The Use of Low-Rejection Nanofiltration Membranes as a Tool to Simplify Pretreatment, Escape Scaling and Radically Increase Recoveries
Abstract
1. Introduction
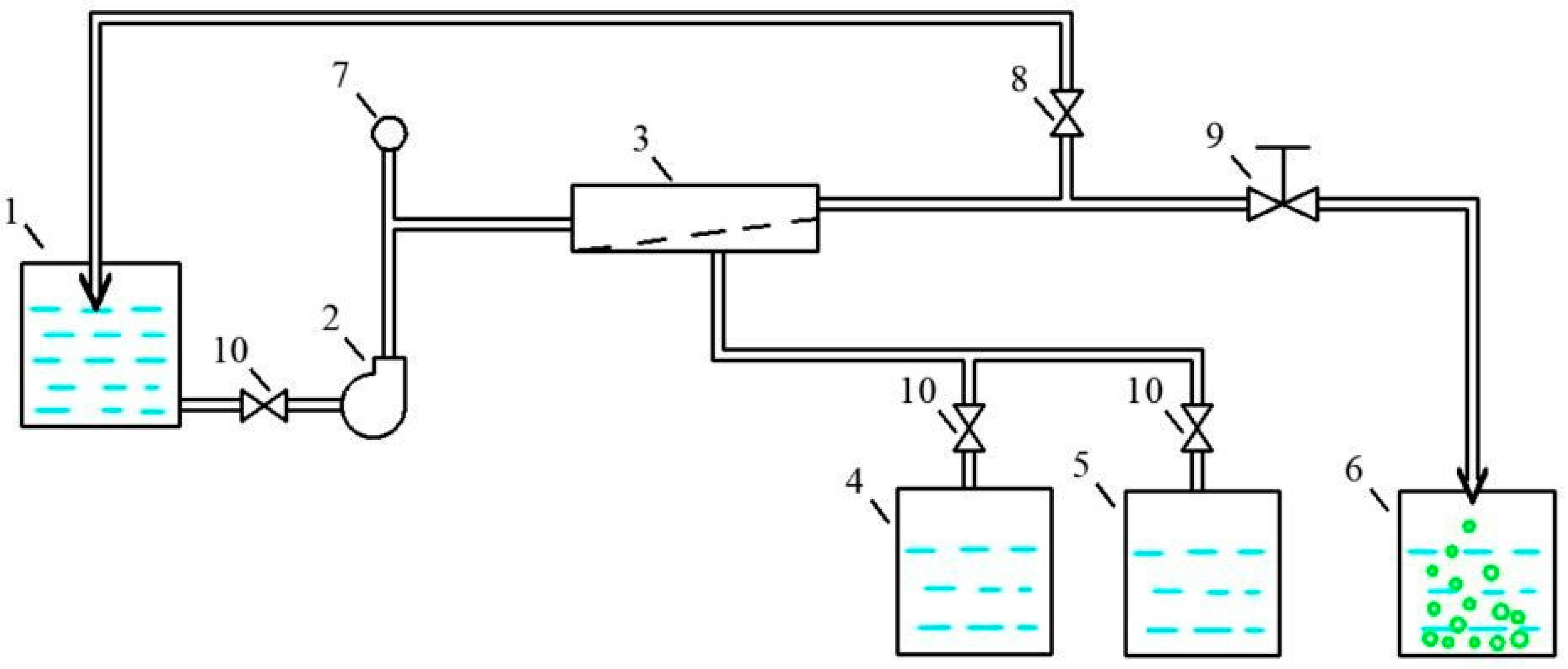
2. Materials and Methods
3. Discussion of the Results
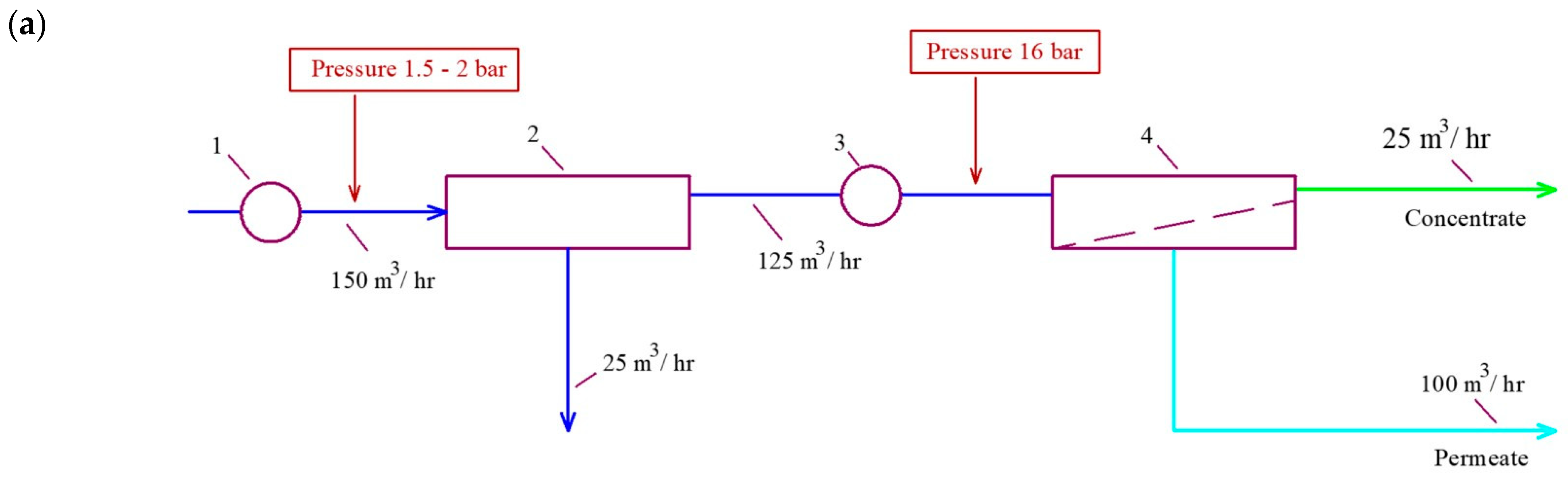
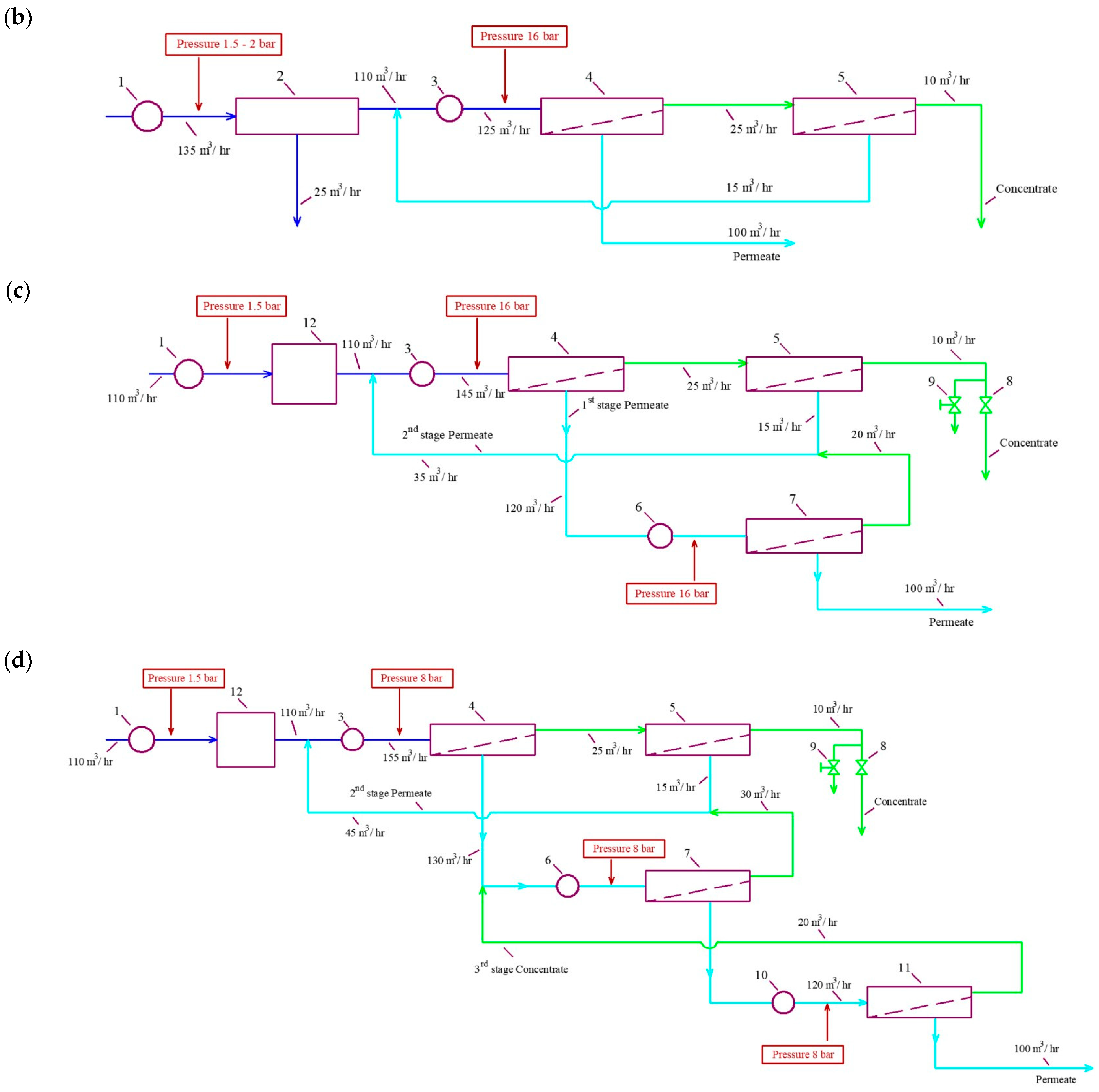
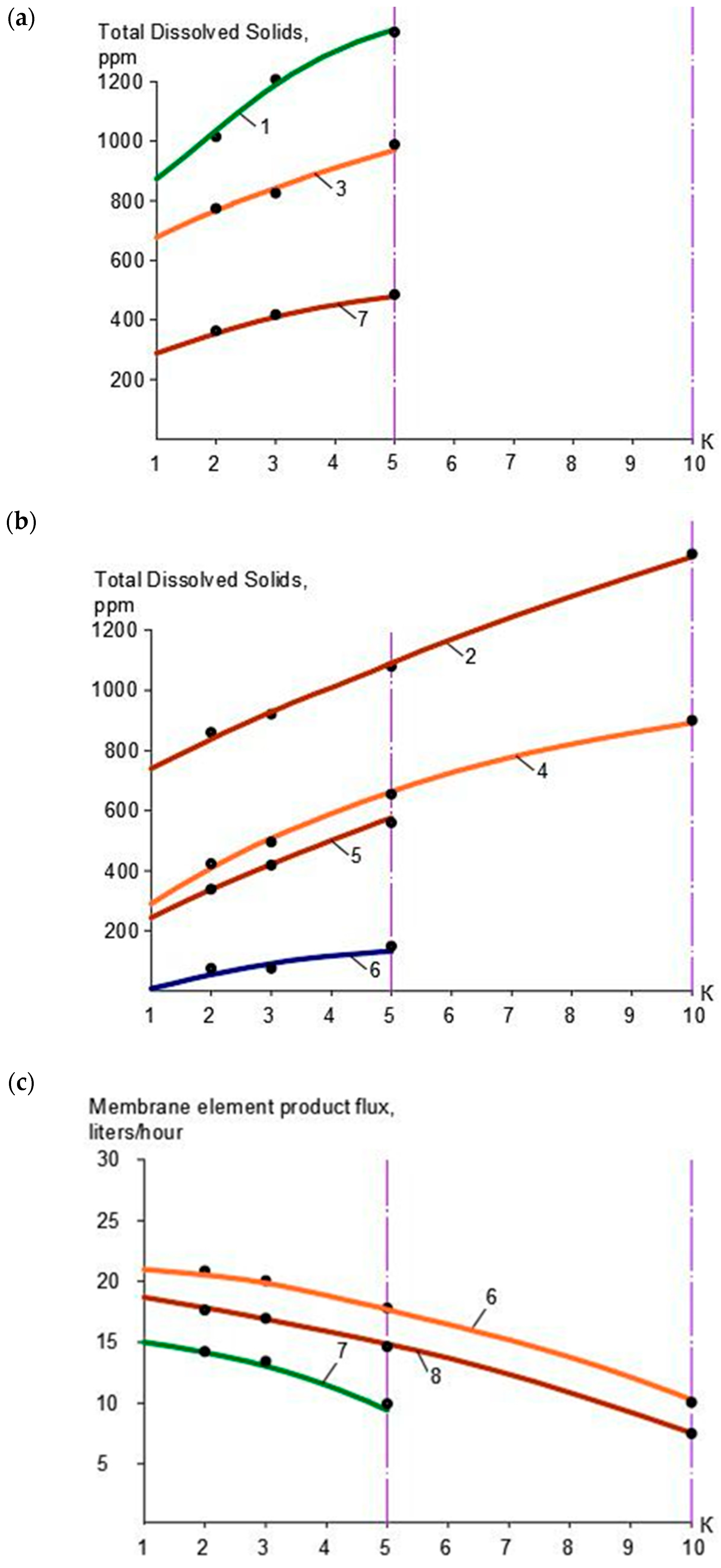
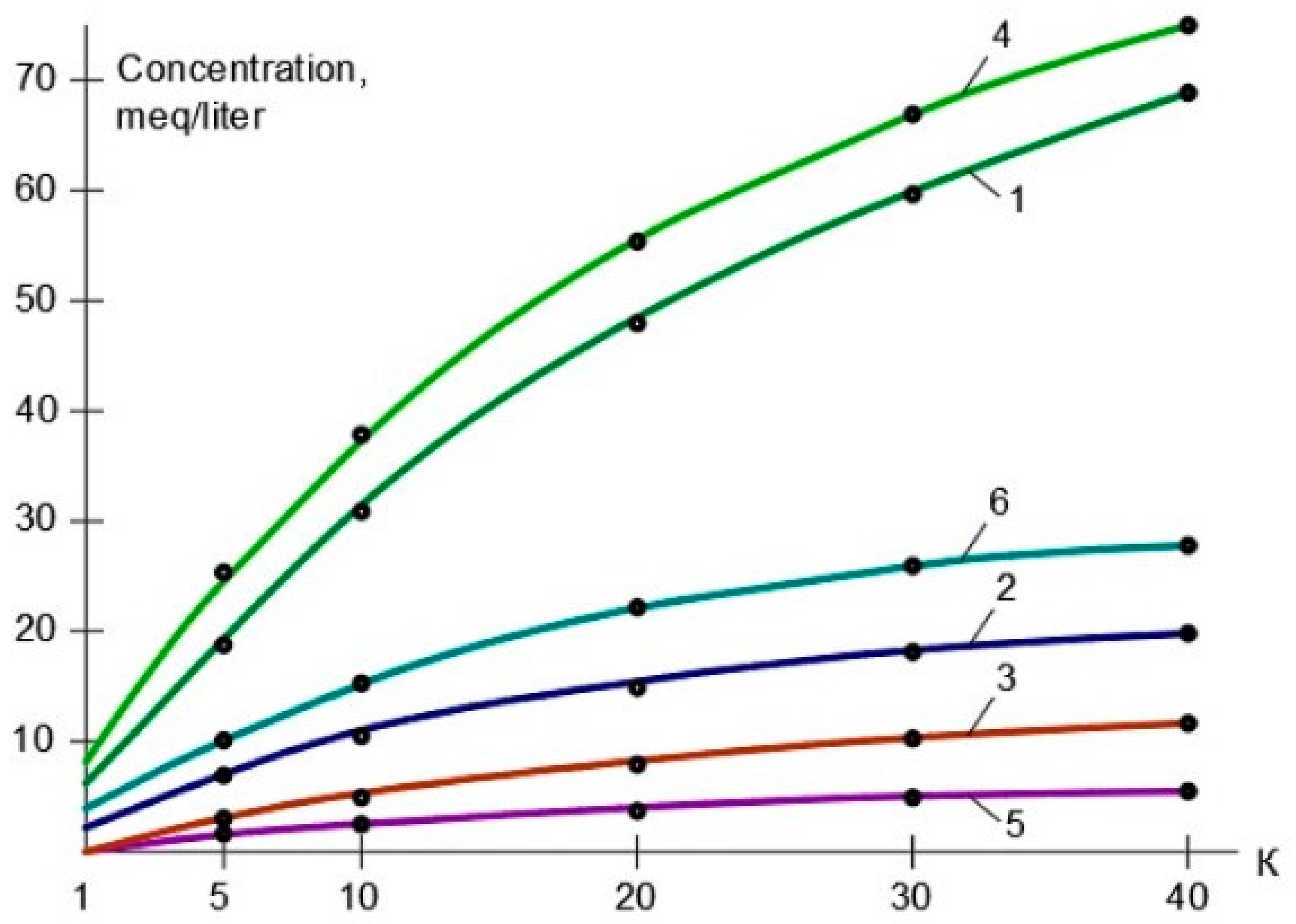
