A Review on Properties of Natural and Synthetic Based Electrospun Fibrous Materials for Bone Tissue Engineering
Abstract
1. Introduction
2. Native Bone Structure and Composition
2.1. Composition of Bone
2.2. Cellular Organization and Bone Remodeling
2.2.1. Osteoblast (Bone Forming Cells)
2.2.2. Osteoclasts (Bone Resorption Cells)
2.2.3. Osteocytes
2.2.4. Bone Lining Cells
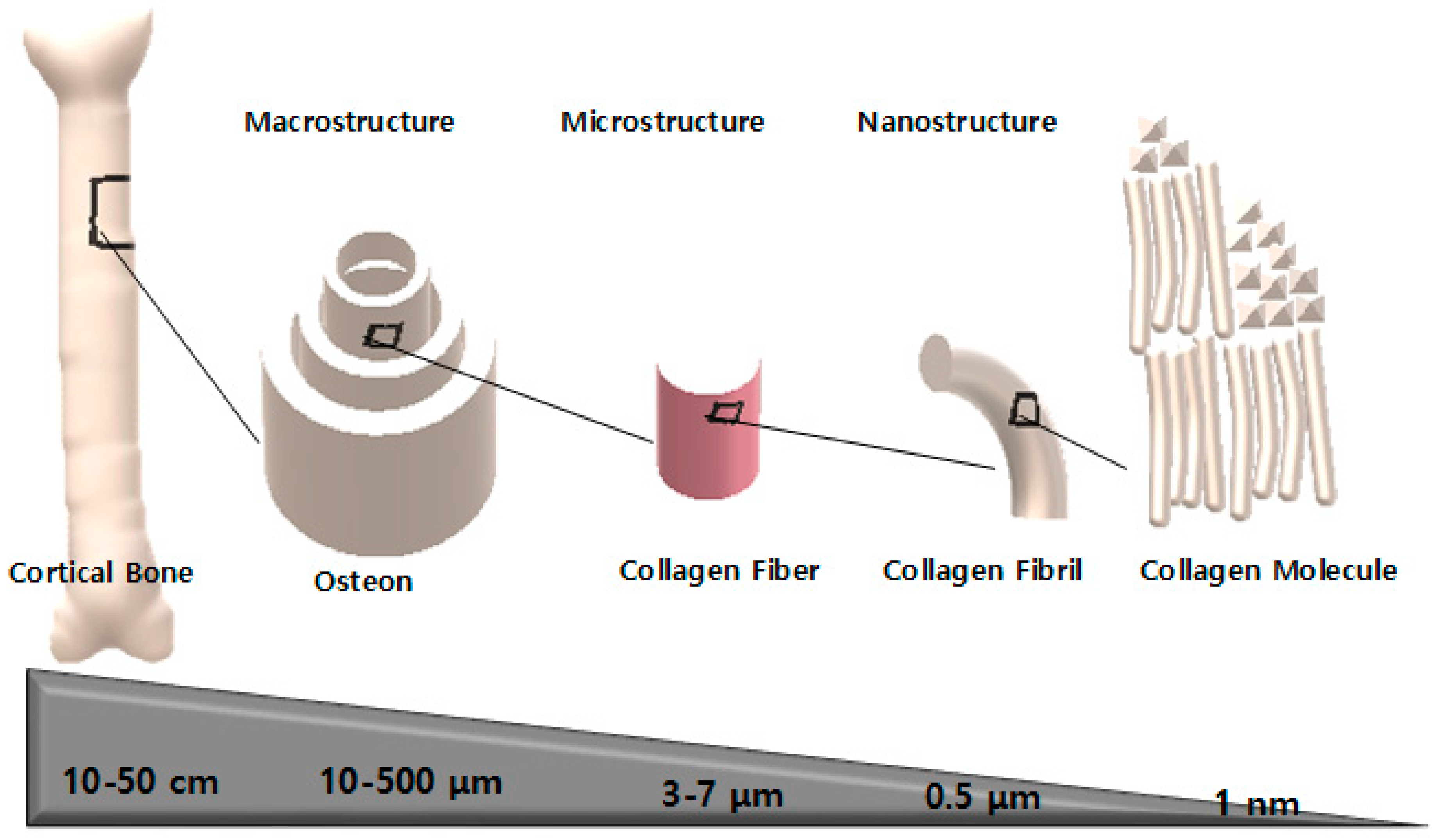
2.3. Hierarchical Bone Structure
2.3.1. Macrostructure
2.3.2. Microstructure
2.3.3. Nanostructure
2.4. Biomimicking and Bone Tissue Engineering
Biomimetic
2.5. Polymers Used in Bone Tissue Engineering
3. Principle of Electrospinning
3.1. Factors Affecting the Electrospinning Process
3.1.1. Solvent Effect
3.1.2. Substrates Effect
3.1.3. Polymer Solution Properties
3.1.4. Ambient Factor
3.1.5. Operation Factor
3.2. Electrospun Materials for Bone Tissue Engineering
3.3. Polymers Used in Electrospinning
3.4. Electrospun Scaffolds from Natural Polymer
3.4.1. Silk
3.4.2. Collagen
3.4.3. Gelatin
3.5. Electrospun Scaffolds from Synthetic Polymer
3.5.1. Polycaprolactone (PCL)
3.5.2. Polylactic Acid (PLA)
3.5.3. Polyglycolic Acid (PGA)
3.5.4. Polyethylene Glycol (PEG)
3.6. Electrospun Scaffolds from Polymer Blends
3.7. Copolymers in Electrospinning
3.7.1. Poly(lactic-co-glycolic acid) (PLGA)
3.7.2. Polylactic Acid-co-polyethylene Glycol (PLA-PEG)
3.8. Polymer-Ceramic Composites
4. Clinical Applications of Scaffolds in Medicine
5. Current Development and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2010, 6, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009, 8, 457. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xia, Z.; Czernuszka, J.T. Design and Development of Three-Dimensional Scaffolds for Tissue Engineering. Chem. Eng. Res. Des. 2007, 85, 1051–1064. [Google Scholar] [CrossRef]
- Lyons, F.G.; Al-Munajjed, A.A.; Kieran, S.M.; Toner, M.E.; Murphy, C.M.; Duffy, G.P.; O’Brien, F.J. The healing of bony defects by cell-free collagen-based scaffolds compared to stem cell-seeded tissue engineered constructs. Biomaterials 2010, 31, 9232–9243. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Valmikinathan, C.M.; Liu, W.; Laurencin, C.T.; Yu, X. Spiral-structured, nanofibrous, 3D scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2010, 93, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.Z.; Ma, J.; He, W.; Ge, Z.L.; Lu, Q.; Kaplan, D.L. Simulation of ECM with silk and chitosan nanocomposite materials. J. Mater. Chem. B 2017, 5, 4789–4796. [Google Scholar] [CrossRef] [PubMed]
- Al-Tamimi, A.A.; Fernandes, P.R.A.; Peach, C.; Cooper, G.; Diver, C.; Bartolo, P.J. Metallic bone fixation implants: A novel design approach for reducing the stress shielding phenomenon. Virtual Phys. Prototyp. 2017, 12, 141–151. [Google Scholar] [CrossRef]
- Maisani, M.; Ziane, S.; Ehret, C.; Levesque, L.; Siadous, R.; Le Meins, J.F.; Chevallier, P.; Barthélémy, P.; De Oliveira, H.; Amédée, J. A new composite hydrogel combining the biological properties of collagen with the mechanical properties of a supramolecular scaffold for bone tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, e1489–e1500. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Tian, T.; Shi, S.; Xie, X.; Ma, Q.; Li, G.; Lin, Y. The fabrication of biomimetic biphasic CAN-PAC hydrogel with a seamless interfacial layer applied in osteochondral defect repair. Bone Res. 2017, 5, 17018. [Google Scholar] [CrossRef] [PubMed]
- Frasca, S.; Norol, F.; Le Visage, C.; Collombet, J.-M.; Letourneur, D.; Holy, X.; Sari Ali, E. Calcium-phosphate ceramics and polysaccharide-based hydrogel scaffolds combined with mesenchymal stem cell differently support bone repair in rats. J. Mater. Sci. Mater. Med. 2017, 28, 35. [Google Scholar] [CrossRef] [PubMed]
- Rong, D.; Chen, P.; Yang, Y.; Li, Q.; Wan, W.; Fang, X.; Zhang, J.; Han, Z.; Tian, J.; Ouyang, J. Fabrication of gelatin/PCL electrospun fiber mat with bone powder and the study of its biocompatibility. J. Funct. Biomater. 2016, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, L.; Cheng, R.; Cui, W. ECM Decorated Electrospun Nanofiber for Improving Bone Tissue Regeneration. Polymers 2018, 10, 272. [Google Scholar] [CrossRef]
- Ramin, K.; Mina, A.; Abbas, B. Electrospun biodegradable nanofibers scaffolds for bone tissue engineering. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Huijie, J.; Pengzhen, C.; Donglin, L.; Junqin, L.; Jimeng, W.; Yi, G.; Shuaishuai, Z.; Tianqing, C.; Chunmei, W.; Liu, Y.; et al. Novel standardized massive bone defect model in rats employing an internal eight-hole stainless steel plate for bone tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, e2162–e2171. [Google Scholar]
- Fousová, M.; Kubásek, J.; Vojtěch, D.; Fojt, J.; Čapek, J. 3D printed porous stainless steel for potential use in medicine. IOP Conf. Ser. Mater. Sci. Eng. 2017, 179, 012025. [Google Scholar] [CrossRef]
- Bhattarai, D.P.; Shrestha, S.; Shrestha, B.K.; Park, C.H.; Kim, C.S. A controlled surface geometry of polyaniline doped titania nanotubes biointerface for accelerating MC3T3-E1 cells growth in bone tissue engineering. Chem. Eng. J. 2018, 350, 57–68. [Google Scholar] [CrossRef]
- Xiong, J.Y.; Li, Y.C.; Wang, X.J.; Hodgson, P.D.; Wen, C.E. Titanium–nickel shape memory alloy foams for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2008, 1, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Tajbakhsh, S.; Hajiali, F. A comprehensive study on the fabrication and properties of biocomposites of poly(lactic acid)/ceramics for bone tissue engineering. Mater. Sci. Eng. C 2017, 70, 897–912. [Google Scholar] [CrossRef] [PubMed]
