A Comparison Between Calcium and Strontium Transport by the (Ca2+ + Mg2+)ATPase of the Basolateral Plasma Membrane of Renal Proximal Convoluted Tubules
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Purified Vesicles Derived from Basolateral Membranes
2.3. 45Ca2+ and 90Sr2+ Uptake
2.4. (Ca2+ + Mg2+)ATPase and (Sr2+ + Mg2+)ATPase Activities
2.5. Data Analysis
3. Results and Discussion
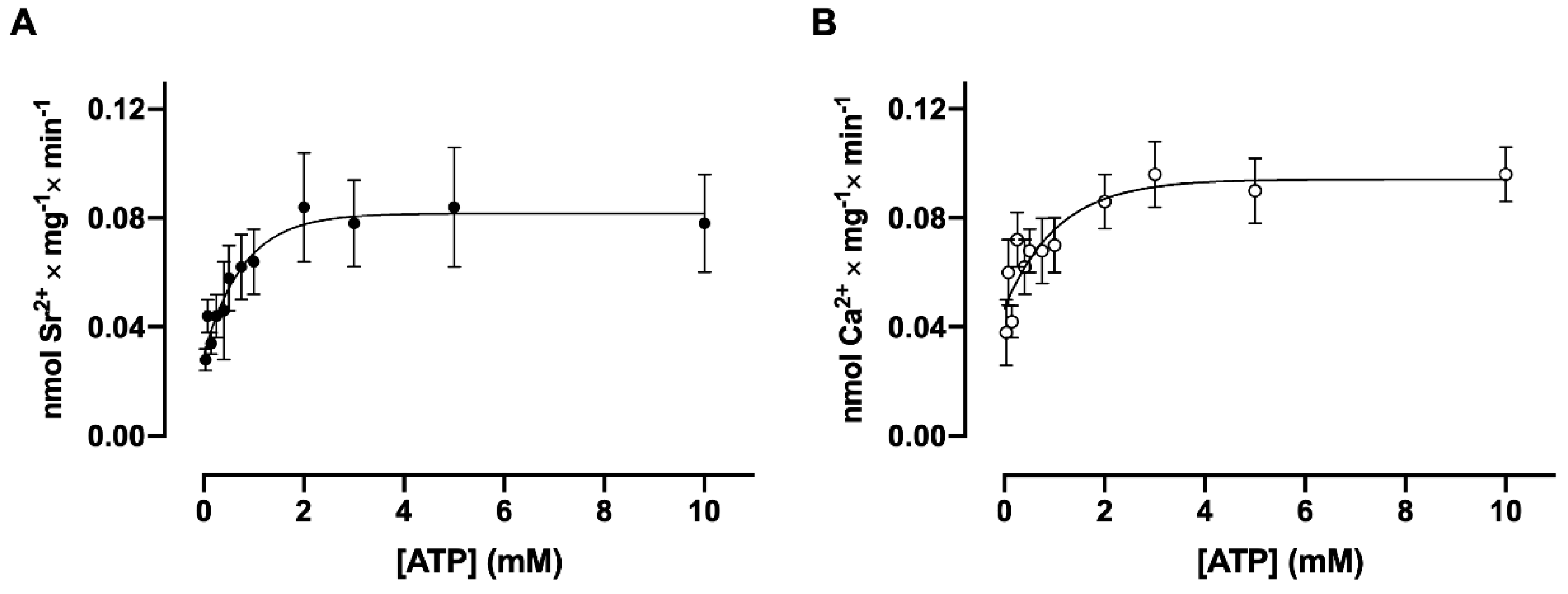
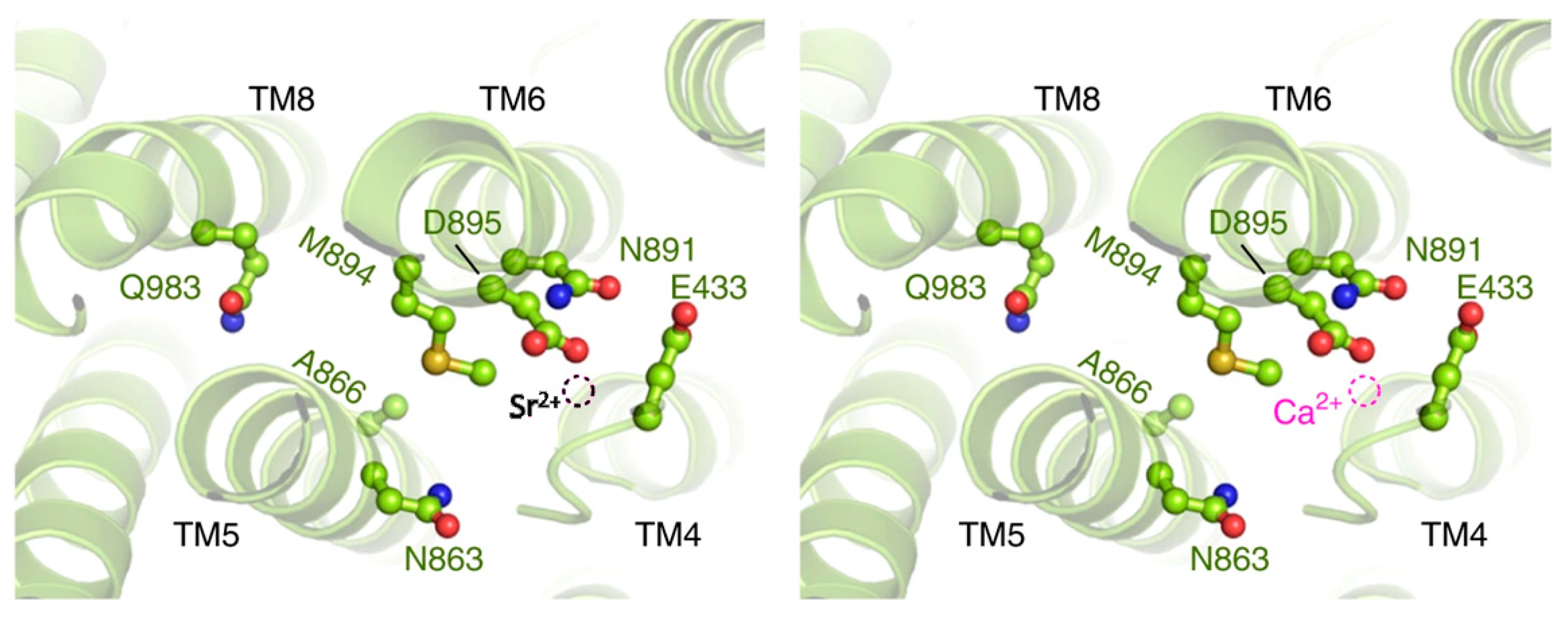
3.1. Ca2+ and Sr2+Transport
3.2. Dependence of Sr2+ and Ca2+ Uptake on Orthophosphate Concentration
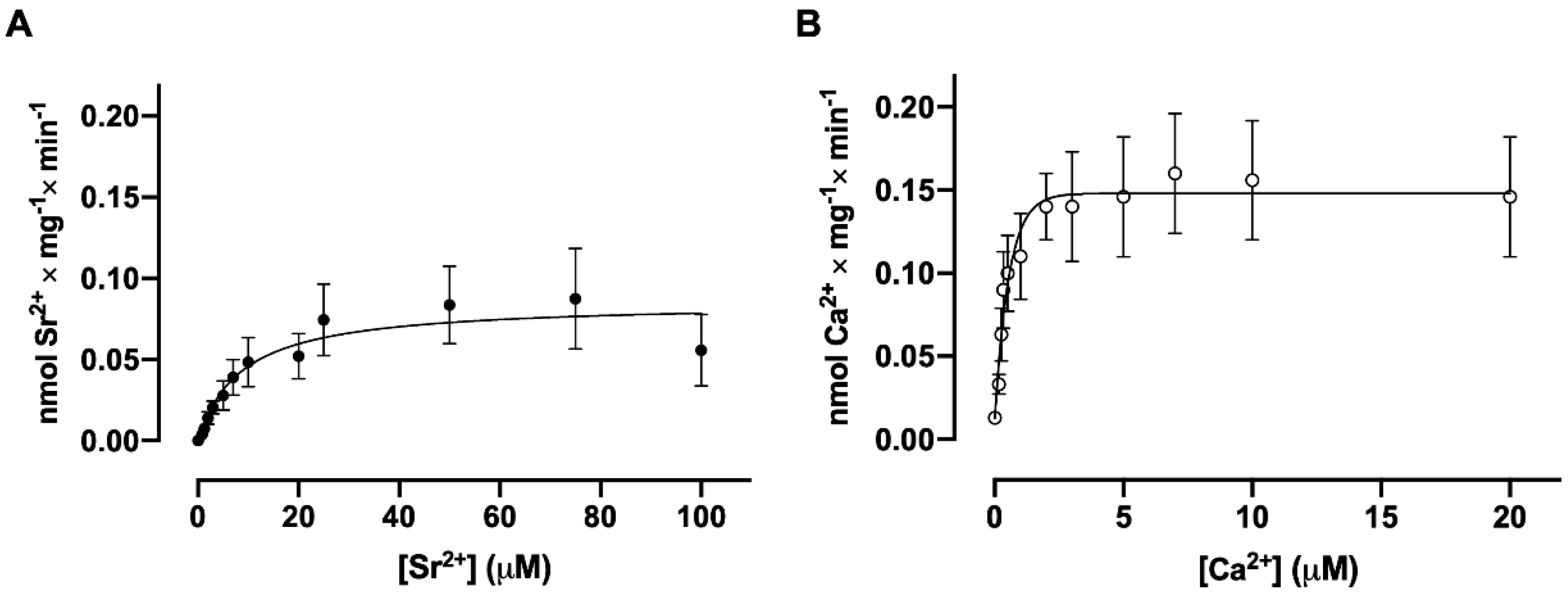
3.3. Dependence of Sr2+ and Ca2+ Uptake on Free Sr2+ and Ca2+ Concentrations, Respectively
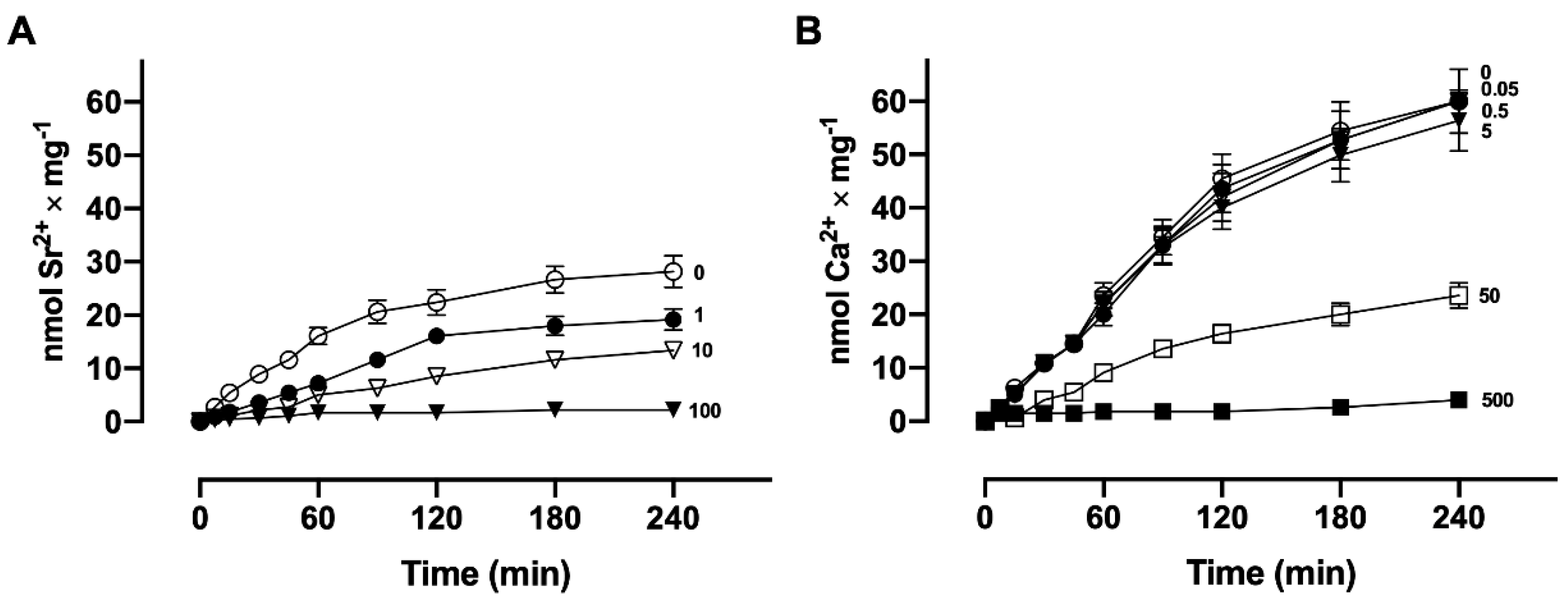
3.4. Sr2+ and Ca2+ Transport Competition Assays
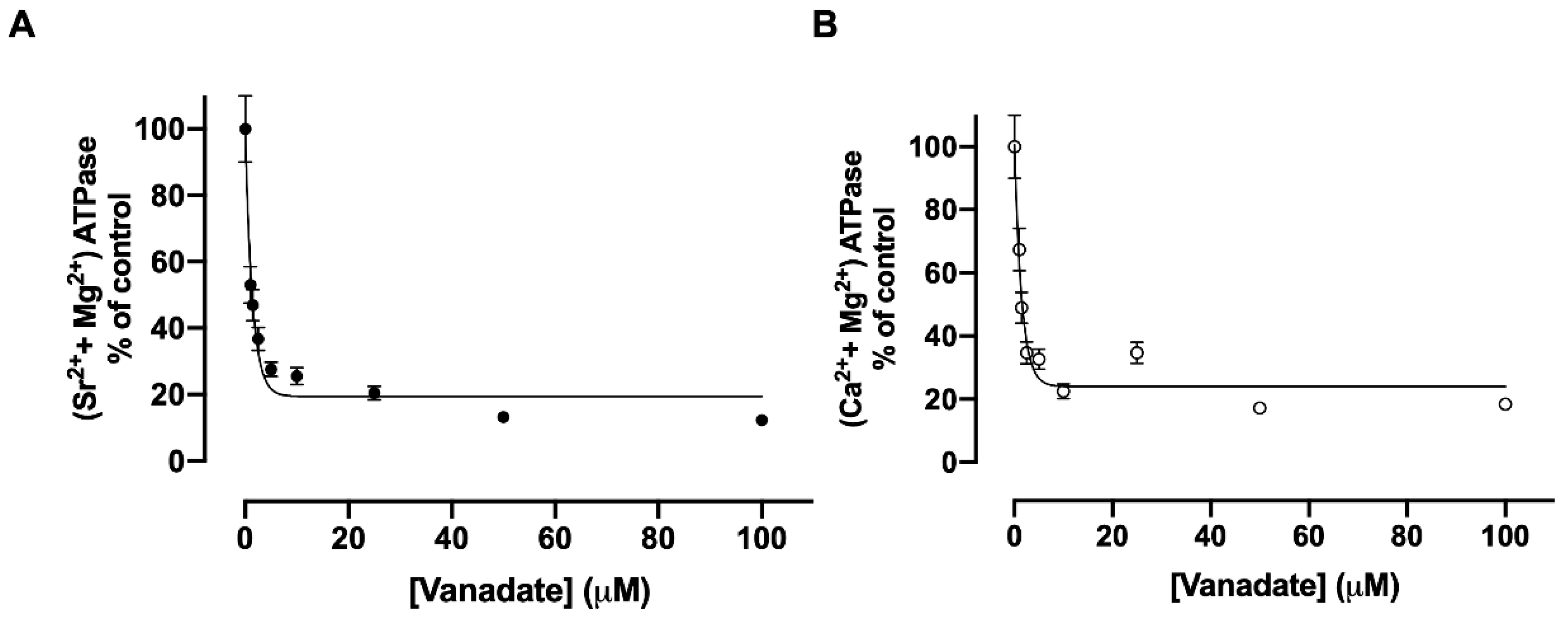
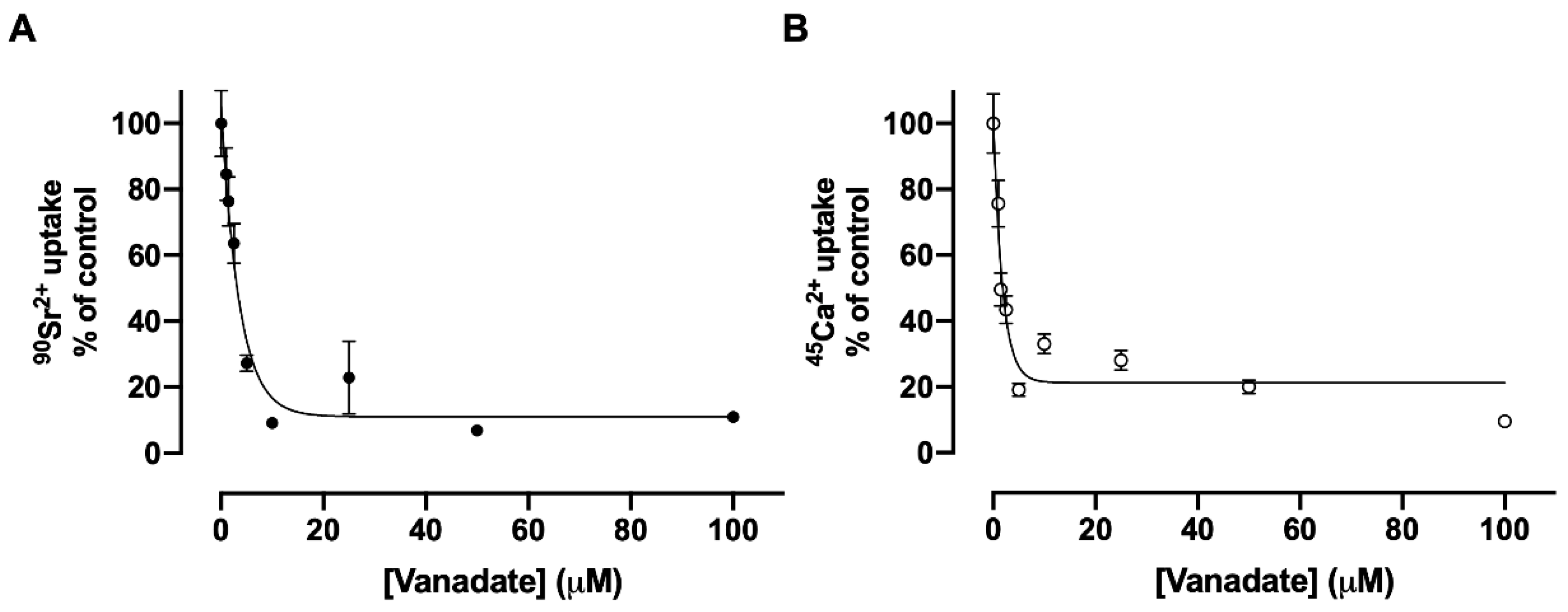
3.5. Inhibition of Sr2+ Transport by Vanadate
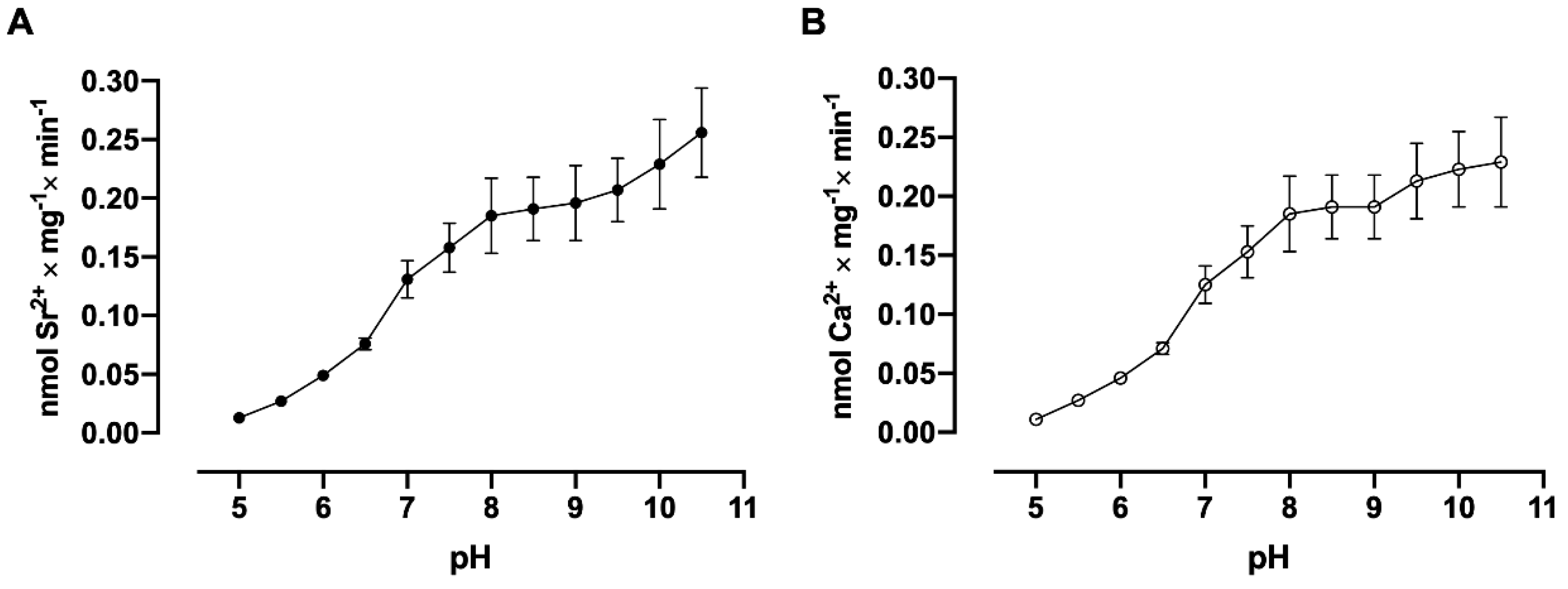