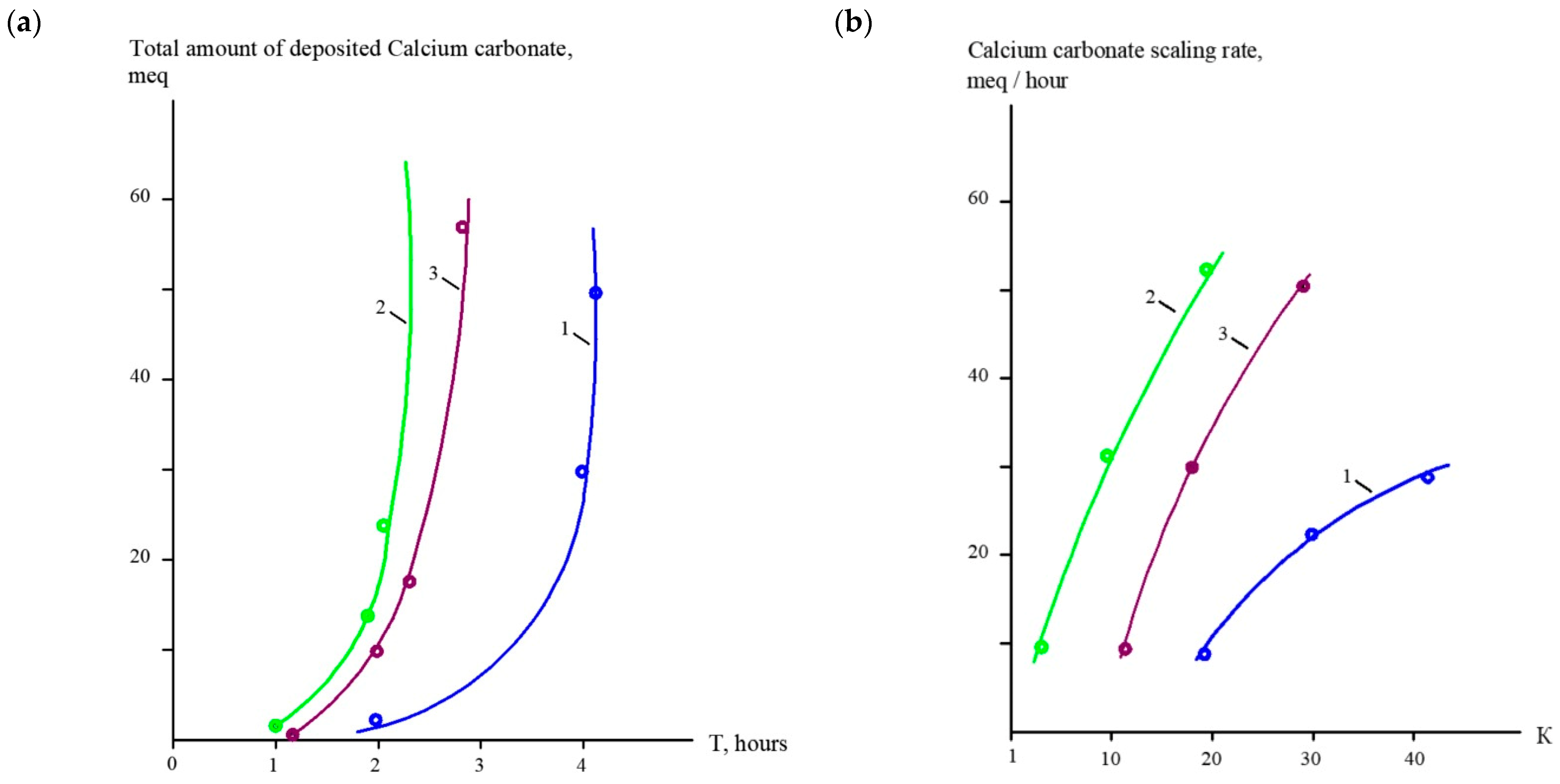
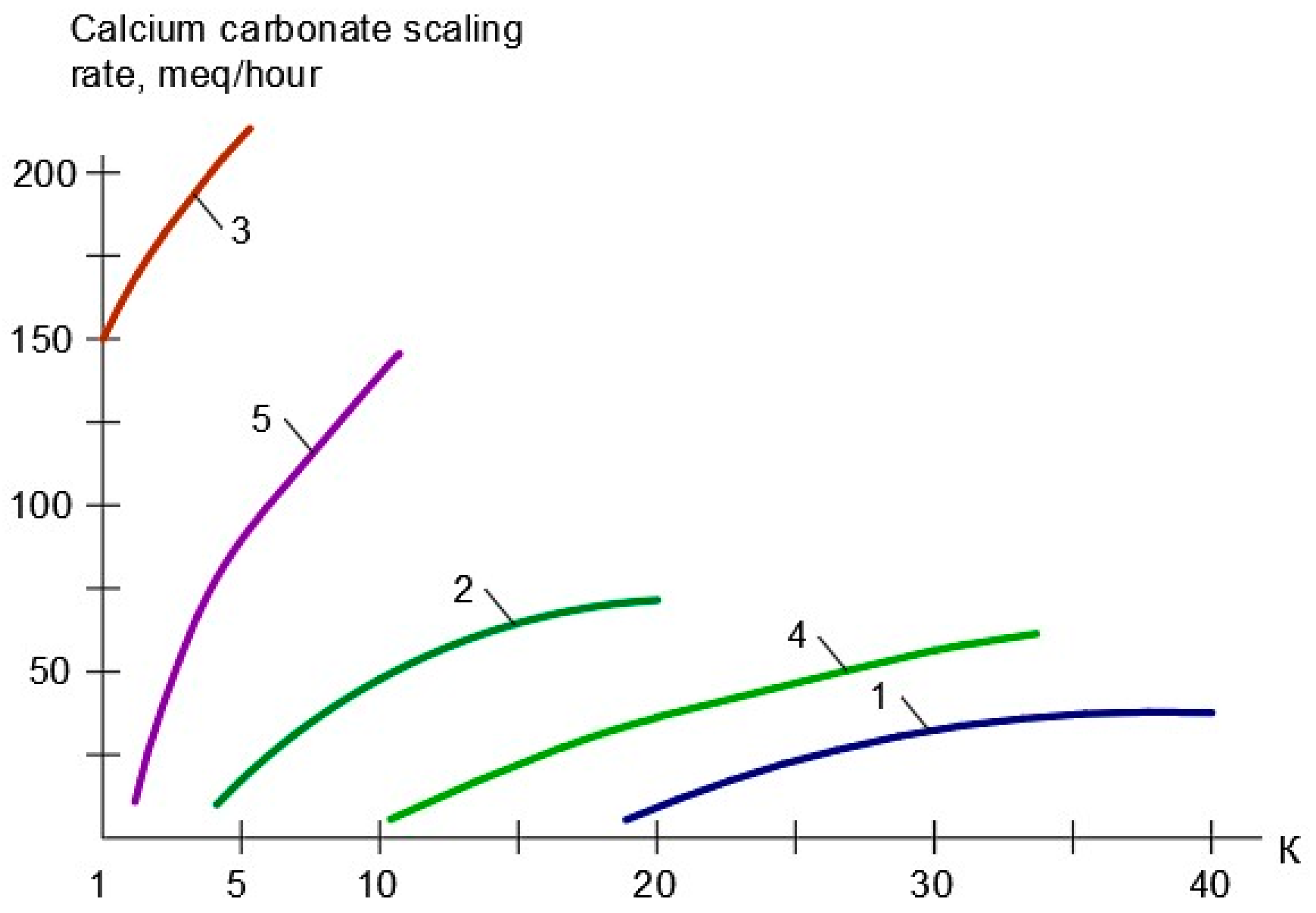
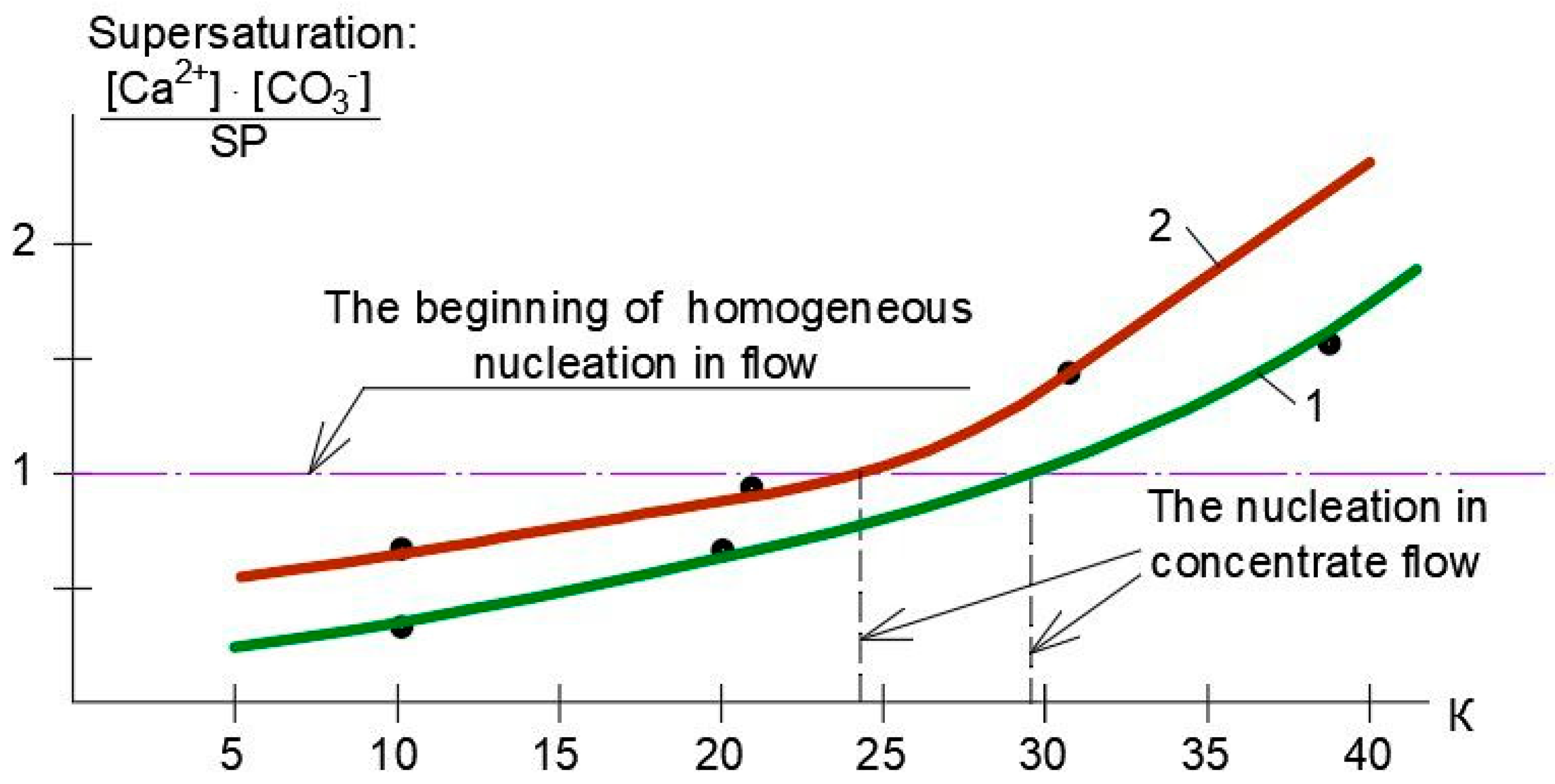
3.1. Results of the First Series of Experiments
3.2. Results of the Second Series of Experiments
3.3. Results of the Third Series of Experiments
- “NanoNF” membranes in the first stage and “BLN” membranes in the second stage;
- “70NE” membranes in the first stage and in the second stage;
- Three stages of the “nanoNF” membranes used to reach the required water quality for boiler feed without antiscalant use.
3.4. Processing of the Results: Feasibility Study of the Developed Technology
- “nanoNF” membranes are used in the first stage and “BLN” membranes are used in the second stage. This can be recognized as the best solution to produce water for boiler feed with hardness not exceeding 150 microgram equivalent/L. But the necessity to add antiscalant and apply timely cleanings to remove calcium carbonate increases operational costs and reduces the reliability of the scheme as well.
- The scheme with nanofiltration membranes “70NE” was used both in the first and in the second stages. This technology also requires antiscalant addition in the first stage due to the high hardness of the feed water. But the scaling rates in “70NE” membrane modules are substantially lower than those in modules tailored with “BLN” membranes. Nanofiltration membranes are operated under the pressure of 8 bar, which provides a substantial reduction in power consumption.
- To ensure “reagent-free” technology without antiscalant addition, a three-stage scheme is designed (Figure 1d). The use of “nanoNF” membranes in each stage is applied, excluding the formation of a calcium carbonate scale in membrane modules in a wide range of feed water compositions.