- Johari, N.; Madaah Hosseini, H.R.; Samadikuchaksaraei, A. Optimized composition of nanocomposite scaffolds formed from silk fibroin and nano-TiO2 for bone tissue engineering. Mater. Sci. Eng. C 2017, 79, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yu, X.; Li, Y.; Su, Z.; Jandt, K.D.; Wei, G. Protein-mimetic peptide nanofibers: Motif design, self-assembly synthesis, and sequence-specific biomedical applications. Prog. Polym. Sci. 2018, 80, 94–124. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, D.; Wang, H.; Li, J.; Nienhaus, G.U.; Su, Z.; Wei, G.; Shang, L. Supramolecular Self-Assembly Bioinspired Synthesis of Luminescent Gold Nanocluster-Embedded Peptide Nanofibers for Temperature Sensing and Cellular Imaging. Bioconj. Chem. 2017, 28, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Natalello, A.; Pugliese, R.; Gelain, F. Fabrication of nanofibrous electrospun scaffolds from a heterogeneous library of co- and self-assembling peptides. Acta Biomater. 2017, 51, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.-Y.; Jing, X.; Napiwocki, B.N.; Li, Z.-T.; Turng, L.-S.; Huang, H.-X. Fabrication of fibrous silica sponges by self-assembly electrospinning and their application in tissue engineering for three-dimensional tissue regeneration. Chem. Eng. J. 2018, 331, 652–662. [Google Scholar] [CrossRef]
- Agathe, G.; Vera, G.; Reine, B.; Valérie, H.; Simon, L.; Nicolas, L.H.; Jean-Christophe, F.; Sylvain, C.; Damien, L.N. Characterization of printed PLA scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2018, 106, 887–894. [Google Scholar]
- Pant, H.R.; Neupane, M.P.; Pant, B.; Panthi, G.; Oh, H.-J.; Lee, M.H.; Kim, H.Y. Fabrication of highly porous poly (ɛ-caprolactone) fibers for novel tissue scaffold via water-bath electrospinning. Colloids Surf. B Biointerfaces 2011, 88, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Abdal-hay, A.; Pant, H.R.; Lim, J.K. Super-hydrophilic electrospun nylon-6/hydroxyapatite membrane for bone tissue engineering. Eur. Polym. J. 2013, 49, 1314–1321. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Mousa, H.M.; Tiwari, A.P.; Ko, S.W.; Park, C.H.; Kim, C.S. Development of polyamide-6, 6/chitosan electrospun hybrid nanofibrous scaffolds for tissue engineering application. Carbohydr. Polym. 2016, 148, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.P.; Joshi, M.K.; Kim, J.I.; Unnithan, A.R.; Lee, J.; Park, C.H.; Kim, C.S. Bimodal fibrous structures for tissue engineering: Fabrication, characterization and in vitro biocompatibility. J. Colloid Interface Sci. 2016, 476, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.K.; Tiwari, A.P.; Maharjan, B.; Won, K.S.; Kim, H.J.; Park, C.H.; Kim, C.S. Cellulose reinforced nylon-6 nanofibrous membrane: Fabrication strategies, physicochemical characterizations, wicking properties and biomimetic mineralization. Carbohydr. Polym. 2016, 147, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Hendrikson, W.J.; van Blitterswijk, C.A.; Rouwkema, J.; Moroni, L. The Use of Finite Element Analyses to Design and Fabricate Three-Dimensional Scaffolds for Skeletal Tissue Engineering. Front. Bioeng. Biotechnol. 2017, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Duan, Y.; Wei, D.; Wang, L.; Wang, H.; Gu, Z.; Kong, D. Co-electrospun fibrous scaffold-adsorbed DNA for substrate-mediated gene delivery. J. Biomed. Mater. Res. A 2011, 96, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Pauly, H.M.; Sathy, B.N.; Olvera, D.; McCarthy, H.O.; Kelly, D.J.; Popat, K.C.; Dunne, N.J.; Haut Donahue, T.L. Hierarchically Structured Electrospun Scaffolds with Chemically Conjugated Growth Factor for Ligament Tissue Engineering. Tissue Eng. Part A 2017, 23, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.K.; Tiwari, A.P.; Pant, H.R.; Shrestha, B.K.; Kim, H.J.; Park, C.H.; Kim, C.S. In situ generation of cellulose nanocrystals in polycaprolactone nanofibers: Effects on crystallinity, mechanical strength, biocompatibility, and biomimetic mineralization. ACS Appl. Mater. Interfaces 2015, 7, 19672–19683. [Google Scholar] [CrossRef] [PubMed]
- Chieruzzi, M.; Pagano, S.; Moretti, S.; Pinna, R.; Milia, E.; Torre, L.; Eramo, S. Nanomaterials for Tissue Engineering In Dentistry. Nanomaterials 2016, 6, 134. [Google Scholar] [CrossRef] [PubMed]
- Lafon, J.P.; Champion, E.; Bernache-Assollant, D. Processing of AB-type carbonated hydroxyapatite Ca10−x(PO4)6−x(CO3)x(OH)2−x−2y(CO3)y ceramics with controlled composition. J. Eur. Ceram. Soc. 2008, 28, 139–147. [Google Scholar] [CrossRef]
- Christopher, A.; Ronald, N.; Steven, T.; Soyon, K.; Benjamin, W.; Min, L. Photopolymerizable chitosan–collagen hydrogels for bone tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 164–174. [Google Scholar]
- Figueiredo, M.; Gamelas, J.; Martins, A. Characterization of bone and bone-based graft materials using FTIR spectroscopy. In Infrared Spectroscopy-Life and Biomedical Sciences; InTech: Vienna, Austria, 2012. [Google Scholar]
- Aubin, J.E.; Triffitt, J.T. Mesenchymal stem cells and osteoblast differentiation. In Principles of Bone Biology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2002; pp. 59–81. [Google Scholar]
- Manolagas, S.C. Birth and Death of Bone Cells: Basic Regulatory Mechanisms and Implications for the Pathogenesis and Treatment of Osteoporosis. Endocr. Rev. 2000, 21, 115–137. [Google Scholar] [CrossRef] [PubMed]
