Surfactant-Enhanced Cleaning Solutions for Ceramic Membranes: A Comparative Study on Humic Acid and BSA Fouling
Abstract
1. Introduction
2. Materials and Methods
3. Results
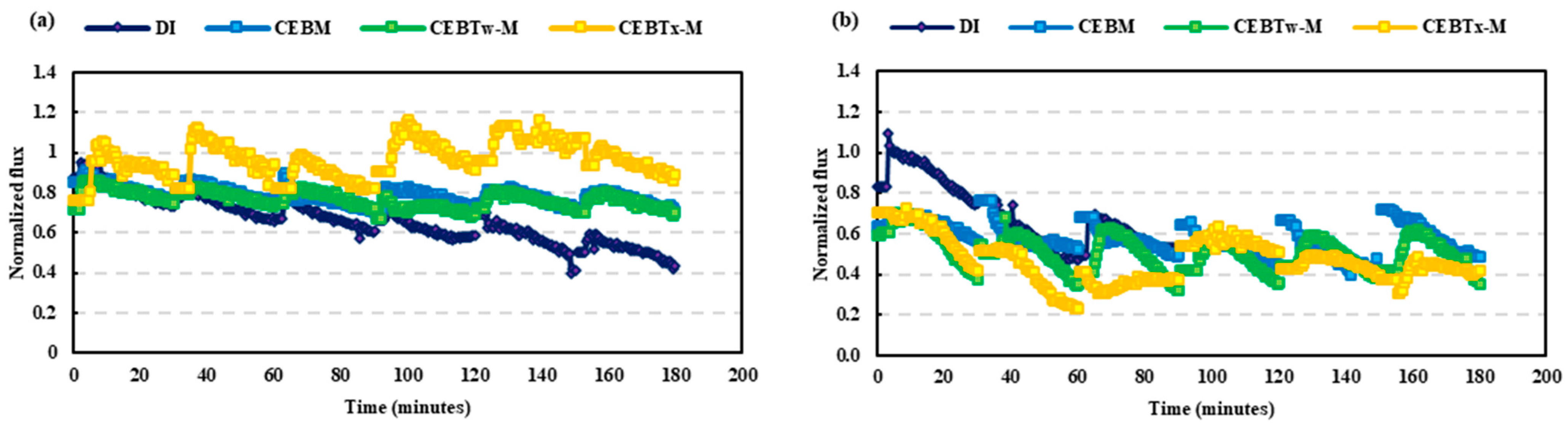
3.1. HA and BSA Fouling on Ceramic UF Membrane
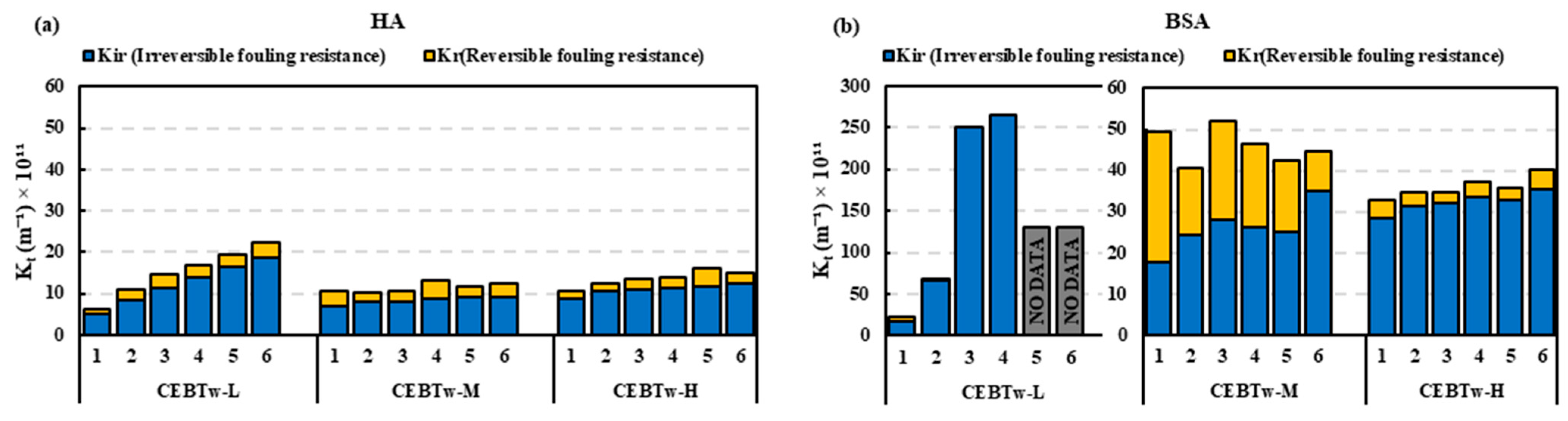
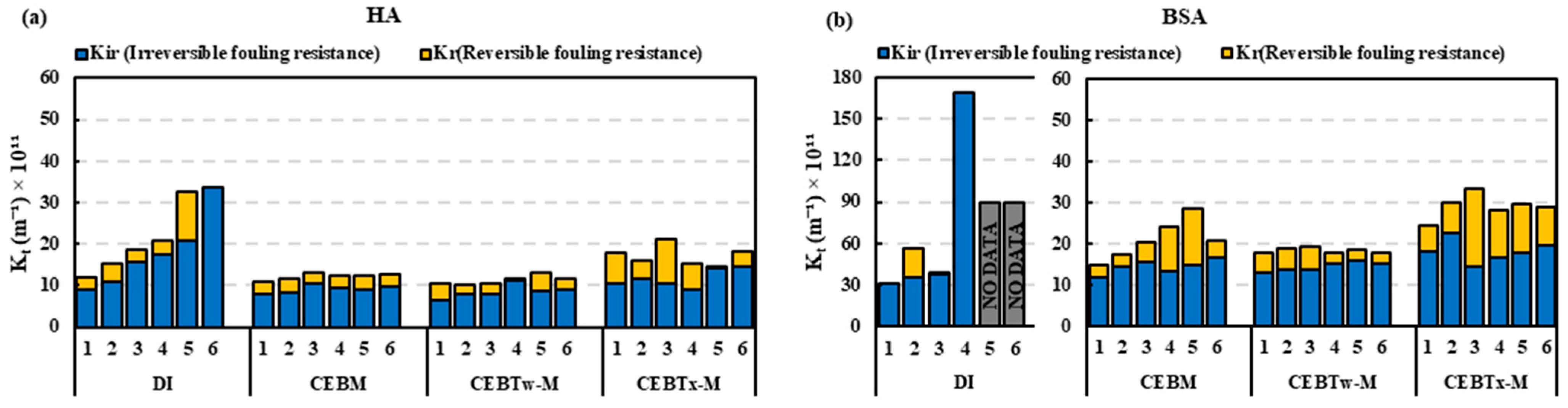
3.2. Removal Efficiency of Surfactant-Enhanced Cleaning Solutions on Ceramic UF Membrane
