Important Approaches to Enhance Reverse Osmosis (RO) Thin Film Composite (TFC) Membranes Performance
Abstract
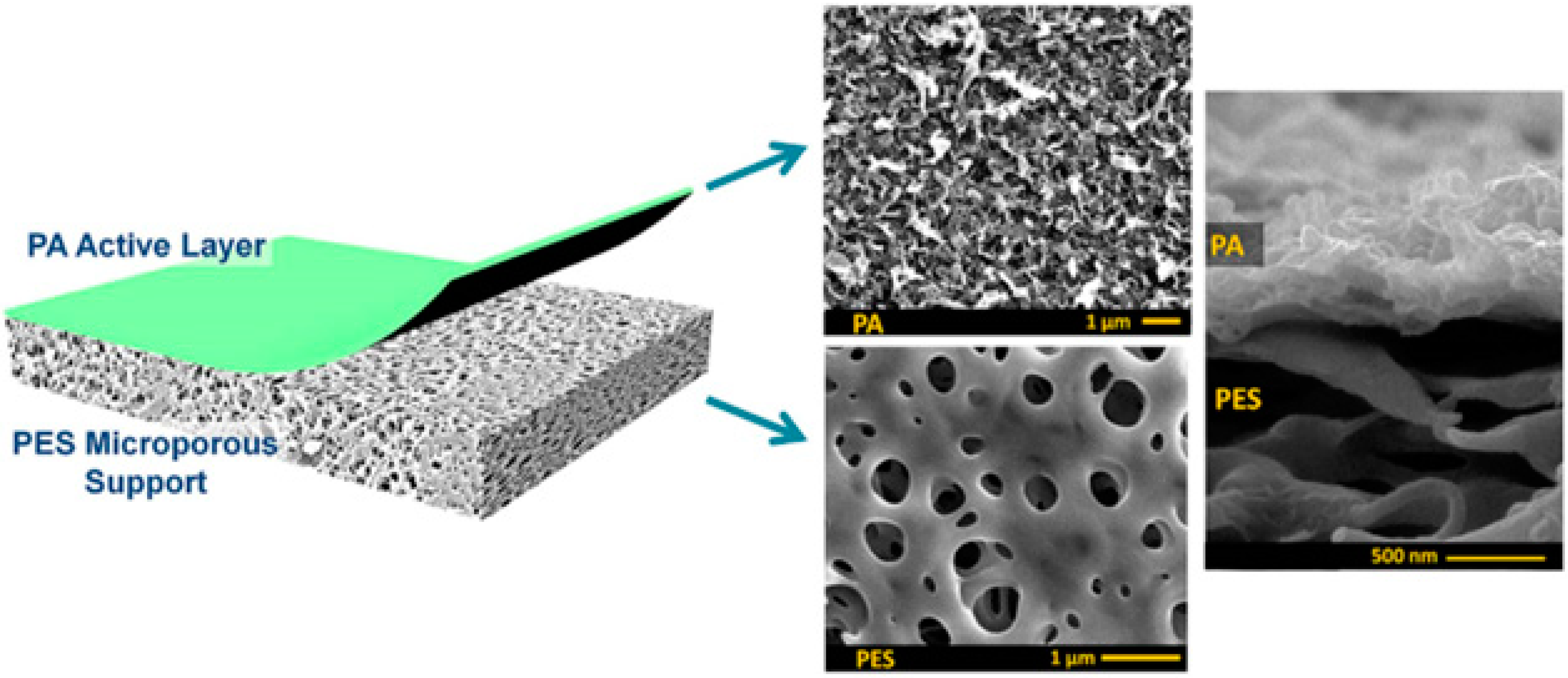
:1. Introduction
2. Using Alternative Monomers to Prepare the Active Layer
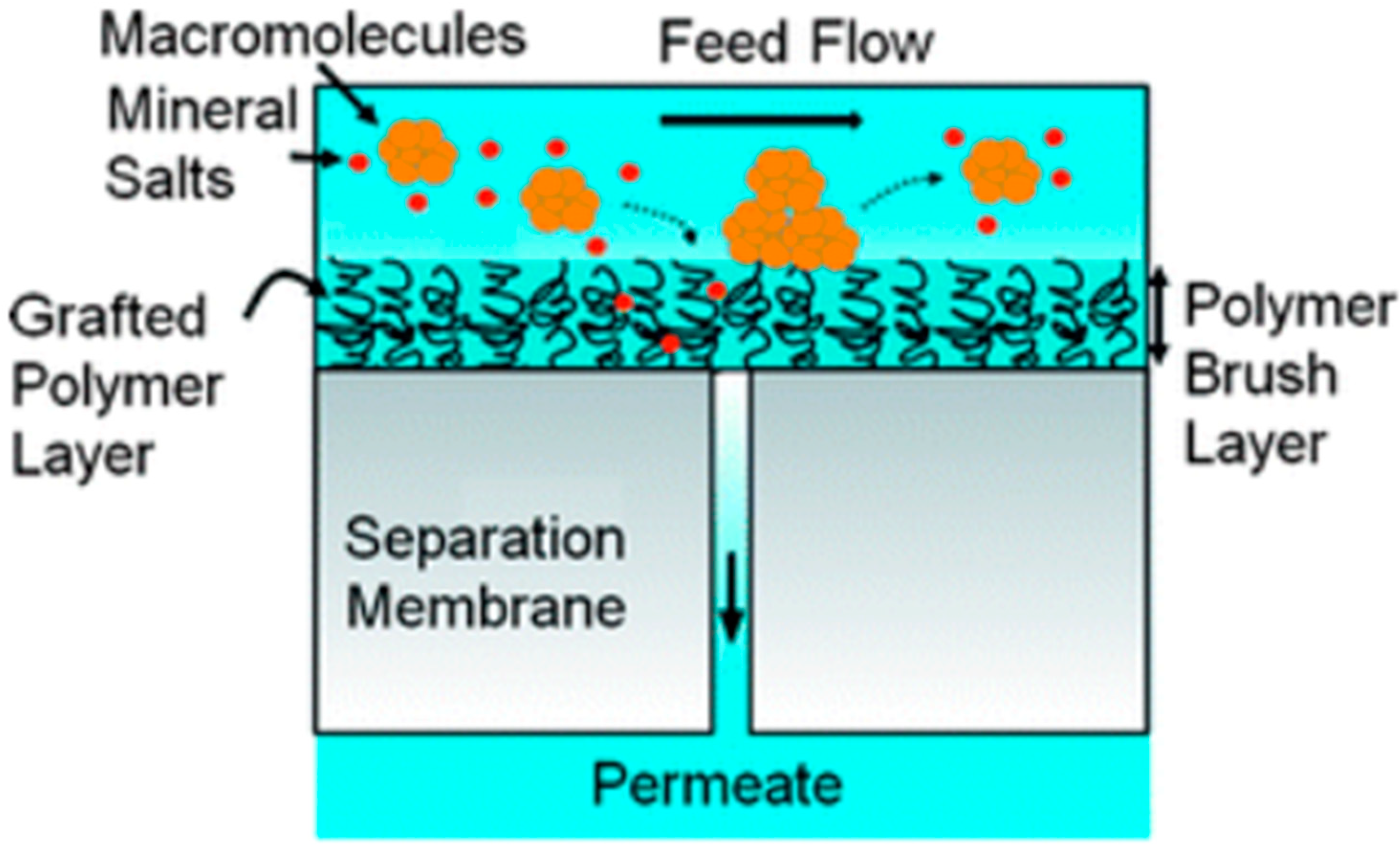
3. Modification of Membrane Surface
4. Optimization of Polymerization Reactions
5. Incorporation of Nanoparticles (NPs) into Membrane PA Layer
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AGO | Aminated-graphene oxide |
| Ag | Silver |
| Al-ZnO | Aluminum doped zinc oxide |
| BDSA | 2,2′-benzidinedisulfonic acid |
| BHAC | 2,2′,4,4′,6,6′-biphenyl hexaacyl chloride |
| BHDT | Bis-2,6-N,N-(2-hydroxyethyl) diaminotoluene |
| BPA | Bisphenol |
| CFIC | 5-chloroformyloxy-isophthaloyl chloride |
| CNT | Carbon nanotube |
| CQDs | Carbon quantum dots |
| Cu | Copper |
| DABA | Triamine 3,5-diamino-N-(4-aminophenyl)-benzamide |
| DMF | N,N-dimethylformamide |
| DMSO | 2,4,6-pyridinetricarboxylic acid chloride |
| DETA | Diethylenetriamine |
| DPA | Dopamine |
| F-MWCNTs | Functionalized multi wall carbon nanotubes |
| F-silica | Functionalized silica |
| GO | Graphene oxide |
| HBP-g-silica | Hyper-branched aromatic polyamide-grafted silica |
| HNTs | Halloysite nanotubes |
| ICIC | 5-isocyanato-isophtahloyl chloride |
| IP | Interfacial polymerization |
| iLSMM | In-situ hydrophilic surface modifying macromolecules |
| MOFs | Metal–organic frameworks |
| MPD | m-phenylenediamine |
| mm-BTEC | 3,3′,5,5′-biphenyl tetraacyl chloride |
| MWCNTs | Multiwall carbon nanotubes |
| NPs | Nanoparticles |
| OA-SiO2 | Oleic acid modified silica |
| om-BTEC | 2,2′,4,4′-biphenyl tetraacyl chloride |
| MOFs | Metal–organic framework |
| op-BTEC | 2,2′,5,5′-biphenyl tetraacyl chloride |
| PA | Polyamide |
| PAMAM | Ethylenediamine cored poly(amidoamine) |
| PDMAEMA | Poly(N,N-dimethylaminoethyl methacrylate) |
| PSU | Polysulfone |
| RO | Reverse osmosis |
| SiO2 | Silicon dioxide |
| SMPD | Sulfonated m-phenylenediamine |
| SWCNTs | Single-wall carbon nanotubes |
| TFC | Thin film composite |
| TFN | Thin film nanocomposite |
| TiO2 | Titanium dioxide |
