Single Gas Permeance Performance of High Silica SSZ-13 Zeolite Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation Procedure of SSZ-13 Zeolite and High Silica SSZ-13 Membranes
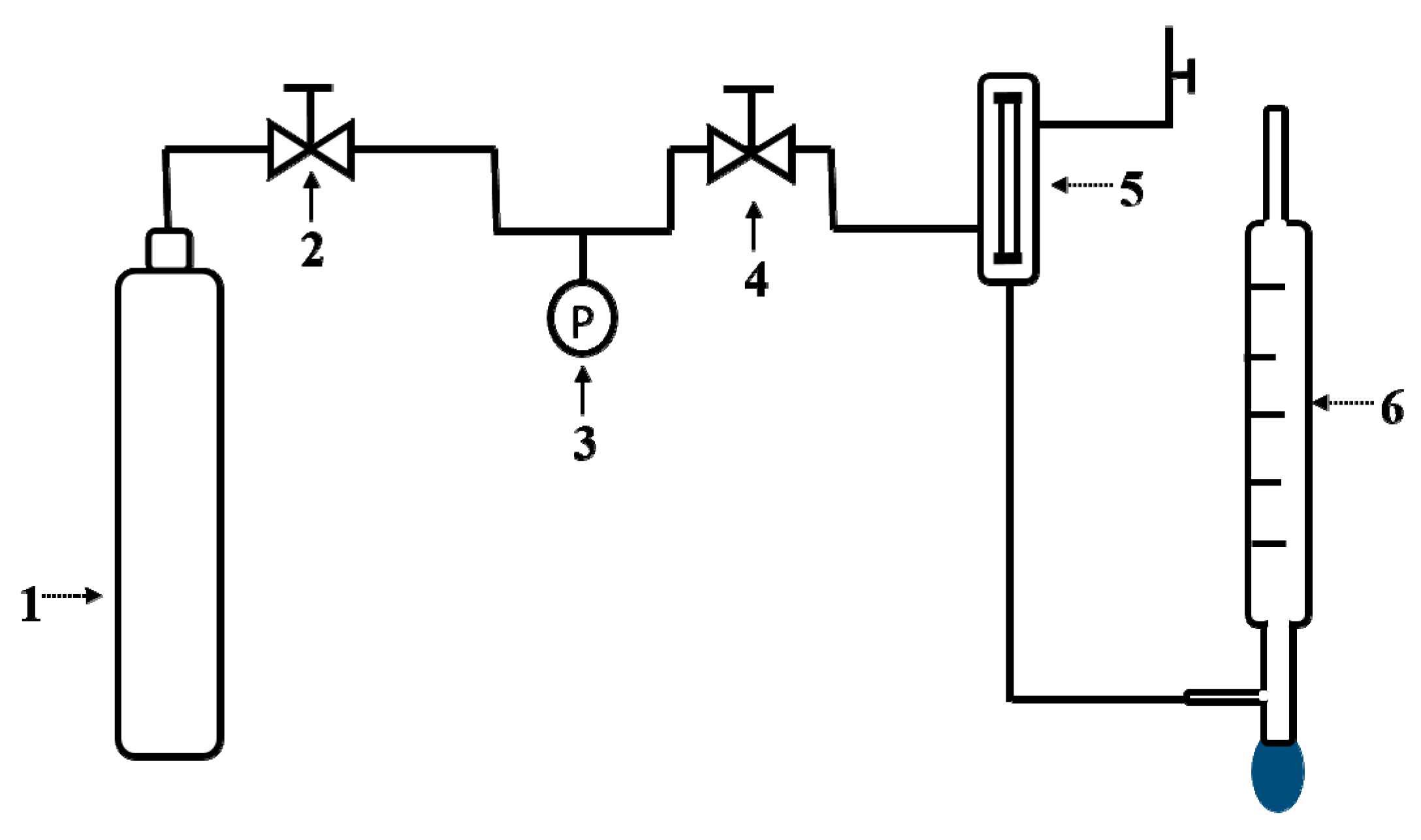
2.2. Characterization and Single-Gas Permeation
3. Results and Discussion
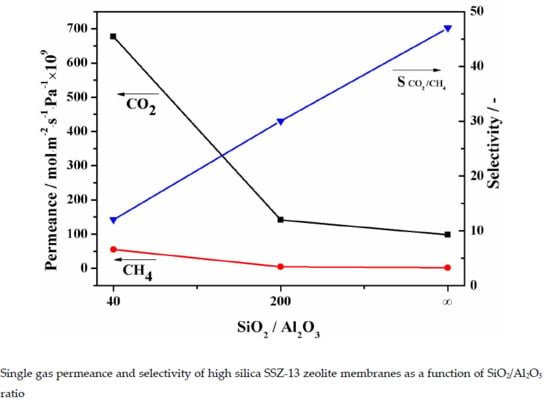
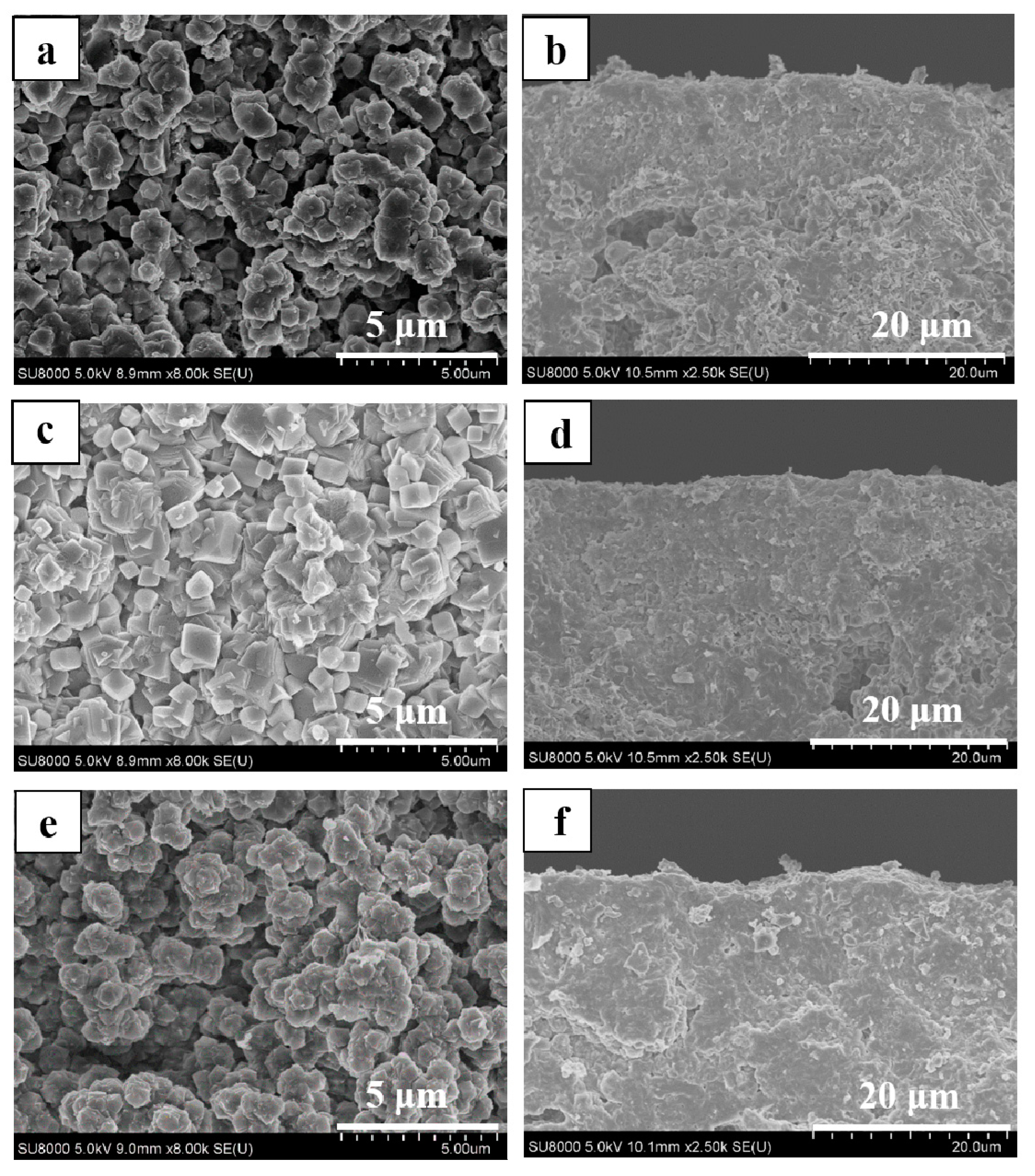
3.1. Effect of the SiO2/Al2O3 Ratio
3.2. Effect of H2O/SiO2 Ratio
3.3. Effect of TMAdaOH/SiO2 Ratio
3.4. Effect of Synthesis Time
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xiao, Y.; Low, B.T.; Hosseini, S.S.; Chung, T.S.; Paul, D.R. The strategies of molecular architecture and modification of polyimide-based membranes for CO2 removal from natural gas—A review. Prog. Polym. Sci. 2009, 34, 561–580. [Google Scholar] [CrossRef]
- NKEA: National Key Economic Area. Available online: http://kada.gov.my/en/web/guest/bidang-ekonomi-utama-negara-nkea (accessed on 2 April 2018).
