Extraction Kinetics of As(V) by Aliquat-336 Using Asymmetric PVDF Hollow-Fiber Membrane Contactors
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
2.2. Preparation of PVDF Microporous Hollow-Fiber (HF) Membranes by Dry-Wet Spinning Process
2.3. Non-Dispersed Solvent Extraction of As(V) by Aliquat-336
- d: inside hollow fiber diameter;
- v: aqueous flow velocity;
- L: length of the fiber;
- H: partition coefficient;
- and are the aqueous and the organic flow rates, respectively;
- and are the aqueous and the organic reservoir volumes, respectively;
- , and are the aqueous feed concentration at time t, the initial aqueous feed concentration and initial organic concentration, respectively.
3. Results and Discussion
3.1. SEM Analysis
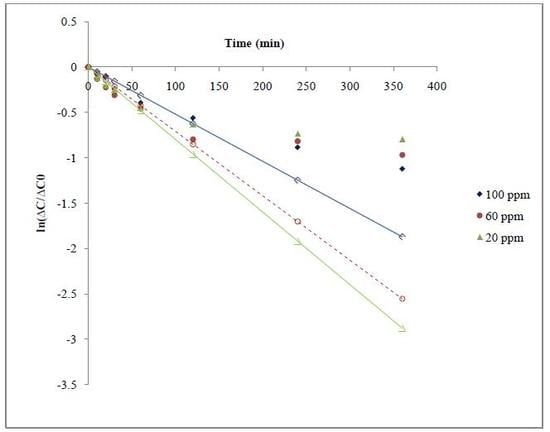
3.2. Solvent Extraction Studies of As(V) by Means of Hollow-Fiber Membrane Contactors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jeon, C.S.; Kitae, B.; Park, J.K.; Oh, Y.K.; Lee, S.D. Adsorption characteristics of As(V) on iron-coated zeolite. J. Hazard. Mater. 2009, 163, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Wang, J.; Hou, D.; Luan, Z.; Fan, B.; Zhao, C. Experimental study of arsenic removal by direct contact membrane distillation. J. Hazard. Mater. 2009, 163, 874–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazari, A.M.; Radzinski, R.; Ghahreman, A. Review of arsenic metallurgy: Treatment of arsenical minerals and the immobilization of arsenic. Hydrometallurgy 2017, 174, 258–281. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Zouboulis, A.I. Removal of arsenic from contaminated water sources by sorption onto iron-oxide-coated polymeric materials. Water Res. 2002, 36, 5141–5155. [Google Scholar] [CrossRef]
- Chuang, C.L.; Fan, M.; Xu, M.; Brown, R.C.; Sung, S.; Saha, B.; Huang, C.P. Adsorption of arsenic(V) by activated carbon prepared from oat hulls. Chemosphere 2005, 61, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Stüben, D.; Berner, Z. Arsenic removal from water using natural iron mineral-quartz sand columns. Sci. Total Environ. 2007, 377, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Lohokare, H.R.; Muthu, M.R.; Agarwal, G.P.; Kharul, U.K. Effective arsenic removal using polyacrylonitrile-based ultrafiltration (UF) membrane. J. Membr. Sci. 2008, 320, 159–166. [Google Scholar] [CrossRef]
- Lv, J.; Wang, K.Y.; Chung, T.S. Investigation of amphoteric polybenzimidazole (PBI) nanofiltration hollow fiber membrane for both cation and anions removal. J. Membr. Sci. 2008, 310, 557–566. [Google Scholar] [CrossRef]
- Perez, M.E.M.; Reyes-Aguilera, J.A.; Saucedo, T.I.; Gonzalez, M.P.; Navarro, R.; Avila-Rodriguez, M. Study of As(V) transfer through a supported liquid membrane impregnated with trioctylphosphine oxide (Cyanex 921). J. Membr. Sci. 2007, 302, 119–126. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Tresintsi, S.; Simeonidis, K.; Katsikini, M.; Palourac, E.C.; Bantsis, G.; Mitrakas, M. A novel approach for arsenic adsorbents regeneration using MgO. J. Hazard. Mater. 2014, 265, 217–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Wang, J.; Wang, L.; Sheng, G.; Liu, J.; Yu, H.; Huang, X.J. Enhanced arsenic removal from water by hierarchically porous CeO2-ZrO2 nanospheres: Role of surface- and structure-dependent properties. J. Hazard. Mater. 2013, 260, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Pancharoena, U.; Poonkuma, W.; Lothongkum, A.W. Treatment of arsenic ions from produced water through hollow fiber supported liquid membrane. J. Alloy. Compd. 2009, 482, 328–334. [Google Scholar] [CrossRef]
- Güella, R.; Fontàs, C.; Anticó, E.; Salvadó, V.; Crespo, J.G.; Velizarov, S. Transport and separation of arsenate and arsenite from aqueous media by supported liquid and anion-exchange membranes. Sep. Purif. Technol. 2011, 80, 428–434. [Google Scholar] [CrossRef]