3.3. Evaluation of Cleaning Efficiency Across Solutions with Equivalent Concentrations
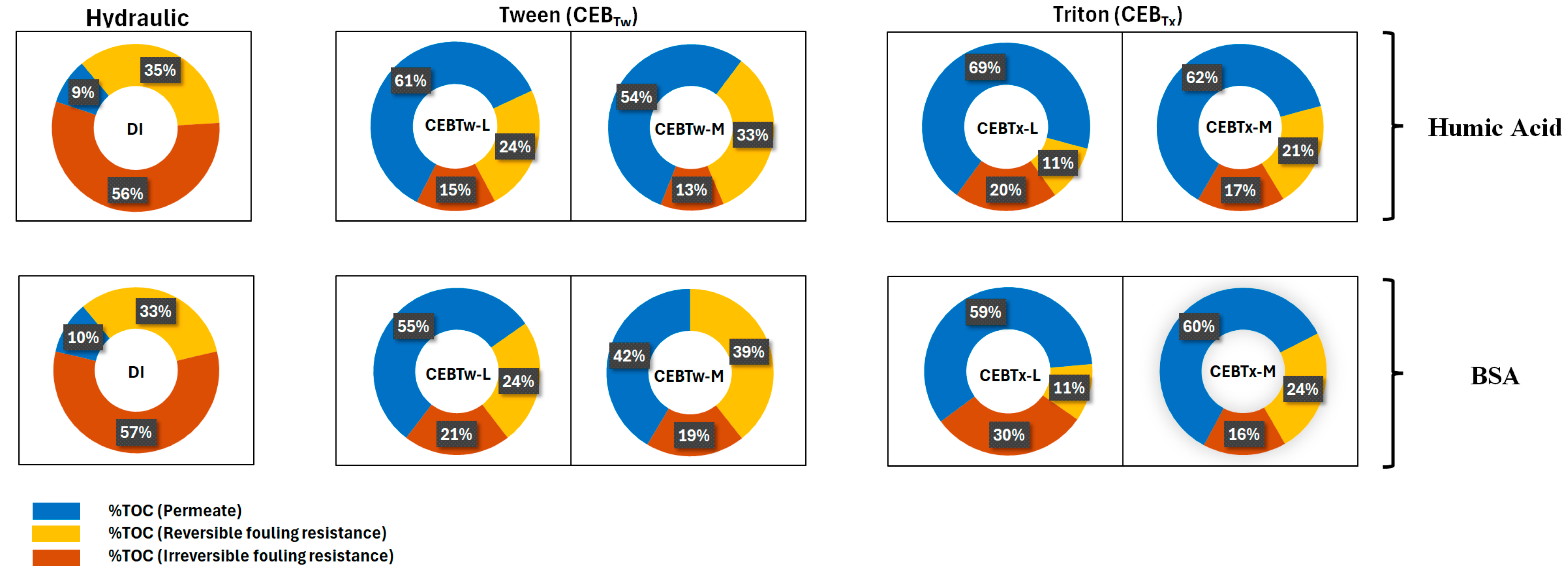
3.4. Reversible and Irreversible Carbon Mass Balance in Wash Waters
4. Conclusions
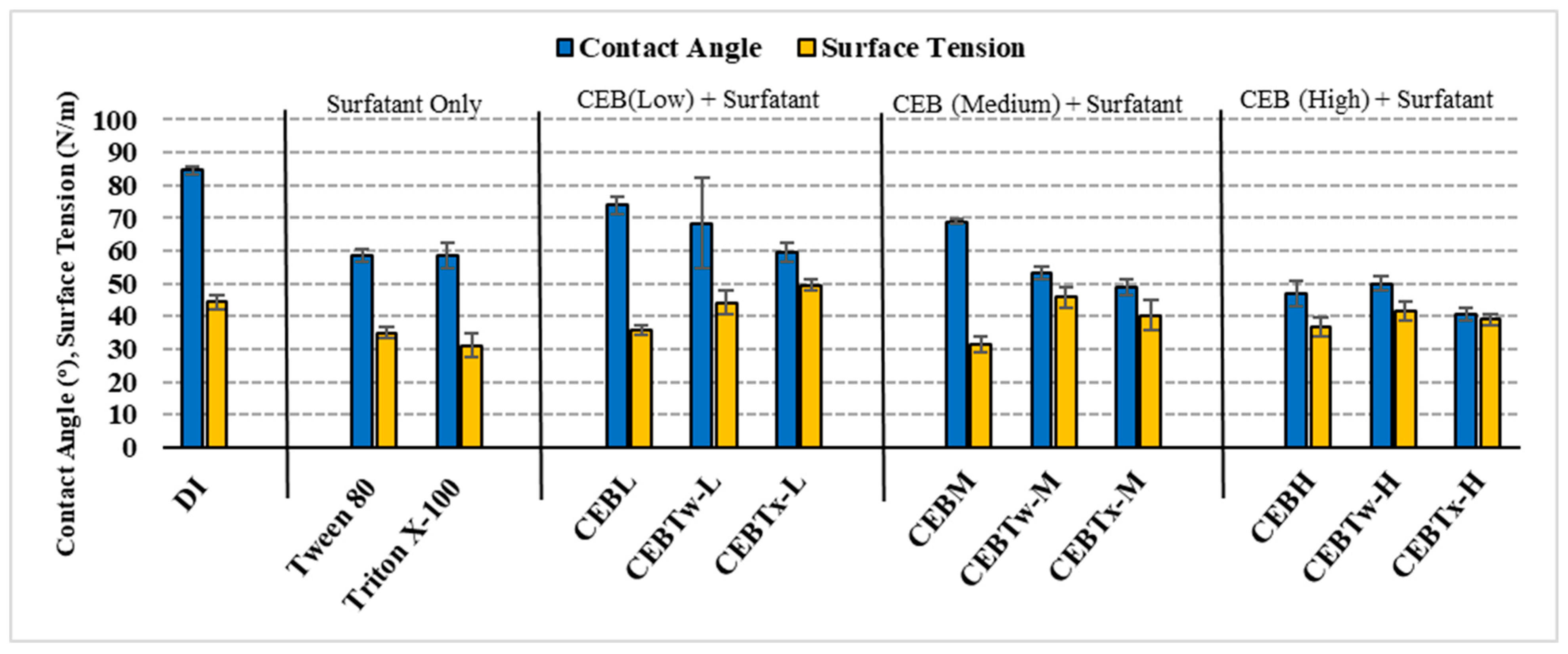
- The lower contact angles of surfactant-enhanced solutions, measuring 53 ± 2° for CEBTw-M and 48 ± 2° for CEBTx-M, compared to 69 ± 2° for conventional cleaning solution (CEBM) and 84 ± 1° for DI water, indicate improved wettability and surface hydrophilicity. Hence, enhancing the cleaning potential by allowing for better penetration and interaction with foulants, resulting in improved fouling control and membrane performance than conventional cleaning solutions and deionized (DI) water.