3.6. pH Dependence Assays
3.7. Specificity for Different Energy-Donor Substrates
3.8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gmaj, P.; Murer, H.; Kinne, R. Calcium ion transport across plasma membranes isolated from rat kidney cortex. Biochem. J. 1979, 178, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Gmaj, P.; Murer, H.; Carafoli, E. Localization and properties of a high affinity (Ca2+ + Mg2+)ATPase in isolated kidney cortex plasma membranes. FEBS Lett. 1982, 144, 226–230. [Google Scholar] [CrossRef]
- Vieyra, A.; Nachbin, L.; de Dios-Abad, E.; Goldfeld, M.; Meyer-Fernandes, J.R.; de Moraes, L. Comparison between calcium transport and adenosine triphosphatase activity in membrane vesicles derived from rabbit kidney proximal tubules. J. Biol. Chem. 1986, 261, 4247–4255. [Google Scholar] [CrossRef] [PubMed]
- Suki, W.N. Calcium transport in the nephron. Am. J. Physiol. 1979, 237, F1–F6. [Google Scholar] [CrossRef] [PubMed]
- Niggli, V.; Sigel, E. Anticipating antiport in P-type ATPases. Trends Biochem. Sci. 2008, 33, 156–160. [Google Scholar] [CrossRef]
- Gmaj, P.; Zurini, M.; Murer, H.; Carafoli, E. A high-affinity, calmodulin-dependent Ca2+ pump in the basal-lateral plasma membranes of kidney cortex. Eur. J. Biochem. 1983, 136, 71–76. [Google Scholar] [CrossRef]
- Pedersen, P.L. Transport ATPases into the year 2008: A brief overview related to types, structures, functions and roles in health and disease. J. Bioenerg. Biomembr. 2007, 39, 349–355. [Google Scholar] [CrossRef]
- Guimarães-Motta, H.; Sande-Lemos, M.P.; de Meis, L. Energy interconversion in sarcoplasmic reticulum vesicles in the presence of Ca2+ and Sr2+ gradients. J. Biol. Chem. 1984, 259, 8699–8705. [Google Scholar] [CrossRef]
- Glynn, I.M.; Chappell, J.B. A simple method for the preparation of 32P-labelled adenosine triphosphate of high specific activity. Biochem. J. 1964, 90, 147–149. [Google Scholar] [CrossRef]