| TMC | Trimesoyl chloride |
| TETA | Triethylenetetramine |
| TEPA | Tetraethylenepentamine |
| TNTs | Titanate nanotubes |
| UV | Ultraviolet |
| ZIF | Zeolitic imidazolate framework |
| ZnO | Zinc oxide |
References
- Marry, P.; Hoek, E. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Fane, A.; Hu, W.; Matsuura, T. Advanced Membrane Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Shenvi, S.; Isloor, A.; Ismail, A. A review on RO membrane technology: Developments and challenges. Desalination 2015, 368, 10–26. [Google Scholar] [CrossRef]
- Malaeb, L.; Ayoub, G. Reverse osmosis technology for water treatment: State of the art review. Desalination 2011, 267, 1–8. [Google Scholar] [CrossRef]
- Mai, Z. Membrane Processes for Water and Wastewater Treatment: Study and Modeling of Interactions between Membrane and Organic Matter. Ph.D. Thesis, LGPM—Laboratoire de Génie des Procédés et Matériaux, Paris, France, 2013. [Google Scholar]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Soltanieh, M.; Gill, W.N. Review of reverse osmosis membrane and transport models. Chem. Eng. Commun. 1981, 12, 279–363. [Google Scholar] [CrossRef]
- Lee, C.H. Theory of reverse osmosis and some other membrane permeation operations. J. Appl. Polym. Sci. 1975, 19, 83–95. [Google Scholar] [CrossRef]
- Reid, C.E.; Breton, E.J. Water and ion flow across cellulosic membranes. J. Appl. Polym. Sci. 1959, 1, 133–143. [Google Scholar] [CrossRef]
- Sourirajan, S. Separation of hydrocarbon liquids by flow under pressure through porous membranes. Nature 1964, 203, 1348–1349. [Google Scholar] [CrossRef]
- Loeb, S. Sea Water Demineralization by Means of a Semipermeable Membrane: Progress Report July 1, 1962-December 31; University of California: Berkeley, CA, USA, 1963. [Google Scholar]
- Porter, M.C. What, when and why of membranes MF, UF, and RO. ALChEsymp. Ser. 1977, 73, 83–103. [Google Scholar]
- Burns and Roe Industrial Services Corporation. Reverse Osmosis Technical Manual; United States Office of Water Research and Technology: Washington, DC, USA, 1979. [Google Scholar]