- Mackie, E.J. Osteoblasts: Novel roles in orchestration of skeletal architecture. Int. J. Biochem. Cell Boil. 2003, 35, 1301–1305. [Google Scholar] [CrossRef]
- Jang, J.-H.; Castano, O.; Kim, H.-W. Electrospun materials as potential platforms for bone tissue engineering. Adv. Drug Deliv. Rev. 2009, 61, 1065–1083. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, Y.; Udagawa, N.; Suda, T.; Takahashi, N. Mechanisms involved in bone resorption regulated by vitamin D. J. Steroid Biochem. Mol. Biol. 2018, 177, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Dallas, S.L.; Prideaux, M.; Bonewald, L.F. The Osteocyte: An Endocrine Cell … and More. Endocr. Rev. 2013, 34, 658–690. [Google Scholar] [CrossRef] [PubMed]
- Streicher, C.; Heyny, A.; Andrukhova, O.; Haigl, B.; Slavic, S.; Schüler, C.; Kollmann, K.; Kantner, I.; Sexl, V.; Kleiter, M.; et al. Estrogen Regulates Bone Turnover by Targeting RANKL Expression in Bone Lining Cells. Sci. Rep. 2017, 7, 6460. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H. Morphology, function, and differentiation of bone cells. J. Hard Tissue Boil. 2007, 16, 15–22. [Google Scholar] [CrossRef]
- Hou, Y.-C.; Wu, C.-C.; Liao, M.-T.; Shyu, J.-F.; Hung, C.-F.; Yen, T.-H.; Lu, C.-L.; Lu, K.-C. Role of nutritional vitamin D in osteoporosis treatment. Clin. Chim. Acta 2018, 484, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.-Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Jasiuk, I.M. Analysis of Trabecular Bone as a Hierarchial Material; XXI ICTAM: Warsaw, Poland, 2004; pp. 15–21. [Google Scholar]
- Salifu, A.A.; Lekakou, C.; Labeed, F.H. Electrospun oriented gelatin-hydroxyapatite fiber scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2017, 105, 1911–1926. [Google Scholar] [CrossRef] [PubMed]
- Uebersax, L.; Apfel, T.; Nuss, K.M.R.; Vogt, R.; Kim, H.Y.; Meinel, L.; Kaplan, D.L.; Auer, J.A.; Merkle, H.P.; von Rechenberg, B. Biocompatibility and osteoconduction of macroporous silk fibroin implants in cortical defects in sheep. Eur. J. Pharm. Biopharm. 2013, 85, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-S.; Sun Park, M.; Jeon, O.; Yong Choi, C.; Kim, B.-S. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Johnathan, N.; Kara, S.; Jonathan, B.; Gordana, V.-N. Biomimetic Approaches for Bone Tissue Engineering. Tissue Eng. Part B Rev. 2017, 23, 480–493. [Google Scholar]
- Maji, S.; Agarwal, T.; Das, J.; Maiti, T.K. Development of gelatin/carboxymethyl chitosan/nano-hydroxyapatite composite 3D macroporous scaffold for bone tissue engineering applications. Carbohydr. Polym. 2018, 189, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.R.; Reis, R.L.; Martins, A.; Neves, N.M. The Use of Electrospinning Technique on Osteochondral Tissue Engineering. In Osteochondral Tissue Engineering: Nanotechnology, Scaffolding-Related Developments and Translation; Oliveira, J.M., Pina, S., Reis, R., San Roman, J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 247–263. [Google Scholar]
- Rezk, A.I.; Rajan Unnithan, A.; Hee Park, C.; Sang Kim, C. Rational design of bone extracellular matrix mimicking tri-layered composite nanofibers for bone tissue regeneration. Chem. Eng. J. 2018, 350, 812–823. [Google Scholar] [CrossRef]
- Pacelli, S.; Basu, S.; Whitlow, J.; Chakravarti, A.; Acosta, F.; Varshney, A.; Modaresi, S.; Berkland, C.; Paul, A. Strategies to develop endogenous stem cell-recruiting bioactive materials for tissue repair and regeneration. Adv. Drug Deliv. Rev. 2017, 120, 50–70. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.R.; Ruckh, T.T.; Popat, K.C. Bone tissue engineering: A review in bone biomimetics and drug delivery strategies. Biotechnol. Prog. 2009, 25, 1539–1560. [Google Scholar] [CrossRef] [PubMed]
- Mitsak, A.G.; Kemppainen, J.M.; Harris, M.T.; Hollister, S.J. Effect of Polycaprolactone Scaffold Permeability on Bone Regeneration In Vivo. Tissue Eng. Part A 2011, 17, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Han, J.; Zhang, S.; Liu, F.; Wang, S.; Duan, J.; Sang, Y.; Jiang, H.; Li, D.; Ge, S.; et al. Hydroxyapatite nanobelt/polylactic acid Janus membrane with osteoinduction/barrier dual functions for precise bone defect repair. Acta Biomater. 2018, 71, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Daculsi, G.; Fellah, B.H.; Miramond, T.; Durand, M. Osteoconduction, Osteogenicity, Osteoinduction, what are the fundamental properties for a smart bone substitutes. IRBM 2013, 34, 346–348. [Google Scholar] [CrossRef]
- Ruslan, A.M.; Amirhossein, G.; Rafiq, A.K.M.; Uzir, W.M. Biomechanical and bioactivity concepts of polyetheretherketone composites for use in orthopedic implants—A review. J. Biomed. Mater. Res. Part A 2015, 103, 3689–3702. [Google Scholar]