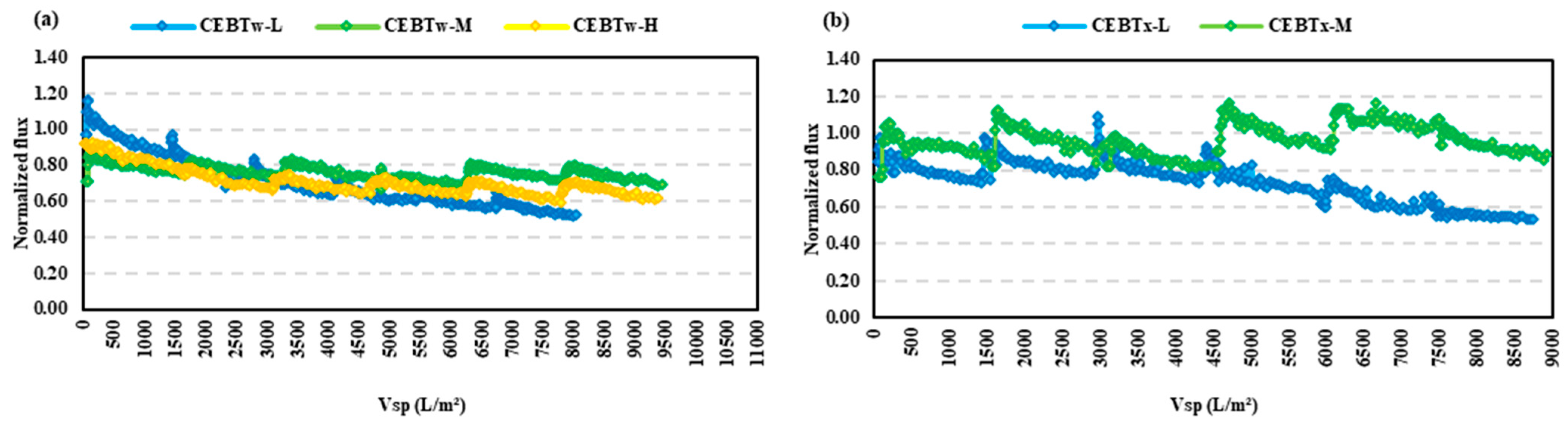
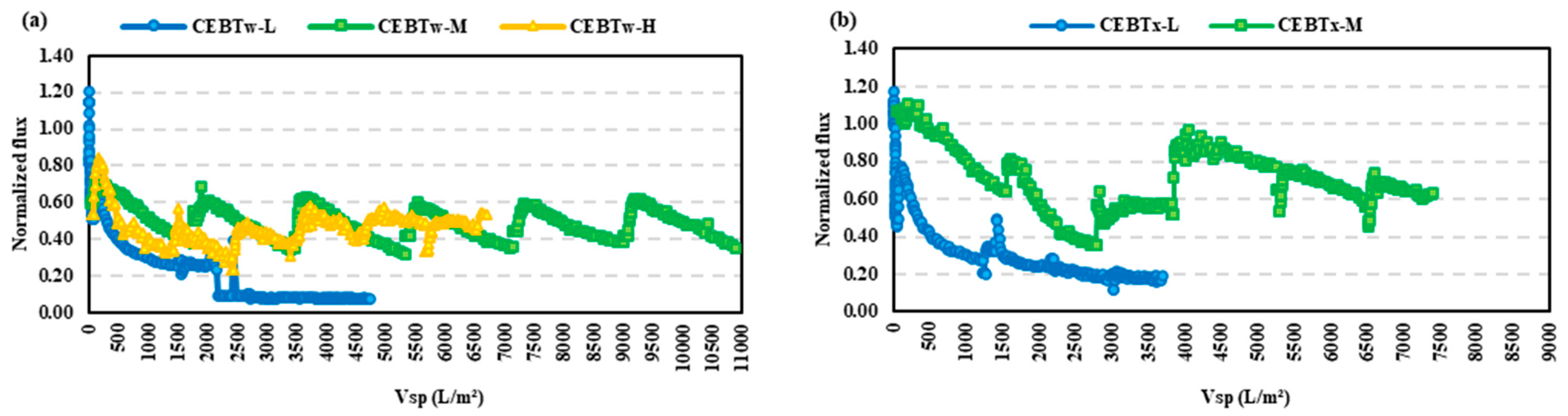
- The resistance in the series analysis demonstrated that fouling control was most effective in the order of CEBTw-M > CEBM > CEBTx-M, indicating that CEBTw-M provided the highest reduction in overall fouling.
