Google Scholar]
- Hu, X.; Wang, J.; Liu, Y.; Li, X.; Zeng, G.; Bao, Z.; Zeng, X.; Chen, A.; Long, F. Adsorption of chromium (VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: Isotherms, kinetics and thermodynamics. J. Hazard. Mater. 2011, 185, 306–314. [Google Scholar] [CrossRef]
- Nascimento, V.X.; Pinto, D.; Lütke, S.F.; Da Silva, M.C.F.; Machado, F.M.; Lima, É.C.; Silva, L.F.O.; Dotto, G.L. Brilliant blue FCF dye adsorption using magnetic activated carbon from Sapelli wood sawdust. Environ. Sci. Pollut. Res. Int. 2023, 30, 58684–58696. [Google Scholar] [CrossRef] [PubMed]





| Universal Constants | |||
| (C) | 1.6 × 10−19 | ||
| (mol−1) | 6.022 × 1023 | ||
| (F/m) | 8.85 × 10−12 | ||
| (J/K) | 1.38 × 10−23 | ||
| Ions Parameters | |||
| BB− | Na+ | ||
| (nm) | 1.0 | (nm) | 0.45 |
| −1 | +1 | ||
| (mmol/l) | 0.0032 | (mmol/l) | 0.0032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usman, M.; Vahedi, S.; Glass, S.; Filiz, V.; Ernst, M. Elucidating the Mechanism of Electro-Adsorption on Electrically Conductive Ultrafiltration Membranes via Modified Poisson-Boltzmann Equation. Membranes 2024, 14, 175. https://doi.org/10.3390/membranes14080175
Usman M, Vahedi S, Glass S, Filiz V, Ernst M. Elucidating the Mechanism of Electro-Adsorption on Electrically Conductive Ultrafiltration Membranes via Modified Poisson-Boltzmann Equation. Membranes. 2024; 14(8):175. https://doi.org/10.3390/membranes14080175
Chicago/Turabian StyleUsman, Muhammad, Shahrokh Vahedi, Sarah Glass, Volkan Filiz, and Mathias Ernst. 2024. "Elucidating the Mechanism of Electro-Adsorption on Electrically Conductive Ultrafiltration Membranes via Modified Poisson-Boltzmann Equation" Membranes 14, no. 8: 175. https://doi.org/10.3390/membranes14080175
APA StyleUsman, M., Vahedi, S., Glass, S., Filiz, V., & Ernst, M. (2024). Elucidating the Mechanism of Electro-Adsorption on Electrically Conductive Ultrafiltration Membranes via Modified Poisson-Boltzmann Equation. Membranes, 14(8), 175. https://doi.org/10.3390/membranes14080175