- Yuan, B.; Cheng, Q.; Zhao, R.; Zhu, X.; Yang, X.; Yang, X.; Zhang, K.; Song, Y.; Zhang, X. Comparison of osteointegration property between PEKK and PEEK: Effects of surface structure and chemistry. Biomaterials 2018, 170, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Yang, S.; Li, W.; Li, H.; Feng, S.; Zeng, R.; Yu, B.; Xiao, L.; Nie, H.-Y.; Tu, M. Scaffold composed of porous vancomycin-loaded poly(lactide-co-glycolide) microspheres: A controlled-release drug delivery system with shape-memory effect. Mater. Sci. Eng. C 2017, 78, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.N.; Hartgerink, J.D. Self-Assembling Multidomain Peptide Nanofibers for Delivery of Bioactive Molecules and Tissue Regeneration. Acc. Chem. Res. 2017, 50, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Singh, K.J.; Anand, V.; Bhatia, G.; Kaur, R.; Kaur, M.; Nim, L.; Arora, D.S. Scaffolds of hydroxyl apatite nanoparticles disseminated in 1,6-diisocyanatohexane-extended poly(1,4-butylene succinate)/poly(methyl methacrylate) for bone tissue engineering. Mater. Sci. Eng. C 2017, 71, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.C.; Dey, M.; Dutta, A.K.; Basu, B. Competent processing techniques for scaffolds in tissue engineering. Biotechnol. Adv. 2017, 35, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Cosme, J.G.L.; Xu, T.; Miszuk, J.M.; Picciani, P.H.S.; Fong, H.; Sun, H. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials 2017, 115, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, S.T.; Quinnell, S.P.; Wei, M. Development of a novel alginate-polyvinyl alcohol-hydroxyapatite hydrogel for 3D bioprinting bone tissue engineered scaffolds. J. Biomed. Mater. Res. Part A 2017, 105, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Hamlet, S.M.; Vaquette, C.; Shah, A.; Hutmacher, D.W.; Ivanovski, S. 3-Dimensional functionalized polycaprolactone-hyaluronic acid hydrogel constructs for bone tissue engineering. J. Clin. Periodontol. 2017, 44, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Holzwarth, J.M.; Ma, P.X. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials 2011, 32, 9622–9629. [Google Scholar] [CrossRef] [PubMed]
- El-Ghannam, A. Bone reconstruction: From bioceramics to tissue engineering. Expert Rev. Med. Devices 2005, 2, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Peng, C.; Zhou, X.; Chen, L.; Wang, W.; Zhang, Y.; Ma, P.X.; He, C. Three-dimensional porous scaffold by self-assembly of reduced graphene oxide and nano-hydroxyapatite composites for bone tissue engineering. Carbon 2017, 116, 325–337. [Google Scholar] [CrossRef]
- Yang, W.-F.; Long, L.; Wang, R.; Chen, D.; Duan, S.; Xu, F.-J. Surface-Modified Hydroxyapatite Nanoparticle-Reinforced Polylactides for Three-Dimensional Printed Bone Tissue Engineering Scaffolds. J. Biomed. Nanotechnol. 2018, 14, 294–303. [Google Scholar]
- Turk, M.; Deliormanlı, A.M. Electrically conductive borate-based bioactive glass scaffolds for bone tissue engineering applications. J. Biomater. Appl. 2017, 32, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Waibhaw, G.; Saxena, V.; Pandey, L.M. Nano-biocomposite scaffolds of chitosan, carboxymethyl cellulose and silver nanoparticle modified cellulose nanowhiskers for bone tissue engineering applications. Int. J. Boil. Macromol. 2018, 111, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Song, Y.; Zhang, J.; Liu, W.; Cui, J.; Li, H.; Chen, F. Laminated electrospun nHA/PHB-composite scaffolds mimicking bone extracellular matrix for bone tissue engineering. Mater. Sci. Eng. C 2017, 72, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, X.; Yu, K.; Meng, H.; Zheng, Y.; Peng, J.; Lu, S.; Liu, X.; Xie, Y.; Qiao, K. Fabrication of nanofibrous microcarriers mimicking extracellular matrix for functional microtissue formation and cartilage regeneration. Biomaterials 2018, 171, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Subhapradha, N.; Abudhahir, M.; Aathira, A.; Srinivasan, N.; Moorthi, A. Polymer coated mesoporous ceramic for drug delivery in bone tissue engineering. Int. J. Boil. Macromol. 2018, 110, 65–73. [Google Scholar] [CrossRef] [PubMed]
- La Mantia, F.P.; Morreale, M.; Botta, L.; Mistretta, M.C.; Ceraulo, M.; Scaffaro, R. Degradation of polymer blends: A brief review. Polym. Degrad. Stab. 2017, 145, 79–92. [Google Scholar] [CrossRef]
- Chang, H.-M.; Huang, C.-C.; Parasuraman, V.R.; Jhu, J.-J.; Tsai, C.-Y.; Chao, H.-Y.; Lee, Y.-L.; Tsai, H.-C. In vivo degradation of poly (ε-caprolactone) films in Gastro Intestinal (GI) tract. Mater. Today Commun. 2017, 11, 18–25. [Google Scholar] [CrossRef]
- Bürck, J.; Aras, O.; Bertinetti, L.; Ilhan, C.A.; Ermeydan, M.A.; Schneider, R.; Ulrich, A.S.; Kazanci, M. Observation of triple helix motif on electrospun collagen nanofibers and its effect on the physical and structural properties. J. Mol. Struct. 2018, 1151, 73–80. [Google Scholar] [CrossRef]
- Cengiz, F.; Jirsak, O. The effect of salt on the roller electrospinning of polyurethane nanofibers. Fibers Polym. 2009, 10, 177–184. [Google Scholar] [CrossRef]
- Geng, X.; Kwon, O.-H.; Jang, J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials 2005, 26, 5427–5432. [Google Scholar] [CrossRef] [PubMed]
- Demir, M.M.; Yilgor, I.; Yilgor, E.; Erman, B. Electrospinning of polyurethane fibers. Polymer 2002, 43, 3303–3309. [Google Scholar] [CrossRef]
- Thompson, C.J.; Chase, G.G.; Yarin, A.L.; Reneker, D.H. Effects of parameters on nanofiber diameter determined from electrospinning model. Polymer 2007, 48, 6913–6922. [Google Scholar] [CrossRef]
- Kunawan, A.; Nuanchan, C.; Duangdao, A.o.; Chidchanok, M.; Pitt, S. Effects of Poly(ethylene glycol), Inorganic Salt, Sodium Dodecyl Sulfate, and Solvent System on Electrospinning of Poly(ethylene oxide). Macromol. Mater. Eng. 2006, 291, 581–591. [Google Scholar]
- Casper, C.L.; Stephens, J.S.; Tassi, N.G.; Chase, D.B.; Rabolt, J.F. Controlling surface morphology of electrospun polystyrene fibers: Effect of humidity and molecular weight in the electrospinning process. Macromolecules 2004, 37, 573–578. [Google Scholar] [CrossRef]