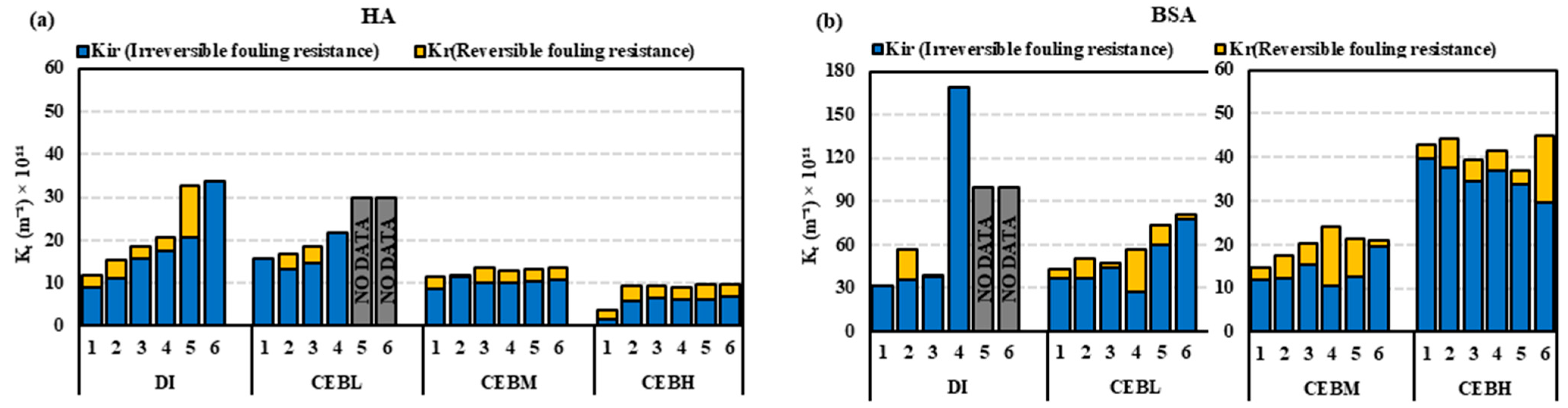
- Overall, the irreversibility was lowest for the medium surfactant-enhanced concentrations, where the irreversible fouling retained on membrane with Tween 80-enhanced CEB was 15–21% and Triton X-100-enhanced CEB was 16–30% for HA and BSA as determined by TOC analysis.
- Among all tested cleaning solutions, CEBTw-M exhibited the highest efficacy and reliability in fouling mitigation for both HA and BSA, establishing it as the optimal choice for efficient membrane cleaning in long-term filtration applications; while CEB with surfactants performed better than a hydraulic backwash alone, HA was overall easier to control than BSA.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alresheedi, M.T.; Barbeau, B.; Basu, O.D. Comparisons of NOM fouling and cleaning of ceramic and polymeric membranes during water treatment. Sep. Purif. Technol. 2019, 209, 452–460. [Google Scholar] [CrossRef]
- Wang, R.; Liang, D.; Liu, X.; Fan, W.; Meng, S.; Cai, W. Effect of magnesium ion on polysaccharide fouling. Chem. Eng. J. 2020, 379, 122351. [Google Scholar] [CrossRef]
- Guerra, K.; Pellegrino, J.; Drewes, J.E. Impact of operating conditions on permeate flux and process economics for cross flow ceramic membrane ultrafiltration of surface water. Sep. Purif. Technol. 2012, 87, 47–53. [Google Scholar] [CrossRef]
- Alresheedi, M.T.; Basu, O.D.; Barbeau, B. Chemical cleaning of ceramic ultrafiltration membranes—Ozone versus conventional cleaning chemicals. Chemosphere 2019, 226, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Merlet, R.B.; Pizzoccaro-Zilamy, M.-A.; Nijmeijer, A.; Winnubst, L. Hybrid ceramic membranes for organic solvent nanofiltration: State-of-the-art and challenges. J. Membr. Sci. 2020, 599, 117839. [Google Scholar] [CrossRef]
- Gruskevica, K.; Mezule, L. Cleaning Methods for Ceramic Ultrafiltration Membranes Affected by Organic Fouling. Membranes 2021, 11, 131. [Google Scholar] [CrossRef]
- Gul, A.; Hruza, J.; Dvorak, L.; Yalcinkaya, F. Chemical Cleaning Process of Polymeric Nanofibrous Membranes. Polymers 2022, 14, 1102. [Google Scholar] [CrossRef] [PubMed]
- Regula, C.; Carretier, E.; Wyart, Y.; Gésan-Guiziou, G.; Vincent, A.; Boudot, D.; Moulin, P. Chemical cleaning/disinfection and ageing of organic UF membranes: A review. Water Res. 2014, 56, 325–365. [Google Scholar] [CrossRef]
- Aguiar, A.; Andrade, L.; Grossi, L.; Pires, W.; Amaral, M. Acid mine drainage treatment by nanofiltration: A study of membrane fouling, chemical cleaning, and membrane ageing. Sep. Purif. Technol. 2018, 192, 185–195. [Google Scholar] [CrossRef]
- Ullah, A.; Tanudjaja, H.J.; Ouda, M.; Hasan, S.W.; Chew, J.W. Membrane fouling mitigation techniques for oily wastewater: A short review. J. Water Process Eng. 2021, 43, 102293. [Google Scholar] [CrossRef]
- Wang, Q.; Zeng, H.; Wu, Z.; Cao, J. Impact of sodium hypochlorite cleaning on the surface properties and performance of PVDF membranes. Appl. Surf. Sci. 2018, 428, 289–295. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, F.; He, X.; Zhou, Z.; Huang, L.-N.; Liang, S. Optimisation and performance of NaClO-assisted maintenance cleaning for fouling control in membrane bioreactors. Water Res. 2014, 53, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, A.; Saghravani, S.F.; Ghasemipanah, K.; Dahrazma, B.; Rasekh, B. Evaluation of the performance of air micro-nano bubbles for cleaning in place to reduce the reverse osmosis membrane clogging. Desalination Water Treat. 2024, 320, 100599. [Google Scholar] [CrossRef]