- Bey, S.; Criscuoli, A.; Figoli, A.; Leopold, A.; Simone, S.; Benamor, M.; Drioli, E. Removal of As(V) by PVDF hollow fibers membrane contactors using Aliquat-336 as extractant. Desalination 2010, 264, 193–200. [Google Scholar] [CrossRef]
- Srivastava, A.; Bhagat, A.; Sharma, U.; Dohare, R.K.; Singh, K.; Upadhyaya, S. Comparative study of arsenic(V) removal from aqueous solution usingAliquat-336 and 2-ethyl hexanol through emulsion liquid membrane. J. Water Process Eng. 2017, 16, 64–68. [Google Scholar] [CrossRef]
- Marino, T.; Figoli, A. Arsenic Removal by Liquid Membranes. Membranes 2015, 5, 150–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lourdes, B.M.; de Rodríguez, S.M.E.; de MT, J.R.; Silva, O.; Muñoz, M.; de Gyves, J. Arsenic(V) removal with polymer inclusion membranes from sulfuric acid media using DBBP as carrier. Environ. Sci. Technol. 2004, 38, 886–891. [Google Scholar]
- Elia, N.A.D.; Dahuron, L.; Cussler, E.L. Liquid-liquid extractions with microporous hollow fibers. J. Membr. Sci. 1986, 29, 309–319. [Google Scholar] [CrossRef]
- Yang, C.; Cussler, E.L. Reaction dependent extraction of copper and nickel using hollow fibers. J. Membr. Sci. 2000, 166, 229–238. [Google Scholar] [CrossRef]




| Liquid Membranes | Feed Phase | Organic Phase | Membrane | Stripping Phase | Ref. |
|---|---|---|---|---|---|
| SLM | As(V) [H2SO4] = 2 M | Cyanex-921 | PVDF-millipore GVHPO4700 | [Na2SO4] = 1 M | [9] |
| HF SLM | Produced water in gas plant [As] = 1.2842 ppm | Cyanex-923; Cyanex-301 TOA; Aliquat-336 | Celgard-X-30240-PP HF | [NaOH] = 0.5 M | [13] |
| SLM | [As] = 10 ppm | Aliquat-336: 0.5 M | PVDF Durapore | [NaCl] = 0.1 M | [14] |
| PIM | [As(V)] = 3000 ppm [H2SO4] = 2 M | DBBP | Cellulose tri-acetate | [LiCl] = 2 M | [18] |
| Membrane contactors | [As] = 50 ppm | Aliquat-336 | PVDF asymmetric hollow fiber | - | [15] |
| ELM | [As] = 100 ppm | Aliquat-336 + Span 80 2-ethyl hexanol + Span 80 | - | [NaOH] = 0.5 M | [16] |
| Properties | Description |
|---|---|
| Material | PVDF |
| Hollow fiber inner diameter (mm) | 1.35 |
| Pore size (µm) | 0.11 |
| Number of fibers | 3 |
| Porosity (%) | 80 |
| Surface (cm2) | 22.9 |
| Module diameter (cm) | 1 |
| Module length (cm) | 18 |
| Parameters | K × 106 (cm/s) | |
|---|---|---|
| pH | 12.5 | 0 |
| 8.2 | 7.54% ± 2% | |
| 6.98 | 6.05% ± 2% | |
| 4.5 | 2.11% ± 1% | |
| Initial As(V) concentration (ppm) | 100 | 7.04% ± 3% |
| 60 | 6.05% ± 2% | |
| 20 | 4.96% ± 1.5% | |
| Temperature (°C) | 25 | 6.05% ± 2% |
| 40 | 6.45% ± 2.5% | |
| 50 | 3.45% ± 1% | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bey, S.; Semghouni, H.; Criscuoli, A.; Benamor, M.; Drioli, E.; Figoli, A. Extraction Kinetics of As(V) by Aliquat-336 Using Asymmetric PVDF Hollow-Fiber Membrane Contactors. Membranes 2018, 8, 53. https://doi.org/10.3390/membranes8030053
Bey S, Semghouni H, Criscuoli A, Benamor M, Drioli E, Figoli A. Extraction Kinetics of As(V) by Aliquat-336 Using Asymmetric PVDF Hollow-Fiber Membrane Contactors. Membranes. 2018; 8(3):53. https://doi.org/10.3390/membranes8030053
Chicago/Turabian StyleBey, Said, Hassina Semghouni, Alessandra Criscuoli, Mohamed Benamor, Enrico Drioli, and Alberto Figoli. 2018. "Extraction Kinetics of As(V) by Aliquat-336 Using Asymmetric PVDF Hollow-Fiber Membrane Contactors" Membranes 8, no. 3: 53. https://doi.org/10.3390/membranes8030053
APA StyleBey, S., Semghouni, H., Criscuoli, A., Benamor, M., Drioli, E., & Figoli, A. (2018). Extraction Kinetics of As(V) by Aliquat-336 Using Asymmetric PVDF Hollow-Fiber Membrane Contactors. Membranes, 8(3), 53. https://doi.org/10.3390/membranes8030053