- Henriques, C.; Vidinha, R.; Botequim, D.; Borges, J.P.; Silva, J.A.M.C. A Systematic Study of Solution and Processing Parameters on Nanofiber Morphology Using a New Electrospinning Apparatus. J. Nanosci. Nanotechnol. 2009, 9, 3535–3545. [Google Scholar] [CrossRef] [PubMed]
- Jarusuwannapoom, T.; Hongrojjanawiwat, W.; Jitjaicham, S.; Wannatong, L.; Nithitanakul, M.; Pattamaprom, C.; Koombhongse, P.; Rangkupan, R.; Supaphol, P. Effect of solvents on electro-spinnability of polystyrene solutions and morphological appearance of resulting electrospun polystyrene fibers. Eur. Polym. J. 2005, 41, 409–421. [Google Scholar] [CrossRef]
- Zeng, J.; Haoqing, H.; Schaper, A.; Wendorff, J.H.; Greiner, A. Poly-l-lactide nanofibers by electrospinning–influence of solution viscosity and electrical conductivity on fiber diameter and fiber morphology. e-Polymers 2003, 3. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Beck Tan, N.C. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Shahreen, L.; Chase, G.G. Effects of Electrospinning Solution Properties on Formation of Beads in TiO2 Fibers with PdO Particles. J. Eng. Fibers Fabr. 2015, 10, 136–145. [Google Scholar]
- Adomavičiūtė, E.; Milašius, R. The influence of applied voltage on poly (vinyl alcohol)(PVA) nanofibre diameter. Fibres Text. East. Eur. 2007, 15, 63. [Google Scholar]
- Jalili, R.; Hosseini, S.A.A.; Morshed, M. The effects of operating parameters on the morphology of electrospun polyacrilonitrile nanofibres. Iran. Polym. J. 2005, 14, 1074. [Google Scholar]
- Dulnik, J.; Kołbuk, D.; Denis, P.; Sajkiewicz, P. The effect of a solvent on cellular response to PCL/gelatin and PCL/collagen electrospun nanofibres. Eur. Polym. J. 2018, 104, 147–156. [Google Scholar] [CrossRef]
- Erencia, M.; Cano, F.; Tornero, J.A.; Fernandes, M.M.; Tzanov, T.; Macanás, J.; Carrillo, F. Electrospinning of gelatin fibers using solutions with low acetic acid concentration: Effect of solvent composition on both diameter of electrospun fibers and cytotoxicity. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Gianluca, C.; Alberta, L.; Salvatore, M.; Giulia, O.; Ignazio, B. Influence of Soluble Electrospun Co-Polyethersulfone Veils on Dynamic Mechanical and Morphological Properties of Epoxy Composites: Effect of Polymer Molar Mass. Adv. Polym. Technol. 2018, 37, 798–809. [Google Scholar]
- Okutan, N.; Terzi, P.; Altay, F. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocoll. 2014, 39, 19–26. [Google Scholar] [CrossRef]
- Bak, S.Y.; Yoon, G.J.; Lee, S.W.; Kim, H.W. Effect of humidity and benign solvent composition on electrospinning of collagen nanofibrous sheets. Mater. Lett. 2016, 181, 136–139. [Google Scholar] [CrossRef]
- Theron, S.A.; Zussman, E.; Yarin, A.L. Experimental investigation of the governing parameters in the electrospinning of polymer solutions. Polymer 2004, 45, 2017–2030. [Google Scholar] [CrossRef]
- Ma, Z.; Kotaki, M.; Inai, R.; Ramakrishna, S. Potential of Nanofiber Matrix as Tissue-Engineering Scaffolds. Tissue Eng. 2005, 11, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Segal, M.; Avinery, R.; Buzhor, M.; Shaharabani, R.; Harnoy, A.J.; Tirosh, E.; Beck, R.; Amir, R.J. Molecular Precision and Enzymatic Degradation: From Readily to Undegradable Polymeric Micelles by Minor Structural Changes. J. Am. Chem. Soc. 2017, 139, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Gunatillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cell Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.P.; Joshi, M.K.; Park, C.H.; Kim, C.S. Nano-Nets Covered Composite Nanofibers with Enhanced Biocompatibility and Mechanical Properties for Bone Tissue Engineering. J. Nanosci. Nanotechnol. 2018, 18, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Silva, R.; Boccaccini, A.R. 7-Fibrous protein-based biomaterials (silk, keratin, elastin, and resilin proteins) for tissue regeneration and repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; MBarbosa, A., Martins, M.C.L., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 175–204. [Google Scholar]
- Gulati, K.; Meher, M.K.; Poluri, K.M. Glycosaminoglycan-based resorbable polymer composites in tissue refurbishment. Regen. Med. 2017, 12, 431–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, H.J.; Vunjak-Novakovic, G.; Kaplan, D.L. Stem cell-based tissue engineering with silk biomaterials. Biomaterials 2006, 27, 6064–6082. [Google Scholar] [CrossRef] [PubMed]
- Wray, L.S.; Hu, X.; Gallego, J.; Georgakoudi, I.; Omenetto, F.G.; Schmidt, D.; Kaplan, D.L. Effect of Processing on Silk-Based Biomaterials: Reproducibility and Biocompatibility. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 99, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials Fabrication from Bombyx mori Silk Fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [PubMed]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Meinel, L.; Betz, O.; Fajardo, R.; Hofmann, S.; Nazarian, A.; Cory, E.; Hilbe, M.; McCool, J.; Langer, R.; Vunjak-Novakovic, G.; et al. Silk based biomaterials to heal critical sized femur defects. Bone 2006, 39, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Kirker-Head, C.; Karageorgiou, V.; Hofmann, S.; Fajardo, R.; Betz, O.; Merkle, H.P.; Hilbe, M.; von Rechenberg, B.; McCool, J.; Abrahamsen, L.; et al. BMP-silk composite matrices heal critically sized femoral defects. Bone 2007, 41, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Chen, J.; Karageorgiou, V.; Altman, G.H.; Kaplan, D.L. Human bone marrow stromal cell responses on electrospun silk fibroin mats. Biomaterials 2004, 25, 1039–1047. [Google Scholar] [CrossRef]