- -
- Annual power consumption costs;
- -
- Annual membrane replacement costs for replacement every 5 years;
- -
- Annual antiscalant costs;
- -
- Annual cleaning chemical costs.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pervov, A. Investigation of Scaling and Inhibition Mechanisms in Reverse Osmosis Spiral Wound Elements. Membranes 2022, 12, 852. [Google Scholar] [CrossRef] [PubMed]
- Orecki, A.; Tomaszewska, M.; Karakulski, K.; Morawski, A. Surface water treatment by the nanofiltration method. Desalination 2004, 162, 47–54. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Ang, W.L.; Teov, Y.H.; Mohammad, A.W.; Hilal, N. Nanofiltration membrane processes for water recycling, reuse and product recovery within various industries: A review. J. Water Process Eng. 2022, 45, 102478. [Google Scholar] [CrossRef]
- Pervov, A.G. Modernization of Conventinal Spiral Wound Channel—A Tol to Escape Fouling, Expand Membrane Life and Increase Recovery. J. Membr. Sep. Technol. 2014, 3, 56–66. [Google Scholar] [CrossRef]
- Yokoyama, F.; Nakajima, M.; Ichikawa, S. Analysis of Calcium Sulphate Scaling Phenomena on Reverse Osmosis Membranes by Scaling-Based Flux Model. Membranes 2022, 12, 894. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Liu, Y.; Gao, L. Stormwater treatment for reuse: Current practice and future development—A review. J. Environ. Manag. 2022, 30, 113830. [Google Scholar] [CrossRef]
- Pervov, A.G. Precipitation of Calcium Carbonate in Reverse osmosis Retentate flow by means of Seeded Techniques—A Tool to Increase Recovery. Desalination 2015, 368, 140–151. [Google Scholar] [CrossRef]
- Scholes, R.; Stiegler, A.; Anderson, C.; Sedlak, D. Enabling Water Reuse by Treatment of Reverse Osmosis Concentrate: The Promise of Constructed Wetlands. ACS Environ. Au 2021, 1, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Sanciolo, P.; Sharma, A.K.; Navaratna, D.; Muthukumaran, S. Stormwater Treatment Using Natural and Engineered Options in an Urban Growth Area: A Case Study in the West of Melbourne. Water 2023, 15, 4047. [Google Scholar] [CrossRef]
- Jafarinejad, S.; Esfahani, M.R. A Review on the Nanofiltration Process for Treating Wastewaters from the Petroleum Industry. Separations 2021, 8, 206. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Hilal, N.; Darwish, N.; Al-Zoubi, H. Prediction of permeate fluxes and rejections of highly concentrated salts in nanofiltration membranes. J. Membr. Sci. 2007, 289, 40–50. [Google Scholar] [CrossRef]
- Al-Qadami, E.; Ahsan, A.; Mustafa, Z.; Abdurrasheed, A.S.; Yusof, K.W.; Shah, S.M.H. Nanofiltration membrane technology and its applications in surface water treatment: A review. J. Desalin. Water Purif. 2020, 18, 3–9. [Google Scholar]
- Guo, H.; Li, X.; Yang, W.; Yao, Z.; Mei, Y.; Peng, L.E.; Yang, Z.; Shao, S.; Tang, C.Y. Nanofiltration for drinking water treatment: A review. Front. Chem. Sci. Eng. 2021, 16, 681–698. [Google Scholar] [CrossRef] [PubMed]
