Air Backwash Efficiency on Organic Fouling of UF Membranes Applied to Shellfish Hatchery Effluents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seawater and Effluents
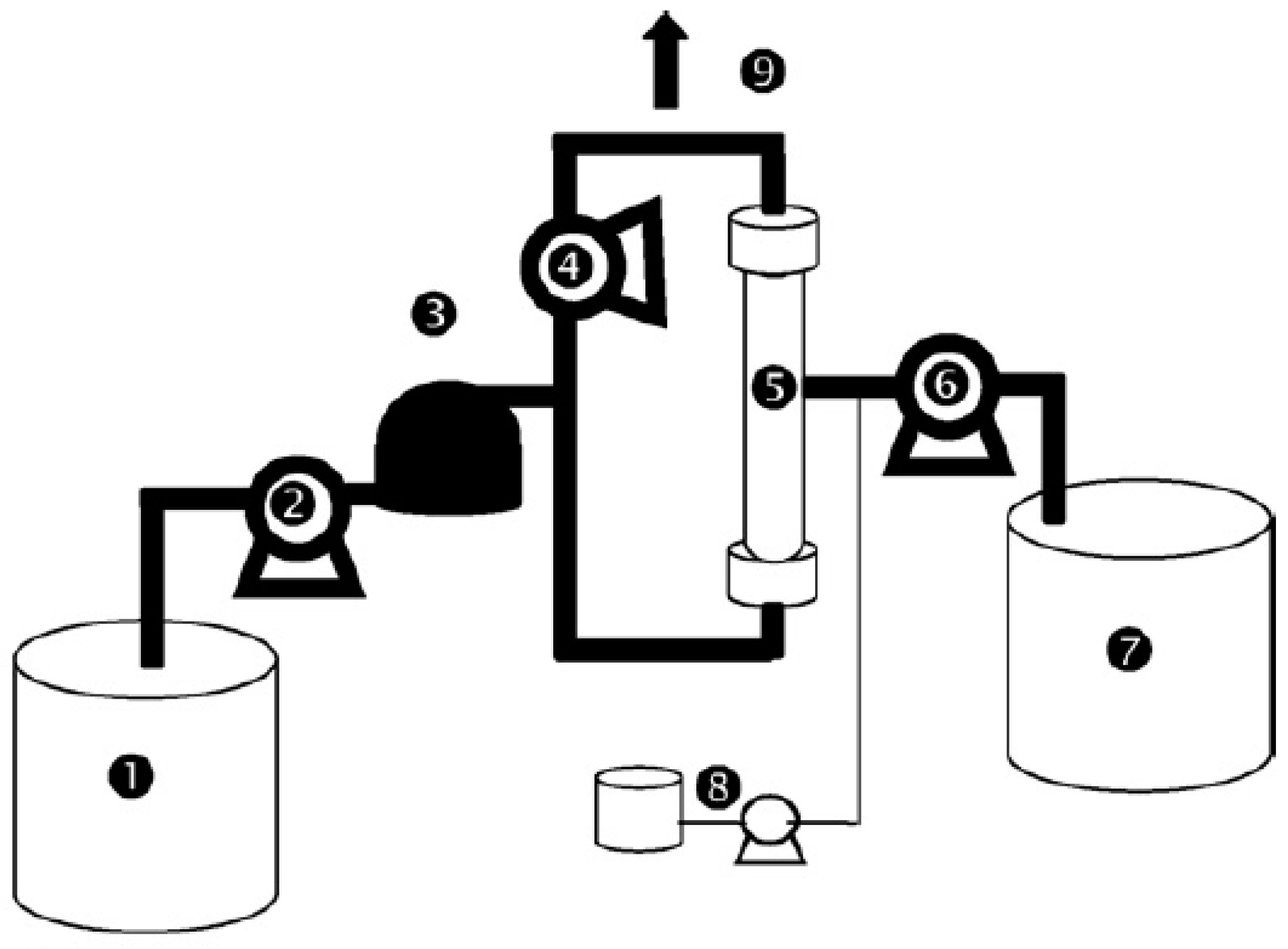
2.2. Ultrafiltration: Membrane and Pilot Plant
2.3. Membrane Cleanings
2.4. Analyses
2.5. Operating Conditions
3. Results
3.1. Seawater
3.2. Shellfish Hatchery Effluent
3.2.1. Hydraulic Effect
3.2.2. Impact on Biologic Matter Integrity
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Di Zio, A.; Prisciandaro, M.; Barba, D. Disinfection of surface waters with UF membranes. Desalination 2005, 179, 297–305. [Google Scholar] [CrossRef]
- Van Hoof, S.C.J.M.; Minnery, J.G.; Mack, B. Dead-end ultrafiltration as alternative pre-treatment to reverse osmosis in seawater desalination: A case study. Desalination 2001, 139, 161–168. [Google Scholar] [CrossRef]
- Brehant, A.; Bonnelye, V.; Perez, M. Comparison of MF/UF pretreatment with conventional filtration prior to RO membranes for surface seawater desalination. Desalination 2002, 144, 353–360. [Google Scholar] [CrossRef]
- Pérez-Gálvez, R.; Guadix, E.M.; Bergé, J.-P.; Guadix, A. Operation and cleaning of ceramic membranes for the filtration of fish press liquor. J. Membr. Sci. 2011, 384, 142–148. [Google Scholar] [CrossRef]
- Serra, C.; Clifton, M.J.; Moulin, P.; Rouch, J.-C.; Aptel, P. Dead-end ultrafiltration in hollow fiber modules: Module design and process simulation. J. Membr. Sci. 1998, 145, 159–172. [Google Scholar] [CrossRef]
- Laîné, J.-M.; Vial, D.; Moulart, P. Status after 10 years of operation—Overview of UF technology today. Desalination 2000, 131, 17–25. [Google Scholar] [CrossRef]
- Shi, X.; Tal, G.; Hankins, N.P.; Gitis, V. Fouling and cleaning of ultrafiltration membranes: A review. J. Water Process Eng. 2014, 1, 121–138. [Google Scholar] [CrossRef]
- Chang, H.; Liang, H.; Qu, F.; Liu, B.; Yu, H.; Du, X.; Li, G.; Snyder, S.A. Hydraulic backwashing for low-pressure membranes in drinking water treatment: A review. J. Membr. Sci. 2017, 540, 362–380. [Google Scholar] [CrossRef]
- Ma, H.; Bowman, C.N.; Davis, R.H. Membrane fouling reduction by backpulsing and surface modification. J. Membr. Sci. 2000, 173, 191–200. [Google Scholar] [CrossRef]
- Gu, H.; Rahardianto, A.; Gao, L.X.; Christofides, P.D.; Cohen, Y. Ultrafiltration with self-generated RO concentrate pulse backwash in a novel integrated seawater desalination UF-RO system. J. Membr. Sci. 2016, 520, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Krishna, P.; Hobbs, C.; Kim, D.; Cho, J. Variations in backwash efficiency during colloidal filtration of hollow-fiber microfiltration membranes. Desalination 2005, 173, 257–268. [Google Scholar] [CrossRef]
- Xu, Y.; Dodds, J.; Leclerc, D. Optimization of a discontinuous microfiltration-backwash process. Chem. Eng. J. Biochem. Eng. J. 1995, 57, 247–251. [Google Scholar] [CrossRef]