- Vassilis, K.; Michael, T.; Robert, F.; Lorenz, M.; Brian, S.; Katherine, W.; Jake, C.; Gordana, V.-N.; Kaplan, D.L. Porous silk fibroin 3-D scaffolds for delivery of bone morphogenetic protein-2 in vitro and in vivo. J. Biomed. Mater. Res. Part A 2006, 78, 324–334. [Google Scholar]
- Wang, G.; Yang, H.; Li, M.; Lu, S.; Chen, X.; Cai, X. The use of silk fibroin/hydroxyapatite composite co-cultured with rabbit bone-marrow stromal cells in the healing of a segmental bone defect. J. Bone Jt. Surgery Br. 2010, 92, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shao, W.; Qian, W.; He, J.; Zhou, Y.; Qi, K.; Wang, L.; Cui, S.; Wang, R. Biomineralized poly (l-lactic-co-glycolic acid)-tussah silk fibroin nanofiber fabric with hierarchical architecture as a scaffold for bone tissue engineering. Mater. Sci. Eng. C 2018, 84, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Ekaputra, A.K.; Prestwich, G.D.; Cool, S.M.; Hutmacher, D.W. The three-dimensional vascularization of growth factor-releasing hybrid scaffold of poly (ɛ-caprolactone)/collagen fibers and hyaluronic acid hydrogel. Biomaterials 2011, 32, 8108–8117. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Wong, M.; Tabata, Y.; Mikos, A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J. Control. Release 2005, 109, 256–274. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-M.; Zhang, Y.Z.; Ramakrishna, S.; Lim, C.T. Electrospinning and mechanical characterization of gelatin nanofibers. Polymer 2004, 45, 5361–5368. [Google Scholar] [CrossRef]
- Song, J.-H.; Kim, H.-E.; Kim, H.-W. Production of electrospun gelatin nanofiber by water-based co-solvent approach. J. Mater. Sci. Mater. Med. 2008, 19, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Naghieh, S.; Badrossamay, M.; Foroozmehr, E.; Kharaziha, M. Combination of PLA Micro-fibers and PCL-Gelatin Nano-fibers for Development of Bone. Tissue Eng. Scaffolds 2017, 6, 1–4. [Google Scholar]
- Gosline, J.M.; Guerette, P.A.; Ortlepp, C.S.; Savage, K.N. The mechanical design of spider silks: From fibroin sequence to mechanical function. J. Exp. Boil. 1999, 202, 3295. [Google Scholar]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Vepari, C.; Kaplan, D.L. Silk as a Biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.P.; Hwang, T.I.; Oh, J.-M.; Maharjan, B.; Chun, S.; Kim, B.S.; Joshi, M.K.; Park, C.H.; Kim, C.S. pH/NIR-Responsive Polypyrrole-Functionalized Fibrous Localized Drug-Delivery Platform for Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 20256–20270. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Mei, L.; Song, C.; Cui, X.; Wang, P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials 2006, 27, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.-J.; Meredith, C.; Johnson, C.; Galis, Z.S. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials 2004, 25, 5735–5742. [Google Scholar] [CrossRef] [PubMed]
- Oksman, K.; Skrifvars, M.; Selin, J.F. Natural fibres as reinforcement in polylactic acid (PLA) composites. Compos. Sci. Technol. 2003, 63, 1317–1324. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/ polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Zhu, K.J.; Lin, X.; Yang, S. Preparation, characterization, and properties of polylactide (PLA)–poly(ethylene glycol) (PEG) copolymers: A potential drug carrier. J. Appl. Polym. Sci. 1990, 39, 1–9. [Google Scholar] [CrossRef]
- Chai, Y.; Lin, D.; Ma, Y.; Yuan, Y.; Liu, C. RhBMP-2 loaded MBG/PEGylated poly (glycerol sebacate) composite scaffolds for rapid bone regeneration. J. Mater. Chem. B 2017, 5, 4633–4647. [Google Scholar] [CrossRef]
- Domingues, R.M.; Chiera, S.; Gershovich, P.; Motta, A.; Reis, R.L.; Gomes, M.E. Fabrication of anisotropically aligned nanofibrous scaffolds based on natural/synthetic polymer blends reinforced with cellulose nanocrystals for tendon tissue engineering. Front. Bioeng. Biotechnol. 2016, 4. [Google Scholar] [CrossRef]
- Sun, K.; Li, H.; Li, R.; Nian, Z.; Li, D.; Xu, C. Silk fibroin/collagen and silk fibroin/chitosan blended three-dimensional scaffolds for tissue engineering. Eur. J. Orthop. Surg. Traumatol. 2015, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.P.; Joshi, M.K.; Lee, J.; Maharjan, B.; Ko, S.W.; Park, C.H.; Kim, C.S. Heterogeneous electrospun polycaprolactone/polyethylene glycol membranes with improved wettability, biocompatibility, and mineralization. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 105–113. [Google Scholar] [CrossRef]
- Eslami, H.; Azimi Lisar, H.; Jafarzadeh Kashi, T.S.; Tahriri, M.; Ansari, M.; Rafiei, T.; Bastami, F.; Shahin-Shamsabadi, A.; Mashhadi Abbas, F.; Tayebi, L. Poly(lactic-co-glycolic acid)(PLGA)/TiO2 nanotube bioactive composite as a novel scaffold for bone tissue engineering: In vitro and in vivo studies. Biologicals 2018, 53, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Telemeco, T.A.; Ayres, C.; Bowlin, G.L.; Wnek, G.E.; Boland, E.D.; Cohen, N.; Baumgarten, C.M.; Mathews, J.; Simpson, D.G. Regulation of cellular infiltration into tissue engineering scaffolds composed of submicron diameter fibrils produced by electrospinning. Acta Biomater. 2005, 1, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Babaie, E.; Bhaduri, S.B. Fabrication Aspects of Porous Biomaterials in Orthopedic Applications: A Review. ACS Biomater. Sci. Eng. 2018, 4, 1–39. [Google Scholar] [CrossRef]