- Zheng, Y.; Hu, N.; Wang, H.; Bu, N.; Zhang, F.; Zhou, R. Preparation of steam-stable high-silica CHA (SSZ-13) membranes for CO2/CH4 and C2H4/C2H6 separation. J. Membr. Sci. 2015, 475, 303–310. [Google Scholar] [CrossRef]
- An, W.; Swenson, P.; Wu, L.; Waller, T.; Ku, A.; Kuznicki, S.M. Selective separation of hydrogen from C1/C2 hydrocarbons and CO2 through dense natural zeolite membranes. J. Membr. Sci. 2011, 69, 414–419. [Google Scholar] [CrossRef]
- Baker, R.W. Future directions of membrane gas separation technology. Ind. Eng. Chem. Res. 2002, 4, 1393–1411. [Google Scholar] [CrossRef]
- Staudt-Bickel, C.; Koros, W.J. Olefin/paraffin gas separations with 6FDA-based polyimide membranes. J. Membr. Sci. 2000, 170, 205–214. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Yeo, Z.Y.; Chew, T.L.; Zhu, P.W.; Mohamed, A.R.; Chai, S.-P. Conventional processes and membrane technology for carbon dioxide removal from natural gas: A review. J. Nat. Gas Chem. 2012, 21, 282–298. [Google Scholar] [CrossRef]
- Cui, Y.; Kita, H.; Okamoto, K.-I. Zeolite T membrane: Preparation, characterization, pervaporation of water/organic liquid mixtures and acid stability. J. Membr. Sci. 2004, 236, 17–27. [Google Scholar] [CrossRef]
- Tomita, T.; Nakayama, K.; Sakai, H. Gas separation characteristics of DDR type zeolite membrane. Microporous Mesoporous Mater. 2004, 68, 71–75. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, G.; Li, H.; Zou, X.; Yin, X.; Yang, W.; Qiu, S.; Xu, R. Hierarchical growth of large-scale ordered zeolite Silicalite-1 membranes with high permeability and selectivity for recycling CO2. Angew. Chem. Int. Ed. 2006, 118, 7211–7214. [Google Scholar] [CrossRef]
- Choi, J.; Jeong, H.K.; Snyder, M.A.; Stoeger, J.A.; Masel, R.I.; Tsapatsis, M. Grain boundary defect elimination in a zeolite membrane by rapid thermal processing. Science 2009, 325, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Falconer, J.L.; Noble, R.D. Improved SAPO-34 membranes for CO2/CH4 separations. Adv. Mater. 2006, 18, 2601–2603. [Google Scholar] [CrossRef]
- Zhang, Y.; Tokay, B.; Funke, H.H.; Falconer, J.L.; Noble, R.D. Template removal from SAPO-34 crystals and membranes. J. Membr. Sci. 2010, 363, 29–35. [Google Scholar] [CrossRef]
- Zhou, R.; Ping, E.W.; Funke, H.H.; Falconer, J.L.; Noble, R.D. Improving SAPO-34 membrane synthesis. J. Membr. Sci. 2013, 444, 384–393. [Google Scholar] [CrossRef]
- Carreon, M.L.; Li, S.; Carreon, M.A. AlPO-18 membranes for CO2/CH4 separation. Chem. Commun. 2012, 48, 2310–2312. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wang, B.; Lu, Z.; Zhou, R.; Chen, X. Alumina-supported AlPO-18 membranes for CO2/CH4 separation. J. Membr. Sci. 2014, 471, 338–346. [Google Scholar] [CrossRef]