- Khan, I.A.; Lee, K.H.; Kim, J.Y.; Lee, J.W.; Kim, J.-O. Effect of preoxidation on fouling mitigation in a low-pressure membrane system with clean-in-place (CIP) treatment. J. Water Process Eng. 2024, 57, 104634. [Google Scholar] [CrossRef]
- Gao, T.; Wang, D.; Xia, L.; Zhao, S.; Xu, R.; Zhang, H.; Meng, F.; Zhou, Z.; Chen, J.; Liu, W. Unveiling the residual membrane foulants in full-scale MBR plant after chemically enhanced backwash: Insights into microbe-associated compounds. Desalination 2023, 551, 116421. [Google Scholar] [CrossRef]
- Levitsky, I.; Duek, A.; Naim, R.; Arkhangelsky, E.; Gitis, V. Cleaning UF membranes with simple and formulated solutions. Chem. Eng. Sci. 2012, 69, 679–683. [Google Scholar] [CrossRef]
- Qiao, Z.; Guo, Y.; Wang, Z.; Hu, G. A chemically enhanced backwash model for predicting the instantaneous transmembrane pressure of flat sheet membranes in constant flow rate mode. J. Membr. Sci. 2023, 666, 121137. [Google Scholar] [CrossRef]
- Park, S.; Kang, J.-S.; Lee, J.J.; Vo, T.-K.-Q.; Kim, H.-S. Application of Physical and Chemical Enhanced Backwashing to Reduce Membrane Fouling in the Water Treatment Process Using Ceramic Membranes. Membranes 2018, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Zeng, G.; Huang, D.; Yang, C.; Lai, C.; Zhang, C.; Liu, Y. Advantages and challenges of Tween 80 surfactant-enhanced technologies for the remediation of soils contaminated with hydrophobic organic compounds. Chem. Eng. J. 2017, 314, 98–113. [Google Scholar] [CrossRef]
- Palanisamy, T.; Tabatabai, S.A.A.; Zhang, T.; Leiknes, T. Role of surfactants in cleaning of PVDF ultrafiltration membranes fouled by emulsified cutting oil. J. Water Process Eng. 2021, 40, 101923. [Google Scholar] [CrossRef]
- Bolandi, S.; Ashtiani, F.Z.; Okhovat, A.; Ghandashtani, M.B. The Effects of Surfactant Additives on Properties and Morphologies of Cellulose Acetate Membranes Prepared by Phase Inversion. Theor. Found. Chem. Eng. 2020, 54, 931–939. [Google Scholar] [CrossRef]
- Aguirre-Ramírez, M.; Silva-Jiménez, H.; Banat, I.M.; Díaz De Rienzo, M.A. Surfactants: Physicochemical interactions with biological macromolecules. Biotechnol. Lett. 2021, 43, 523–535. [Google Scholar] [CrossRef]
- Alves, L.; Magalhães, S.; Esteves, C.; Sebastião, M.; Antunes, F. Synergisms between Surfactants, Polymers, and Alcohols to Improve the Foamability of Mixed Systems. J 2024, 7, 169–182. [Google Scholar] [CrossRef]
- Trentin, A.; Güell, C.; Gelaw, T.; de Lamo, S.; Ferrando, M. Cleaning protocols for organic microfiltration membranes used in premix membrane emulsification. Sep. Purif. Technol. 2012, 88, 70–78. [Google Scholar] [CrossRef]
- Nagasawa, H.; Omura, T.; Asai, T.; Kanezashi, M.; Tsuru, T. Filtration of surfactant-stabilized oil-in-water emulsions with porous ceramic membranes: Effects of membrane pore size and surface charge on fouling behavior. J. Membr. Sci. 2020, 610, 118210. [Google Scholar] [CrossRef]