- Chellam, S.; Jacangelo, J.; Bonacquisti, T. Modeling and experimental verification of pilot-scale hollow fiber, direct flow microfiltration with periodic backwashing. Environ. Sci. Technol. 1998, 32, 75–81. [Google Scholar] [CrossRef]
- Hillis, P.; Padley, M.B.; Powell, N.I.; Gallagher, P.M. Effects of backwash conditions on out-to-in membrane microfiltration. Desalination 1998, 118, 197–204. [Google Scholar] [CrossRef]
- Kennedy, M.; Kim, S.; Mutenyo, I.; Broens, L.; Schippers, J. Intermittent crossflushing of hollow fiber ultrafiltration systems. Desalination 1998, 118, 175–187. [Google Scholar] [CrossRef]
- Decarolis, J.; Hong, S.; Taylor, J. Fouling behavior of a pilot scale inside-out hollow fiber UF membrane during dead-end filtration of tertiary wastewater. J. Membr. Sci. 2001, 191, 165–178. [Google Scholar] [CrossRef]
- Chen, J.P.; Kim, S.L.; Ting, Y.P. Optimization of membrane physical and chemical cleaning by a statistically designed approach. J. Membr. Sci. 2003, 219, 27–45. [Google Scholar] [CrossRef]
- Guigui, C.; Mougenot, M.; Cabassud, C. Air sparging backwash in ultrafiltration hollow fibres for drinking water production. Water Sci. Technol. Water Supply 2003, 3, 415–422. [Google Scholar] [CrossRef]
- Smith, P.J.; Vigneswaran, S.; Ngo, H.H.; Ben-Aim, R.; Nguyen, H. A new approach to backwash initiation in membrane systems. J. Membr. Sci. 2006, 278, 381–389. [Google Scholar] [CrossRef]
- Chang, H.; Qu, F.; Liu, B.; Yu, H.; Li, K.; Shao, S.; Li, G.; Liang, H. Hydraulic irreversibility of ultrafiltration membrane fouling by humic acid: Effects of membrane properties and backwash water composition. J. Membr. Sci. 2015, 493, 723–733. [Google Scholar] [CrossRef]
- Chang, H.; Liang, H.; Qu, F.; Shao, S.; Yu, H.; Liu, B.; Gao, W.; Li, G. Role of backwash water composition in alleviating ultrafiltration membrane fouling by sodium alginate and the effectiveness of salt backwashing. J. Membr. Sci. 2016, 499, 429–441. [Google Scholar] [CrossRef]
- Cabassud, C.; Laborie, S.; Lainé, J.M. How slug flow can improve ultrafiltration flux in organic hollow fibres. J. Membr. Sci. 1997, 128, 93–101. [Google Scholar] [CrossRef]
- Hu, A.; Stuckey, D. Treatment of dilute wastewaters using a novel submerged anaerobic membrane bioreactor. J. Environ. Eng. 2006, 132, 190–198. [Google Scholar] [CrossRef]
- Laborie, S.; Cabassud, C.; Durand-Bourlier, L.; Lainé, J.M. Fouling control by air sparging inside hollow fibre membranes—Effects on energy consumption. Desalination 1998, 118, 189–196. [Google Scholar] [CrossRef]
- Remize, P.J.; Guigui, C.; Cabassud, C. Evaluation of backwash efficiency, definition of remaining fouling and characterisation of its contribution in irreversible fouling: Case of drinking water production by air-assisted ultra-filtration. J. Membr. Sci. 2010, 355, 104–111. [Google Scholar] [CrossRef]
- Serra, C.; Durand-Bourlier, L.; Clifton, M.J.; Moulin, P.; Rouch, J.-C.; Aptel, P. Use of air sparging to improve backwash efficiency in hollow-fiber modules. J. Membr. Sci. 1999, 161, 95–113. [Google Scholar] [CrossRef]