- Fabiato, A.; Fabiato, F. Calculation programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J. Physiol. 1979, 75, 463–505. [Google Scholar]
- Sorenson, M.M.; Coelho, H.S.; Reuben, J.P. Caffeine inhibition of calcium accumulation by the sarcoplasmic reticulum in mammalian skinned fibers. J. Membr. Biol. 1986, 90, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Grassl, S.M.; Aronson, P.S. Na+/HCO3− co-transport in basolateral membrane vesicles isolated from rabbit renal cortex. J. Biol. Chem. 1986, 261, 8778–8783. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randal, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Martonosi, A.; Feretos, R. Sarcoplasmic Reticulum I. The uptake of Ca2+ by Sarcoplasmic reticulum fragments. J. Biol. Chem. 1964, 239, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Grubmeyer, C.; Penefsky, H.S. The presence of two hydrolytic sites on beef heart mitochondrial adenosine triphosphatase. J. Biol. Chem. 1981, 256, 3718–3727. [Google Scholar] [CrossRef]
- Nightingale, E.R., Jr. Phenomenological theory of ion solvation. Effective radii of hydrated ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Graf, E.; Verma, A.K.; Gorski, J.P.; Lopaschuk, G.; Niggli, V.; Zurini, M.; Carafoli, E.; Penniston, J.T. Molecular properties of calcium-pumping ATPase from human erythrocytes. Biochemistry 1982, 21, 4511–4516. [Google Scholar] [CrossRef]
- Caride, A.J.; Rega, A.F.; Garrahan, P.J. Effects of p-nitrophenylphosphate on Ca2+ transport in inside-out vesicles from human red-cell membranes. Biochim. Biophys. Acta 1983, 734, 363–367. [Google Scholar] [CrossRef]
- de Meis, L.; Sorenson, M.M. ATP regulation of calcium transport in back-inhibited sarcoplasmic reticulum vesicles. Biochim. Biophys. Acta 1989, 984, 373–378. [Google Scholar] [CrossRef]