- Belfort, G. Chapter 6: Pressure driven membrane processes and wastewater renovation in water renovation and reuse. In Water Renovation and Reuse, 1st ed.; Shuval, H., Ed.; Academic Press: Cambridge, MA, USA, 1977. [Google Scholar]
- Shields, C.P. Five years’ experience with reverse osmosis systems using DU PONT “Permasp” permeators. Desalination 1979, 28, 157–179. [Google Scholar] [CrossRef]
- Cadotte, J.E. Interfacially Synthesized Reverse Osmosis Membrane. U.S. Patent 4,277,344, 7 July 1979. [Google Scholar]
- Khorshidi, B.; Thundat, T.; Fleck, B.A.; Sadrzadeh, M. A novel approach toward fabrication of high performance thin film composite polyamide membranes. Sci. Rep. 2016, 6, 22029. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Deng, B. Polymer-matrix nanocomposite membranes for water treatment. J. Membr. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Xu, G.R.; Xu, J.M.; Feng, H.J.; Zhao, H.L.; Wu, S.B. Tailoring structures and performance of polyamide thin film composite (PA-TFC) desalination membranes via sublayers adjustment—A review. Desalination 2017, 417, 19–35. [Google Scholar] [CrossRef]
- Gohil, J.M.; Ray, P. A review on semi-aromatic polyamide TFC membranes prepared by interfacial polymerization: Potential for water treatment and desalination. Sep. Purif. Technol. 2017, 181, 159–182. [Google Scholar] [CrossRef]
- Li, L.; Zhang, S.; Zhang, X.; Zheng, G. Polyamide thin film composite membranes prepared from isomeric biphenyl tetraacyl chloride and m-phenylenediamine. J. Membr. Sci. 2008, 315, 20–27. [Google Scholar] [CrossRef]
- Wang, H.; Li, L.; Zhang, X.; Zhang, S. Polyamide thin-film composite membranes prepared from a novel triamine 3,5-diamino-N-(4-aminophenyl)-benzamide monomer and m-phenylenediamin. J. Membr. Sci. 2010, 353, 78–84. [Google Scholar] [CrossRef]
- Nathaniel, G.; Lim, J.; Jung, B. High performance thin film composite polyamide reverse osmosis membrane prepared via m-phenylenediamine and 2,2-benzidinedisulfonic acid. Desalination 2012, 291, 69–77. [Google Scholar]