- Chung, H.J.; Park, T.G. Surface engineered and drug releasing pre-fabricated scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640. [Google Scholar] [CrossRef] [PubMed]
- Jose, M.V.; Thomas, V.; Johnson, K.T.; Dean, D.R.; Nyairo, E. Aligned PLGA/HA nanofibrous nanocomposite scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Ngiam, M.; Liao, S.; Patil, A.J.; Cheng, Z.; Chan, C.K.; Ramakrishna, S. The fabrication of nano-hydroxyapatite on PLGA and PLGA/collagen nanofibrous composite scaffolds and their effects in osteoblastic behavior for bone tissue engineering. Bone 2009, 45, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Abdel-Fattah, W.I.; Laurencin, C.T. In vitro evaluation of chitosan/poly(lactic acid-glycolic acid) sintered microsphere scaffolds for bone tissue engineering. Biomaterials 2006, 27, 4894–4903. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Slamovich, E.B.; Webster, T.J. Less harmful acidic degradation of poly(lactic-co-glycolic acid) bone tissue engineering scaffolds through titania nanoparticle addition. Int. J. Nanomed. 2006, 1, 541–545. [Google Scholar] [CrossRef]
- Hong Nam, P.; Thi Ha Giang, P.; Dac Tu, N.; Quoc Thong, P.; Thi Thu Huong, L.; Phuong Thu, H.; Hung Manh, D.; Thi My Nhung, H.; Xuan Phuc, N. Magnetic inductive heating of organs of mouse models treated by copolymer coated Fe3O4 nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 025013. [Google Scholar]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Z.; Zhou, H.; Li, L.; Zhu, Q.; Zhang, P. An injectable hydroxyapatite/poly(lactide-co-glycolide) composite reinforced by micro/nano-hybrid poly(glycolide) fibers for bone repair. Mater. Sci. Eng. C 2017, 80, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Rezk, A.I.; Mousa, H.M.; Lee, J.; Park, C.H.; Kim, C.S. Composite PCL/HA/simvastatin electrospun nanofiber coating on biodegradable Mg alloy for orthopedic implant application. J. Coat. Technol. Res. 2018. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Shrestha, S.; Tiwari, A.P.; Kim, J.-I.; Ko, S.W.; Kim, H.-J.; Park, C.H.; Kim, C.S. Bio-inspired hybrid scaffold of zinc oxide-functionalized multi-wall carbon nanotubes reinforced polyurethane nanofibers for bone tissue engineering. Mater. Des. 2017, 133, 69–81. [Google Scholar] [CrossRef]
- Muffly, T.M.; Tizzano, A.P.; Walters, M.D. The history and evolution of sutures in pelvic surgery. J. R. Soc. Med. 2011, 104, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Andreas, L. Polymers in Biomedicine. Macromol. Biosci. 2010, 10, 993–997. [Google Scholar]
- Andreas, L.; Matthias, R.; Buchmeiser, M.R.; Haag, R. Polymers in Biomedicine and Electronics. Macromol. Rapid Commun. 2010, 31, 1487–1491. [Google Scholar]
- Bhattacharjee, P.; Kundu, B.; Naskar, D.; Kim, H.-W.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Silk scaffolds in bone tissue engineering: An overview. Acta Biomater. 2017, 63, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, H.; Shin, Y.M.; Terai, H.; Vacanti, J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar] [CrossRef]
- Shi, P.; Teh, T.K.H.; Toh, S.L.; Goh, J.C.H. Variation of the effect of calcium phosphate enhancement of implanted silk fibroin ligament bone integration. Biomaterials 2013, 34, 5947–5957. [Google Scholar] [CrossRef] [PubMed]
- Law, J.X.; Liau, L.L.; Saim, A.; Yang, Y.; Idrus, R. Electrospun Collagen Nanofibers and Their Applications in Skin Tissue Engineering. Tissue Eng. Regen. Med. 2017, 14, 699–718. [Google Scholar] [CrossRef]
- Meechaisue, C.; Wutticharoenmongkol, P.; Waraput, R.; Huangjing, T.; Ketbumrung, N.; Pavasant, P.; Supaphol, P. Preparation of electrospun silk fibroin fiber mats as bone scaffolds: A preliminary study. Biomed. Mater. 2007, 2, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, T.; Li, M.; Fu, N.; Fu, Y.; Ba, K.; Deng, S.; Jiang, Y.; Hu, J.; Peng, Q.; et al. Electrospun fibers for dental and craniofacial applications. Curr. Stem. Cell Res. Ther. 2014, 9, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Morshed, M.; Varshosaz, J.; Jannesari, M. Controlled release of metronidazole benzoate from poly ε-caprolactone electrospun nanofibers for periodontal diseases. Eur. J. Pharm. Biopharm. 2010, 75, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Guazzo, R.; Gardin, C.; Bellin, G.; Sbricoli, L.; Ferroni, L.; Ludovichetti, F.; Piattelli, A.; Antoniac, I.; Bressan, E.; Zavan, B. Graphene-Based Nanomaterials for Tissue Engineering in the Dental Field. Nanomaterials 2018, 8, 349. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, U. Osteoinduction in porous hydroxyapatite implanted in heterotopic sites of different animal models. Biomaterials 1996, 17, 31–35. [Google Scholar] [CrossRef]
- Reinholz, G.G.; Lu, L.; Saris, D.B.F.; Yaszemski, M.J.; O’Driscoll, S.W. Animal models for cartilage reconstruction. Biomaterials 2004, 25, 1511–1521. [Google Scholar] [CrossRef]
- Wang, R.M.; Johnson, T.D.; He, J.; Rong, Z.; Wong, M.; Nigam, V.; Behfar, A.; Xu, Y.; Christman, K.L. Humanized mouse model for assessing the human immune response to xenogeneic and allogeneic decellularized biomaterials. Biomaterials 2017, 129, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Qing, L.; Lei, L.; Tao, Y.; Ting, J.; Yunqing, K. Effect of Brain-Derived Neurotrophic Factor on the Neurogenesis and Osteogenesis in Bone Engineering. Tissue Eng. Part A 2018, 24, 1283–1292. [Google Scholar]