- Wang, B.; Hu, N.; Wang, H.; Zheng, Y.; Zhou, R. Improved AlPO-18 membranes for light gas separation. J. Mater. Chem. A 2015, 3, 12205–12212. [Google Scholar] [CrossRef]
- Kalipcilar, H.; Bowen, T.; Noble, R.; Falconer, J. Synthesis and separation performance of SSZ-13 zeolite membranes on tubular supports. Chem. Mater. 2002, 14, 3458–3464. [Google Scholar] [CrossRef]
- Kosinov, N.; Auffret, C.; Borghuis, G.J.; Sripathi, V.G.P.; Hensen, E.J.M. Influence of the Si/Al ratio on the separation properties of SSZ-13 zeolite membranes. J. Membr. Sci. 2015, 484, 140–145. [Google Scholar] [CrossRef]
- Kida, K.; Maeta, Y.; Yogo, K. Preparation and gas permeation properties on pure silica CHA-type zeolite membranes. J. Membr. Sci. 2017, 522, 363–370. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, R.; Bu, N.; Wang, Q.; Zhong, S.; Wang, B.; Hidetoshi, K. Room-temperature ionic liquids modified zeolite SSZ-13 membranes for CO2/CH4 separation. J. Membr. Sci. 2017, 524, 12–19. [Google Scholar] [CrossRef]
- Venna, S.R.; Jasinski, J.B.; Carreon, M.A. Structural evolution of zeolitic imidazolate framework-8. J. Am. Chem. Soc. 2010, 132, 18030–18033. [Google Scholar] [CrossRef] [PubMed]
- Hedin, N.; DeMartin, G.J.; Roth, W.J.; Strohmaier, K.G.; Reyes, S.C. PFG NMR self-diffusion of small hydrocarbons in high silica DDR, CHA and LTA structures. Microporous Mesoporous Mater. 2008, 109, 327–334. [Google Scholar] [CrossRef]
- Noack, M.; Mabande, G.T.P.; Caro, J.; Georgi, G.; Schwieger, W.; Kölsch, P.; Avhale, A. Influence of Si/Al ratio, pre-treatment and measurement conditions on permeation properties of MFI membranes on metallic and ceramic supports. Microporous Mesoporous Mater. 2005, 82, 147–157. [Google Scholar] [CrossRef]
- Caro, J.; Albrecht, D.; Noack, M. Why is it so extremely difficult to prepare shape-selective Al-rich zeolite membranes like LTA and FAU for gas separation? Sep. Purif. Technol. 2009, 66, 143–147. [Google Scholar] [CrossRef]
- Noack, M.; Kölsch, P.; Dittmar, A.; Stöhr, M.; Georgi, G.; Eckelt, R.; Caro, J. Effect of crystal intergrowth supporting substances (ISS) on the permeation properties of MFI membranes with enhanced Al-content. Microporous Mesoporous Mater. 2006, 97, 88–96. [Google Scholar] [CrossRef]
- Kosinov, N.; Auffret, C.; Sripathi, V.G.P.; Gücüyener, C.; Gascon, J.; Kapteijn, F.; Hensen, E.J.M. Influence of support morphologies on the detemplation and permeation of ZSM-5 and SSZ-13 zeolite membranes. Microporous Mesoporous Mater. 2014, 197, 268–277. [Google Scholar] [CrossRef]
- Huang, A.; Wang, N.; Caro, J. Synthesis of multi-layer zeolite LTA membranes with enhanced gas separation performance by using 3-aminopropyltriethoxysilane as interlayer. Microporous Mesoporous Mater. 2012, 164, 294–301. [Google Scholar] [CrossRef]
- Zones, S.I. Zeolite SSZ-13 and Its Method of Preparation. U.S. Patent 4544538, 1 October 1985. [Google Scholar]