- Turek, M.; Mitko, K.; Piotrowski, K.; Dydo, P.; Laskowska, E.; Jakóbik-Kolon, A. Prospects for high water recovery membrane desalination. Desalination 2017, 401, 180–189. [Google Scholar] [CrossRef]
- Goh, P.S.; Lau, W.J.; Othman, M.H.D.; Ismail, A.F. Membrane fouling in desalination and its mitigation strategies. Desalination 2018, 425, 130–155. [Google Scholar] [CrossRef]
- Shahid, M.K.; Choi, Y.-G. The comparative study for scale inhibition on surface of RO membranes in wastewater reclamation: CO2 purging versus three different antiscalants. J. Membr. Sci. 2018, 546, 61–69. [Google Scholar] [CrossRef]
- Joo, S.H.; Tansel, B. Novel Technologies for Reverse Osmosis Concentrate Treatment: A Review. J. Environ. Manag. 2015, 150, 322–335. [Google Scholar] [CrossRef]
- Zimmer, K.; Hater, W.; Icart, A.; Jaworski, J.; Kruse, N.; Braun, G. The performance of polycarboxylates as inhibitors for CaCO3 scaling in reverse osmosis plants. Desalin. Water Treat. 2016, 57, 48–49. [Google Scholar] [CrossRef]
- Jamaly, S.; Darwish, N.N.; Ahmed, I.; Hasan, S.W. A short review on reverse osmosis pretreatment technologies. Desalination 2014, 354, 30–38. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Y.; Ladewig, B.P. A review of reverse osmosis membrane fouling and control strategies. Sci. Total Environ. 2017, 595, 567–583. [Google Scholar] [CrossRef]



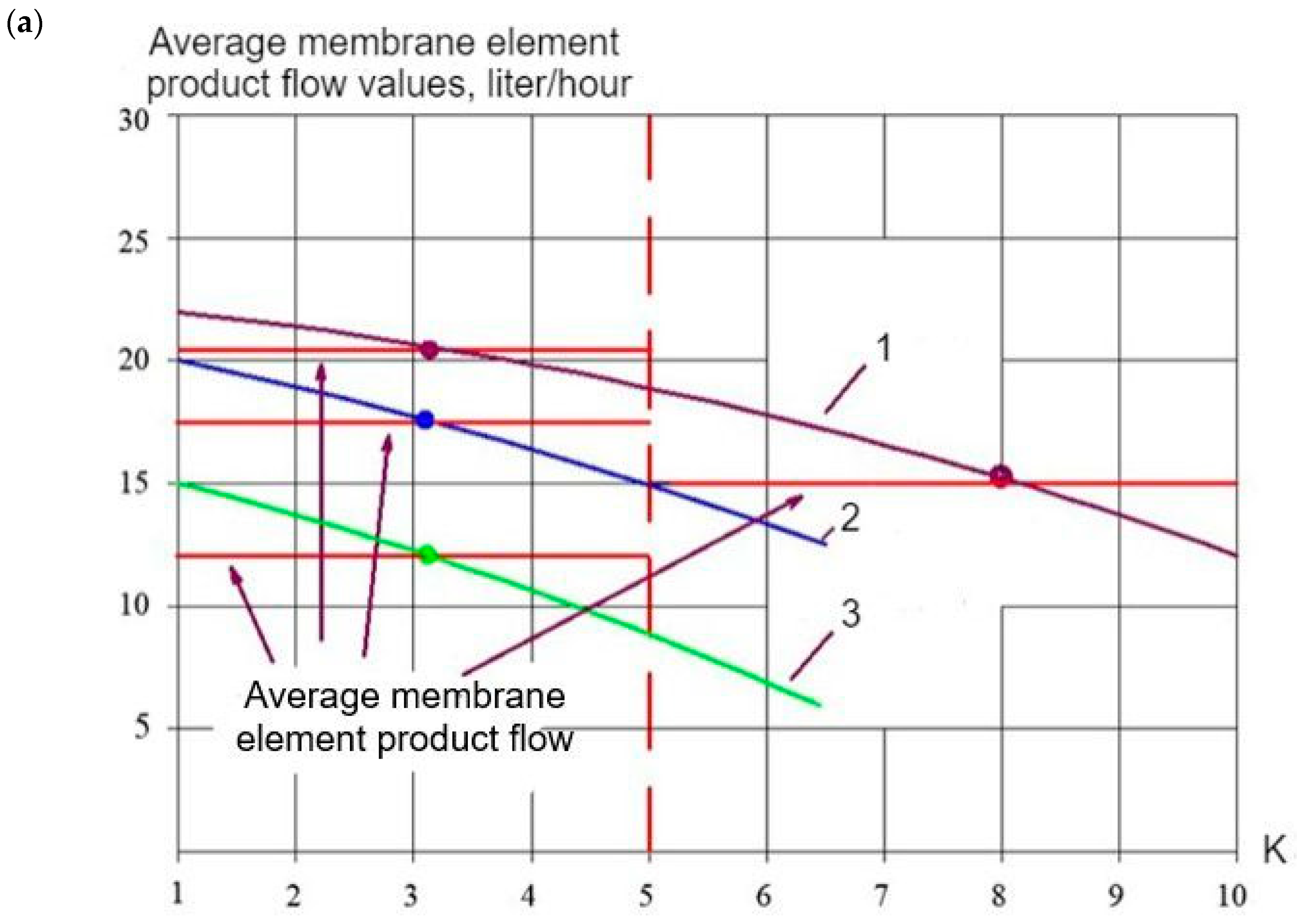
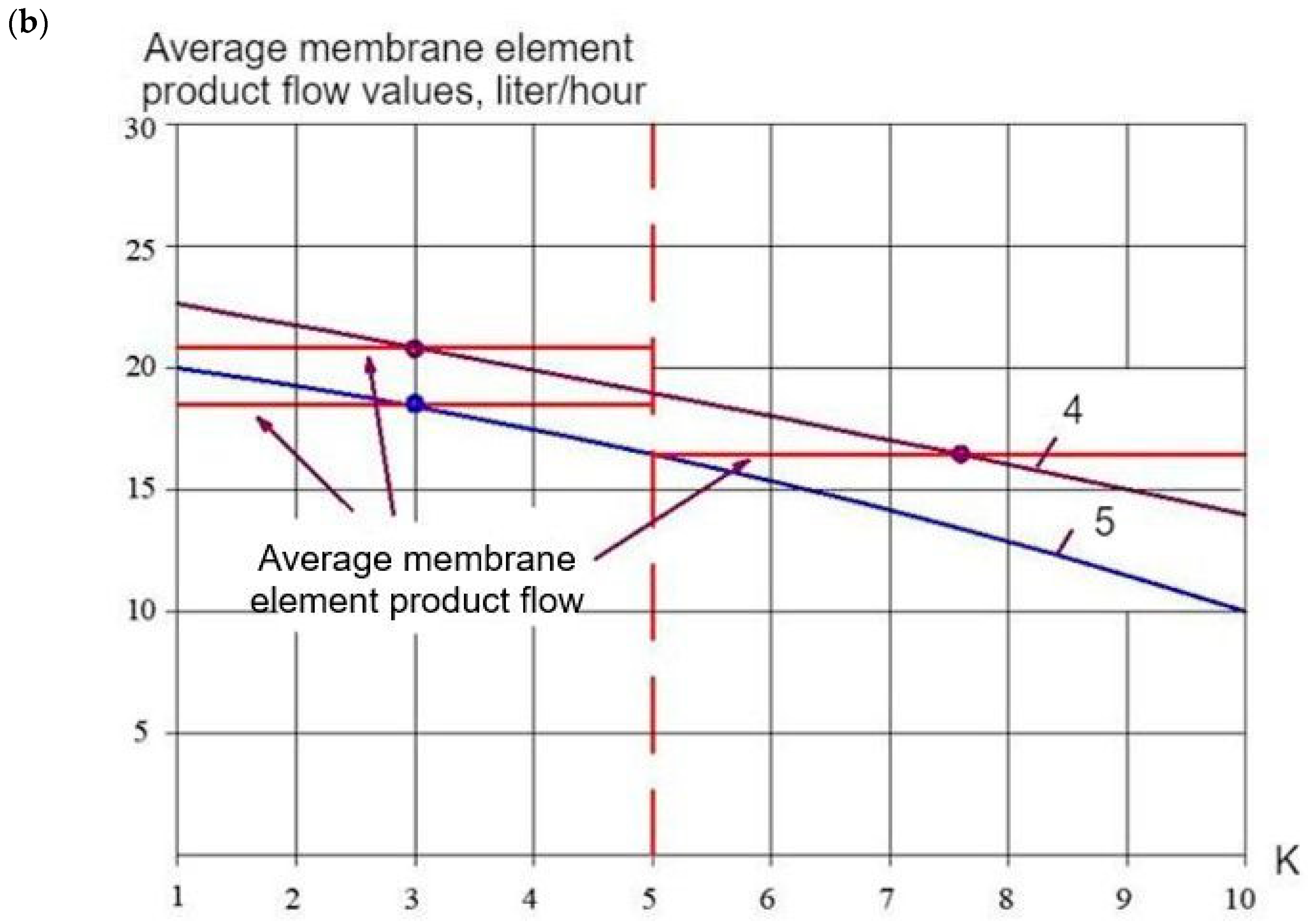
| N | Component | Drainage Wastewater |
|---|---|---|
| 1 | Calcium, ppm | 6.7 |
| 2 | Magnesium, ppm | 0.8 |
| 3 | Total hardness | 7.5 |
| 4 | Chlorides, ppm | 55 |
| 5 | Sulfates, ppm | 180 |
| 6 | Bicarbonates, ppm | 2.6 |
| 7 | Sodium + potassium, ppm | 46 |
| 8 | pH | 7.5 |
| 9 | Ammonium, ppm | 6.5 |
| 10 | Oil product, mg/L | 0.2 |
| 11 | Color units, CUs | 149 |
| 12 | TDS, ppm | 810 |
| N | K | Ca2+, meq/L | HCO3−, meq/L | pH | TDS, ppm | Solubility Product, SP, (meq/L)2 | [CO32−]/[HCO3−] | [CO32−], meq/L | (Supersaturation) |
|---|---|---|---|---|---|---|---|---|---|
| “nanoNF” membranes | |||||||||
| 1 | 10 | 31 | 11 | 7.7 | 2400 | 5.22 × 10−9 | 0.3 | 3.3 | 0.2 × 105 |
| 2 | 20 | 42 | 15 | 8 | 3300 | 0.4 | 7 | 0.6 × 105 | |
| 3 | 30 | 61 | 20 | 8.1 | 4400 | 0.4 | 8.1 | 1.1 × 105 | |
| 4 | 40 | 70 | 22 | 8.2 | 5200 | 0.5 | 10 | 1.4 × 105 | |
| “70NE” membranes | |||||||||
| 5 | 10 | 38 | 15 | 7.7 | 3000 | 0.3 | 5 | 0.4 × 105 | |
| 6 | 20 | 55 | 20 | 8 | 4000 | 0.4 | 8.4 | 0.8 × 105 | |
| 7 | 30 | 65 | 24 | 8.1 | 5100 | 0.5 | 10 | 1.3 × 105 | |
| № | Components of Operating Costs | Flow Diagram 1 | Flow Diagram 2 | Flow Diagram 3 |
|---|---|---|---|---|
| I stage—“nanoNF” II stage—“BLN” | I stage—“70NE” II stage—“70NE” | I stage—“nanoNF” II stage—“nanoNF” III stage—“nanoNF” | ||