- Lv, Z.; Zhang, S.; Jiao, W.; Zuo, X.; Zhang, Y.; Liu, Y. High-efficiency cleaning technology and lifespan prediction for the ceramic membrane treating secondary treated effluent. Water Sci. Technol. 2023, 88, 321–338. [Google Scholar] [CrossRef]
- Sun, W.; Liu, W.; Dai, S.; Yang, T.; Duan, H.; Liu, W. Effect of Tween 80 on flotation separation of magnesite and dolomite using NaOL as the collector. J. Mol. Liq. 2020, 315, 113712. [Google Scholar] [CrossRef]
- Johnson, M. Détergents: Triton X-100, Tween-20, et autres (G. Colette, Trans.). Mater. Methods 2013, 3, 163. [Google Scholar] [CrossRef]
- Al-Koofee, D. Effect of Temperature Changes on Critical Micelle Concentration for Tween Series Surfactant. Glob. J. Sci. Front. Res. Chem. 2013. Available online: https://www.researchgate.net/publication/237841643_Effect_of_Temperature_Changes_on_Critical_Micelle_Concentration_for_Tween_Series_Surfactant (accessed on 27 March 2023).
- Karimi, M.A.; Mozaheb, M.A.; Hatefi-Mehrjardi, A.; Tavallali, H.; Attaran, A.M.; Shamsi, R. A new simple method for determining the critical micelle concentration of surfactants using surface plasmon resonance of silver nanoparticles. J. Anal. Sci. Technol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, K.; Khan, B.; Du, X.; Hou, L.; Zhao, S.; Li, P.; Liu, S.; Song, P.; Zhang, H.; et al. Experimental Study of Fouling Behavior of Main Substances (BSA, HA, SA) of Dissolved Organic Matter (DOM) in Dead-end Membrane Filtration. IOP Conf. Ser. Mater. Sci. Eng. 2018, 301, 012031. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Xu, M.; Qi, F.; Li, C.; Sun, D.; Nan, J.; Li, W.; Guan, X.; Ikhlaq, A. Unveiling the role of cake layer in coagulation-ultrafiltration on membrane fouling and emerging application as dynamic membrane before ultrafiltration. Sep. Purif. Technol. 2024, 350, 127932. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, Z. A three-mechanism (intermediate pore blocking, standard pore blocking and cake filtration) model considering correction of effective filtration area. J. Environ. Chem. Eng. 2024, 12, 113654. [Google Scholar] [CrossRef]
- Kim, H.; Shim, I.; Zhan, M. Chemical Enhanced Backwashing for Controlling Organic Fouling in Drinking Water Treatment Using a Novel Hollow-Fiber Polyacrylonitrile Nanofiltration Membrane. Appl. Sci. 2021, 11, 6764. [Google Scholar] [CrossRef]
- Marais, S.S.; Ncube, E.J.; Msagati, T.A.M.; Mamba, B.B.; Nkambule, T.T.I. Comparison of natural organic matter removal by ultrafiltration, granular activated carbon filtration and full scale conventional water treatment. J. Environ. Chem. Eng. 2018, 6, 6282–6289. [Google Scholar] [CrossRef]


| Categories | Contents |
|---|---|
| Membrane Type | UF ceramic membrane |
| Material | Ceramic |
| Support Material | Al2O3 |
| Surface Material | ZrO2 |
| Area | 95 cm2 |
| Nominal pore size | 0.01 µm |
| Length and Internal diameter | 50 cm and 0.6 cm |
| MWCO | 25 kDa |
| CEB Solution * | Concentration | pH | Abbr. | Feed Solutions |
|---|---|---|---|---|
| Deionized Water | - | 5.4 ± 0.2 | - | HA, BSA |
| NaOH + NaOCl | 90 mg/L + 25 mg/L | 10 ± 0.2 | CEBL | HA, BSA |