- Hasegawa, Y.; Abe, C.; Nishioka, M.; Sato, K.; Nagase, T.; Hanaoka, T. Influence of synthesis gel composition on morphology, composition, and dehydration performance of CHA-type zeolite membranes. J. Membr. Sci. 2010, 363, 256–264. [Google Scholar] [CrossRef]
- Kosinov, N.; Auffret, C.; Gücüyener, C.; Szyja, B.M.; Gascon, J.; Kapteijn, F.; Hensen, E.J.M. High flux high-silica SSZ-13 membrane for CO2 separation. J. Mater. Chem. A 2014, 2, 13083–13092. [Google Scholar] [CrossRef]







| No. | Molar Composition of Precursor Synthesis Gel | Synthesis Time (h) | Gas Permeance | SCO2/CH4 | |||
|---|---|---|---|---|---|---|---|
| SiO2/Al2O3 | H2O/SiO2 | TMAdaOH/SiO2 | CO2 × 107 (mol/(m2∙s∙Pa)) | CH4 × 109 (mol/(m2∙s∙Pa)) | |||
| M-1 | 40 | 80 | 0.10 | 48 | 6.77 | 55.10 | 12 |
| M-2 | 200 | 80 | 0.10 | 48 | 1.41 | 4.67 | 30 |
| M-3 | ∞ | 80 | 0.10 | 48 | 0.98 | 2.10 | 47 |
| M-4 | ∞ | 20 | 0.10 | 48 | 9.20 | 46.00 | 20 |
| M-5 | ∞ | 40 | 0.10 | 48 | 0.73 | 5.55 | 13 |
| M-6 | ∞ | 60 | 0.10 | 48 | 1.00 | 5.20 | 19 |
| M-7 | ∞ | 20 | 0.05 | 48 | 4.15 | 32.00 | 13 |
| M-8 | ∞ | 20 | 0.15 | 48 | 8.20 | 50.00 | 16 |
| M-9 | ∞ | 20 | 0.10 | 24 | 8,50 | 121.00 | 7 |
| M-10 | ∞ | 20 | 0.10 | 36 | 4.94 | 38.00 | 13 |
| M-11 | ∞ | 20 | 0.10 | 72 | 4.05 | 44.00 | 9 |
| Molar Ratio of Precursor Synthesis Gel | Pressure Drop (MPa) | Single Gas Permeance Performance | Reference (-) | ||
|---|---|---|---|---|---|
| H2O/SiO2 | SiO2/Al2O3 | CO2 Permeance × 107 (mol/(m2∙s∙Pa)) | SCO2/CH4 | ||
| 80 | ∞ | 0.40 | 0.98 | 47 | This work |
| 44 | 40 | 0.05 | 1.00 | 11 | [19] |
| 42 | 50 | 0.60 | 3.00 | 20 | [32] |
| 80 | 40 | 0.20 | 2.00 | 360 | [3] |
| 5.7 | ∞ | 0.30 | 15.00 | 45 | [21] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, L.; Zhu, M.; Chen, L.; Zhong, C.; Yang, Y.; Wu, T.; Wang, H.; Kumakiri, I.; Chen, X.; Kita, H. Single Gas Permeance Performance of High Silica SSZ-13 Zeolite Membranes. Membranes 2018, 8, 43. https://doi.org/10.3390/membranes8030043
Liang L, Zhu M, Chen L, Zhong C, Yang Y, Wu T, Wang H, Kumakiri I, Chen X, Kita H. Single Gas Permeance Performance of High Silica SSZ-13 Zeolite Membranes. Membranes. 2018; 8(3):43. https://doi.org/10.3390/membranes8030043
Chicago/Turabian StyleLiang, Li, Meihua Zhu, Le Chen, Caijun Zhong, Yiming Yang, Ting Wu, Heli Wang, Izumi Kumakiri, Xiangshu Chen, and Hidetoshi Kita. 2018. "Single Gas Permeance Performance of High Silica SSZ-13 Zeolite Membranes" Membranes 8, no. 3: 43. https://doi.org/10.3390/membranes8030043
APA StyleLiang, L., Zhu, M., Chen, L., Zhong, C., Yang, Y., Wu, T., Wang, H., Kumakiri, I., Chen, X., & Kita, H. (2018). Single Gas Permeance Performance of High Silica SSZ-13 Zeolite Membranes. Membranes, 8(3), 43. https://doi.org/10.3390/membranes8030043