- Lipp, P.; Baldauf, G. Application of out—In MF/UF-systems for drinking water treatment with air supported backwash—Three case studies. Desalination 2002, 147, 63–68. [Google Scholar] [CrossRef]
- Lipp, P.; Witte, M.; Baldauf, G.; Povorov, A.A. Treatment of reservoir water with a backwashable MF/UF spiral wound membrane. Desalination 2005, 179, 83–94. [Google Scholar] [CrossRef]
- Bessiere, Y.; Guigui, C.; Remize, P.J.; Cabassud, C. Coupling air-assisted backwash and rinsing steps: A new way to improve ultrafiltration process operation for inside-out hollow fibre modules. Desalination 2009, 240, 71–77. [Google Scholar] [CrossRef]
- Xu, J.; Ruan, G.; Gao, X.; Pan, X.; Su, B.; Gao, C. Pilot study of inside-out and outside-in hollow fiber UF modules as direct pretreatment of seawater at low temperature for reverse osmosis. Desalination 2008, 219, 179–189. [Google Scholar] [CrossRef]
- Moll, R.; Veyret, D.; Charbit, F.; Moulin, P. Dean vortices applied to membrane process—Part I. Experimental approach. J. Membr. Sci. 2007, 288, 307–320. [Google Scholar] [CrossRef]
- Le Goic, N.; Hegaret, H.; Boulais, M.; Beguel, J.-P.; Lambert, C.; Fabioux, C.; Soudant, P. Flow cytometric assessment of morphology, viability, and production of reactive oxygen species of Crassostrea gigas oocytes. application to toxic dinoflagellate (Alexandrium minutum) exposure. Cytometry Part A 2014, 85A, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Field, R.W.; Pearce, G.K. Critical, sustainable and threshold fluxes for membrane filtration with water industry applications. Adv. Colloid Interface Sci. 2011, 164, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Guilbaud, J.; Wyart, Y.; Klaag, K.; Moulin, P. Comparison of seawater and freshwater ultrafiltration on semi-industrial scale: Ballast water treatment application. J. Membr. Sci. Res. 2018, 4, 136–145. [Google Scholar]
- Massé, A.; Arab, O.; Séchet, V.; Jaouen, P.; Pontié, M.; Sabiri, N.-E.; Plantier, S. Performances of dead-end ultrafiltration of seawater: From the filtration and backwash efficiencies to the membrane fouling mechanisms. Sep. Purif. Technol. 2015, 156, 512–521. [Google Scholar] [CrossRef]








| Characteristics | Min. | Max. | Average |
|---|---|---|---|
| Turbidity (NFU) | 0.674 | 13.4 | 4.5 |
| Salinity (%) | 30.8 | 34.7 | 33.5 |
| Chlorophyll a (µg L−1) | 0.89 | 7.04 | 2.5 |
| Pheo-pigments (µg L−1) | 0.24 | 1.84 | 0.71 |
| Characteristics | ||
|---|---|---|
| Material | Polyethersulfone |  |
| Inside diameter (mm) | 0.9 | |
| Molecular weight cut off (kDa) | 200 | |
| Numbers of channels | 7 | |
| Module area (m²) | 8 | |
| Water permeability (L h−1 m −2 bar−1) | 1000 | |
| Operating Parameters | CB | AB | CEB |
|---|---|---|---|
| Volume of permeate (L) | 32 | 50 | 100 |
| Time (min) | 1 | 5 | 60 |
| Frequency | 20–60 min | 5 CB | 72 h |
| Time | Membrane Inlet | Backwash | Air Backwash | Permeate |
|---|---|---|---|---|