| 1. | Electric power costs | (Feed water flow, (m3/h) × specific power consumption, (kWh/m3) × 7000 h/year × 0.05 USD/kW) | ||
| 1.1. | I stage | 14.5 | 14.5 | 15.5 |
| 1.2. | II stage | 12.0 | 12.0 | 13.0 |
| 1.3. | III stage | 12.0 | ||
| 1.4. | Annual electric power costs, USD/year | 5687.50 | 4637.50 | 7087.50 |
| 2. | Membrane replacement costs | Number of elements × 500/5 | ||
| 2.1. | Number and model of elements in the first stage | 14.5:0.04:40 = 9 elements nanoNF 8040 | 14.5:0.36:4 = 10.06 elements 70NE 8040 | 15.5:0.4:4 = 9.68 elements nanoNF 8040 |
| 2.2. | Number and model of elements in the second stage | 12.0:0.24:4 = 12.5 elements BLN 8040 | 12.0:0.44:4 = 6.8 elements 70NE 8040 | 13.0:0.44:4 = 7.38 elements nanoNF 8040 |
| 2.3. | Number and model of elements in the third stage | 12.0:0.5:4 = 6 nanoNF 8040 | ||
| 2.4. | Annual membrane replacement costs, USD/year | (9 + 13) × 500/5 = 2200.00 | (11 + 7) × 500/5 = 1800.00 | (10 + 8 + 6) × 500/5 = 2400.00 |
| 3. | Antiscalant annual costs evaluation, USD | Antiscalant consumption, kg/h × 7 000 h × 12 USD/kg | ||
| 3.1. | Antiscalant dose, kg/h | (Feed water flow, (m3/h) × dose, kg/m3) | ||
| 3.2. | I stage | – | 5 | – |
| 3.3. | II stage | 10 | – | – |
| 3.4. | III stage | – | ||
| 3.5. | Antiscalant annual costs, USD | 10,080.00 | 6048.00 | – |
| 4. | Cleaning costs, USD/year | Number ofelements × 2 kg/element × 10 USD/kg × number of cleanings per year | ||
| 4.1. | Cleaning chemical consumption: 2 kg/element | |||
| 4.2. | I stage | 9 × 2 × 10 × 3 = 540 | 11 × 2 × 10 × 4 = 880 | 10 × 2 × 10 × 3 = 600 |
| 4.3. | II stage | 13 × 2 × 10 × 6 = 1 440 | 7 × 2 × 10 × 2 = 280 | – |
| 4.4. | Annual cleaning costs, USD | 1980.00 | 1160.00 | 600.00 |
| 5. | Total annual costs, USD/year | 19,949.50 | 13,645.50 | 7927.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pervov, A.G.; Spitsov, D.; Kulagina, A.; Aung, H.Z. The Use of Low-Rejection Nanofiltration Membranes as a Tool to Simplify Pretreatment, Escape Scaling and Radically Increase Recoveries. Membranes 2025, 15, 96. https://doi.org/10.3390/membranes15040096
Pervov AG, Spitsov D, Kulagina A, Aung HZ. The Use of Low-Rejection Nanofiltration Membranes as a Tool to Simplify Pretreatment, Escape Scaling and Radically Increase Recoveries. Membranes. 2025; 15(4):96. https://doi.org/10.3390/membranes15040096
Chicago/Turabian StylePervov, Alexei G., Dmitry Spitsov, Anna Kulagina, and Htet Zaw Aung. 2025. "The Use of Low-Rejection Nanofiltration Membranes as a Tool to Simplify Pretreatment, Escape Scaling and Radically Increase Recoveries" Membranes 15, no. 4: 96. https://doi.org/10.3390/membranes15040096
APA StylePervov, A. G., Spitsov, D., Kulagina, A., & Aung, H. Z. (2025). The Use of Low-Rejection Nanofiltration Membranes as a Tool to Simplify Pretreatment, Escape Scaling and Radically Increase Recoveries. Membranes, 15(4), 96. https://doi.org/10.3390/membranes15040096