- Marrella, A.; Lee, T.Y.; Lee, D.H.; Karuthedom, S.; Syla, D.; Chawla, A.; Khademhosseini, A.; Jang, H.L. Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater. Today 2018, 21, 362–376. [Google Scholar] [CrossRef]
- Juqing, S.; Guanglin, Z.; Lin, W.; Geng, A.; Xuetao, S.; Yingjun, W. Assembling of electrospun meshes into three-dimensional porous scaffolds for bone repair. Biofabrication 2017, 9, 015018. [Google Scholar]
- Carlier, A.; Geris, L.; Gastel, N.V.; Carmeliet, G.; Oosterwyck, H.V. Oxygen as a critical determinant of bone fracture healing—A multiscale model. J. Theor. Boil. 2015, 365, 247–264. [Google Scholar] [CrossRef] [PubMed]


| Structure | Dimension Range | Structural Unit/Moieties | Dimension | Scale | Ref. |
|---|---|---|---|---|---|
| Macro | Whole bone dimension | Trabecules | Length | 1 mm | [50] |
| Diameter | 0.1 mm | ||||
| Compact (cortical bone) | |||||
| Micro | ~10–500 µm | Mature osteoclasts | 50–100 µm | [41] | |
| Single trabeculae | Diameter | 50–300 µm | [50] | ||
| Haversian system (Osteon) | Diameter | 200–250 µm | |||
| Submicro | 1–10 µm | Lining cells | 1–2 µm | [41] | |
| Single lamellae | Thickness | 3–7 µm | |||
| Haversian canal | 3–7 µm | ||||
| Nano | Few hundred nm—below 1 µm | Collagen fibril | 500 nm | ||
| Subnano | Below few hundred nm | Apatites plates (HA) | Dimension | 2 × 25 ×50 nm | [50] |
| Type I collagen | Diameter | 3–10 nm | |||
| Carbonate apatite | Thickness | 2–3 nm |
| Bone Component | Property | Measurement | Ref. |
|---|---|---|---|
| Large tensile cortical specimens | Young modulus | 14–20 GPa | [51] |
| Microbending cortical specimens | Young modulus | 5.4 GPa. | [52] |
| Osteon lamellar bone | Young modulus | 22 GPa | [45] |
| Osteonal segment (sample with majority of lamellar orientation in the longitudinal direction) | Elastic modulus | 12 GPa | [45] |
| Osteonal segment | Strength | 120 MPa | |
| Osteonal segment | Elastic modulus | 5.5 GPa | |
| Cortical bone | Elastic modulus | 5.4 GPa | [51] |
| SN | Parameters | Effect on Fiber Morphology | References | |
|---|---|---|---|---|
| 1 | Polymer property | Polymer | Fiber morphology is specific to polymer used | [86,90,91] |
| Molecular weight | Increased molecular mass of polymer might reduce the number of beads. Fiber diameter increases with higher molecular mass of polymer. | |||
| 2 | Solvent property | Solvent | Solvent used in electrospinning affect on solution spinnability | [92,93] |
| Boiling point/vapor pressure | ||||
| spinnability | ||||
| 3 | Solution property | |||
| Concentration | Increase in concentration of solution increases the fiber diameter (power law relation). | [91,93,94,95] | ||
| Low concentration of solution led to beaded fibers, Intermediate concentration led to good fiber and high concentration led to bimodal fibers and even higher concentration led to a distributed deposition. | ||||
| Conductivity | Increase in conductivity of solution decreases the fiber diameter | |||
| Viscosity/Surface tension | Formation of an unstable jet as a resultant effect of surface tension and viscosity led to the bead formation [95]. | |||
| 4 | Processing parameter | Spinning voltage | Increase in voltage decreases the fiber diameter and it is strongly correlated to bead formation. | [91,94,96,97] |
| Tip-collector distance | Distance effects on complete evaporation of fiber. Too short and too large distances may generate beads. Increased tip-collector distance represents weak electric field. Greater distance to be covered by the fiber and longer flight time favor the formation of thinner fiber. | |||
| flow rate | Decrease in flow rate decreases the fiber diameter. | |||
| High flow rate might generate beads. Fiber diameter increases with increasing feed rate. | ||||
| 5 | Ambient parameter | Humidity | High humidity might affect solvent evaporation. | [90] |
| Temperature | Increase in temperature decreases the fiber diameter | [87] | ||
| 6 | Supplementary addition | Salt | Addition of salt might help in reduction of beads | [85] |
| Polymers/Substrate | Ultimate Tensile Strength (MPa) | Modulus (GPa) | Breaking Strain (%) | Ref. |
|---|---|---|---|---|
| Bone | 160 | 20 | 3 | [126] |
| Silk with sericin (from B. mori) | 500 | 5–12 | 10–23.4 | [113,127] |
| Silk without sericin (from B. mori) | 740 | 15–17 | 4–16 | [128] |
| Collagen | 0.9–7.4 | 0.0018–0.046 | 24–68 | [113,129] |
| PLA | 28–50 | 1.2–3.0 | 2–6 | [113,129] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattarai, D.P.; Aguilar, L.E.; Park, C.H.; Kim, C.S. A Review on Properties of Natural and Synthetic Based Electrospun Fibrous Materials for Bone Tissue Engineering. Membranes 2018, 8, 62. https://doi.org/10.3390/membranes8030062
Bhattarai DP, Aguilar LE, Park CH, Kim CS. A Review on Properties of Natural and Synthetic Based Electrospun Fibrous Materials for Bone Tissue Engineering. Membranes. 2018; 8(3):62. https://doi.org/10.3390/membranes8030062
Chicago/Turabian StyleBhattarai, Deval Prasad, Ludwig Erik Aguilar, Chan Hee Park, and Cheol Sang Kim. 2018. "A Review on Properties of Natural and Synthetic Based Electrospun Fibrous Materials for Bone Tissue Engineering" Membranes 8, no. 3: 62. https://doi.org/10.3390/membranes8030062
APA StyleBhattarai, D. P., Aguilar, L. E., Park, C. H., & Kim, C. S. (2018). A Review on Properties of Natural and Synthetic Based Electrospun Fibrous Materials for Bone Tissue Engineering. Membranes, 8(3), 62. https://doi.org/10.3390/membranes8030062