| NaOH + NaOCl | 230 mg/L + 250 mg/L | 10.5 ± 0.2 | CEBM | HA, BSA |
| NaOH + NaOCl | 460 mg/L + 500 mg/L | 12.5 ± 0.2 | CEBH | HA, BSA |
| Tween 80 + NaOH + NaOCl | 0.015 mM + 90 mg/L + 25 mg/L | 11.5 ± 0.2 | CEBTw-L | HA, BSA |
| Tween 80 + NaOH + NaOCl | 0.015 mM + 230 mg/L + 250 mg/L | 12 ± 0.2 | CEBTw-M | HA, BSA |
| Tween 80 + NaOH + NaOCl | 0.015 mM + 460 mg/L + 500 mg/L | 12.5 ± 0.2 | CEBTw-H | HA, BSA |
| Triton X-100 + NaOH + NaOCl | 0.26 mM + 90 mg/L + 25 mg/L | 10 ± 0.2 | CEBTx-L | HA, BSA |
| Triton X-100 + NaOH + NaOCl | 0.26 mM + 230 mg/L + 250 mg/L | 12.5 ± 0.2 | CEBTx-M | HA, BSA |
| CIP Solutions | Concentration | pH | ||
| NaOH + NaOCl | 460 mg/L + 500 mg/L | 12.1 ± 0.2 | - |
| Backwash | TMP (kPa) | Jsp Beg (L/m2h /KPa) | Jsp End (L/m2h /KPa) | Volume Permeate Collected (mL) | %Spec Flux Decline | %TMP Rise |
|---|---|---|---|---|---|---|
| Humic Acid (HA) | ||||||
| DI | 127.7 | 1.439 | 0.66 | 2795 | 54 | 94 |
| CEBL | 127.6 | 1.379 | 0.689 | 2916 | 50 | 85 |
| CEBM | 85.1 | 1.407 | 1.122 | 2868 | 20 | 23 |
| CEBH | 76.5 | 1.814 | 1.224 | 2807 | 33 | 55 |
| Protein (BSA) | ||||||
| DI | 128.9 | 1.359 | 0.284 | 1531 | 79 | 89 |
| CEBL | 106.1 | 0.93 | 0.4 | 1640 | 57 | 35 |
| CEBM | 104.0 | 1.234 | 0.884 | 2946 | 28 | 32 |
| CEBH | 96.8 | 0.776 | 0.563 | 2251 | 27 | 11 |
| Backwash Composition | TMP (kPa) | Jsp Beg (LMH/kPa) | Jsp End (LMH/kPa) | Volume Permeate Collected (mL) | %Spec Flux Decline | %TMP Rise |
|---|---|---|---|---|---|---|
| Humic Acid (HA) | ||||||
| Tween 80 + NaOH + NaOCl (CEB) | ||||||
| CEBLTw | 100.00 | 1.379 | 0.689 | 2498 | 50 | 67 |
| CEBMTw | 83.77 | 1.618 | 1.127 | 2881 | 30 | 21 |
| CEBHTw | 85.77 | 1.353 | 1.121 | 2984 | 17 | 43 |
| Triton X100 + NaOH + NaOCl (CEB) | ||||||
| CEBLTx | 100.66 | 1.297 | 0.793 | 2734 | 39 | 43 |
| CEBMTx | 88.46 | 0.948 | 0.894 | 2856 | 6 | 8 |
| Protein (BSA) | ||||||
| Tween 80 + NaOH + NaOCl (CEB) | ||||||
| CEBLTw | 131.00 | 1.156 | 0.127 | 1299 | 89 | 58 |
| CEBMTw | 103.42 | 1.048 | 0.793 | 3372 | 24 | 15 |
| CEBHTw | 95.22 | 0.92 | 0.515 | 2023 | 44 | 15 |
| Triton X100 + NaOH + NaOCl (CEB) | ||||||
| CEBLTx | 116.87 | 1.036 | 0.213 | 1414 | 79 | 32 |
| CEBMTx | 95.91 | 1.216 | 0.727 | 2267 | 40 | 29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kallapalli, N.; Basu, O.D. Surfactant-Enhanced Cleaning Solutions for Ceramic Membranes: A Comparative Study on Humic Acid and BSA Fouling. Membranes 2025, 15, 73. https://doi.org/10.3390/membranes15030073
Kallapalli N, Basu OD. Surfactant-Enhanced Cleaning Solutions for Ceramic Membranes: A Comparative Study on Humic Acid and BSA Fouling. Membranes. 2025; 15(3):73. https://doi.org/10.3390/membranes15030073
Chicago/Turabian StyleKallapalli, Navneet, and Onita D. Basu. 2025. "Surfactant-Enhanced Cleaning Solutions for Ceramic Membranes: A Comparative Study on Humic Acid and BSA Fouling" Membranes 15, no. 3: 73. https://doi.org/10.3390/membranes15030073
APA StyleKallapalli, N., & Basu, O. D. (2025). Surfactant-Enhanced Cleaning Solutions for Ceramic Membranes: A Comparative Study on Humic Acid and BSA Fouling. Membranes, 15(3), 73. https://doi.org/10.3390/membranes15030073