| Day 1 | 30,705 spz mL−1 | 120,560 spz mL−1 | 8,345 spz mL−1 | spz mL−1 < limit of detection |
 |  |  | ||
| Day 2 | 18,305 spz mL−1 | 73,840 spz mL−1 | 10,170 spz mL−1 | spz mL−1 < limit of detection |
 |  |  | ||
| Time | Membrane Inlet | Backwash | Air Backwash | Permeate |
|---|---|---|---|---|
| Day 1 | 45 oo mL−1 16.67% | 165 oo mL−1 17.14% | 185 oo mL−1 62.50% | 0 oo mL−1 - |
 |  |  | ||
| Day 2 | 30 oo mL−1 14.29% | 85 oo mL−1 5.56% | 30 oo mL−1 33% | 0 oo mL−1 - |
 |  |  | ||
| Time | Membrane Inlet | Backwash | Air Backwash | Permeate |
|---|---|---|---|---|
| Day 1 | 5115 spz mL−1 | 22,485 spz mL−1 | 8100 spz mL−1 | spz mL−1 < limit of detection |
 |  |  | ||
| Day 2 | 6770 spz mL−1 | 53,850 spz mL−1 | 10,340 spz mL−1 | spz mL−1 < limit of detection |
 |  |  | ||
| Time | Membrane Inlet | Backwash | Air Backwash | Permeate |
|---|---|---|---|---|
| Day 1 | 55 oo mL−1 0.0% | 530 oo mL−1 4.17% | 390 oo mL−1 5.32% | 0 oo mL−1 - |
 |  |  | ||
| Day 2 | 70 oo mL−1 5.56% | 750 oo mL−1 2.86% | 570 oo mL−1 19.18% | 0 oo mL−1 - |
 |  |  | ||
| Test | Membrane Inlet | Backwash | Air Backwash | Permeate |
|---|---|---|---|---|
| Test 1 | 29,515 spz mL−1 | 121,600 spz mL−1 | 44,175 spz mL−1 | spz mL−1 < limit of detection |
 |  |  | ||
| Test 2 | 192,495 spz mL−1 | 466,715 spz mL−1 | 11,560 spz mL−1 | spz mL−1 < limit of detection |
 |  |  | ||
| Test | Membrane Inlet | Backwash | Air Backwash | Permeate |
|---|---|---|---|---|
| Test 1 | 255 oo.mL−1 8.06% | 1430 oo.mL−1 11.73% | 1355 oo.mL−1 48.47% | 0 oo.mL−1 - |
 |  |  | ||
| Test 2 | 400 oo.mL−1 5.38% | 2360 oo.mL−1 6.14% | 1535 oo.mL−1 49.68% | 0 oo.mL−1 - |
 |  |  | ||
| Water Sample | Sample | Number of Spermatozoa | Moving Spermatozoa (%) |
|---|---|---|---|
| Before filtration | 1 | 79 | 19 |
| 2 | 84 | 24 | |
| 3 | 84 | 15 | |
| CB | 1 | 20 | 10 |
| 2 | 61 | 8 | |
| 3 | 83 | 4 | |
| AB | 1 | 108 | 0 |
| 2 | 134 | 0 | |
| 3 | 98 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordier, C.; Stavrakakis, C.; Sauvade, P.; Coelho, F.; Moulin, P. Air Backwash Efficiency on Organic Fouling of UF Membranes Applied to Shellfish Hatchery Effluents. Membranes 2018, 8, 48. https://doi.org/10.3390/membranes8030048
Cordier C, Stavrakakis C, Sauvade P, Coelho F, Moulin P. Air Backwash Efficiency on Organic Fouling of UF Membranes Applied to Shellfish Hatchery Effluents. Membranes. 2018; 8(3):48. https://doi.org/10.3390/membranes8030048
Chicago/Turabian StyleCordier, Clémence, Christophe Stavrakakis, Patrick Sauvade, Franz Coelho, and Philippe Moulin. 2018. "Air Backwash Efficiency on Organic Fouling of UF Membranes Applied to Shellfish Hatchery Effluents" Membranes 8, no. 3: 48. https://doi.org/10.3390/membranes8030048
APA StyleCordier, C., Stavrakakis, C., Sauvade, P., Coelho, F., & Moulin, P. (2018). Air Backwash Efficiency on Organic Fouling of UF Membranes Applied to Shellfish Hatchery Effluents. Membranes, 8(3), 48. https://doi.org/10.3390/membranes8030048