- Gong, D.; Chi, X.; Ren, K.; Huang, G.; Shou, G.; Yan, N.; Lei, J.; Zhou, Q. Structure of the huma plasma membrane Ca2+-ATPase 1 in complex with its obligatory subunit neuroplastin. Nat. Commun. 2018, 9, 3623. [Google Scholar] [CrossRef]
- Rossi, J.P.; Garrahan, P.J.; Rega, A.F. Vanadate inhibition of active Ca2+ transport across human red cell membranes. Biochem. Biophys. Acta 1981, 648, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.P. The symbiosis of metal and protein functions. Eur. J. Biochem. 1985, 150, 231–248. [Google Scholar] [CrossRef] [PubMed]



| Substrates | Uptake of 90Sr (%) | Uptake of 45Ca (%) |
|---|---|---|
| ATP | 100 | 100 |
| CTP | 2.4 | 5.2 |
| GTP | 2.7 | 1.4 |
| ITP | 1.6 | 4.5 |
| UTP | 1.4 | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer-Fernandes, J.R.; Sola-Penna, M.; Vieyra, A. A Comparison Between Calcium and Strontium Transport by the (Ca2+ + Mg2+)ATPase of the Basolateral Plasma Membrane of Renal Proximal Convoluted Tubules. Membranes 2025, 15, 122. https://doi.org/10.3390/membranes15040122
Meyer-Fernandes JR, Sola-Penna M, Vieyra A. A Comparison Between Calcium and Strontium Transport by the (Ca2+ + Mg2+)ATPase of the Basolateral Plasma Membrane of Renal Proximal Convoluted Tubules. Membranes. 2025; 15(4):122. https://doi.org/10.3390/membranes15040122
Chicago/Turabian StyleMeyer-Fernandes, José Roberto, Mauro Sola-Penna, and Adalberto Vieyra. 2025. "A Comparison Between Calcium and Strontium Transport by the (Ca2+ + Mg2+)ATPase of the Basolateral Plasma Membrane of Renal Proximal Convoluted Tubules" Membranes 15, no. 4: 122. https://doi.org/10.3390/membranes15040122
APA StyleMeyer-Fernandes, J. R., Sola-Penna, M., & Vieyra, A. (2025). A Comparison Between Calcium and Strontium Transport by the (Ca2+ + Mg2+)ATPase of the Basolateral Plasma Membrane of Renal Proximal Convoluted Tubules. Membranes, 15(4), 122. https://doi.org/10.3390/membranes15040122