- Xie, W.; Geise, G.; Freeman, B.; Lee, H.; Byun, G.; McGrath, J. Polyamide interfacial composite membranes prepared from m-phenylene diamine, trimesoyl chloride and a new disulfonated diamine. J. Membr. Sci. 2012, 404, 152–161. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.; Chen, H.; Wang, T. Preparation of polyamide membranes with improved chlorine resistance by bis-2,6-N,N-(2-hydroxyethyl) diaminotoluene and trimesoyl chloride. Desalination 2013, 331, 16–25. [Google Scholar] [CrossRef]
- Sum, J.Y.; Ahmad, A.; Ooi, B.S. Synthesis of thin film composite membrane using mixed dendritic poly(amidoamine) and void filling piperazine monomers. J. Membr. Sci. 2014, 466, 183–191. [Google Scholar] [CrossRef]
- Li, Y.; Su, Y.; Dong, Y.; Zhao, X.; Jiang, Z.; Zhang, R.; Zhao, J. Separation performance of thin-film composite nanofiltration membrane through interfacial polymerization using different amine monomers. Desalination 2014, 333, 59–65. [Google Scholar] [CrossRef]
- Zhao, J.; Su, Y.; He, X.; Zhao, X.; Li, Y.; Zhang, R.; Jiang, Z. Dopamine composite nanofiltration membranes prepared by self-polymerization and interfacial polymerization. J. Membr. Sci. 2014, 465, 41–48. [Google Scholar] [CrossRef]
- Jewrajka, S.; Reddy, A.V.R.; Rana, H.; Mandal, S.; Khullar, S.; Haldar, S.; Joshi, N.; Ghosh, P. Use of 2,4,6-pyridinetricarboxylic acid chloride as a novel co-monomer for the preparation of thin film composite polyamide membrane with improved bacterial resistance. J. Membr. Sci. 2013, 439, 87–95. [Google Scholar] [CrossRef]
- Wang, T.; Dai, L.; Zhang, Q.; Li, A.; Zhang, S. Effects of acyl chloride monomer functionality on the properties of polyamide reverse osmosis (RO) membrane. J. Membr. Sci. 2013, 440, 48–57. [Google Scholar] [CrossRef]
- Yong, Z.; Sanchuan, Y.; Meihong, L.; Congjie, G. Polyamide thin film composite membrane prepared from m-phenylenediamine and m-pheneylenediamine-5-sulfonic acid. J. Membr. Sci. 2006, 270, 162–168. [Google Scholar] [CrossRef]
- Liu, M.; Wu, D.; Yu, S.; Gao, C. Influence of the polyacyl chloride structure on the reverse osmosis performance, surface properties and chlorine stability of the thin-film composite polyamide membranes. J. Membr. Sci. 2009, 326, 205–214. [Google Scholar] [CrossRef]
- La, Y.; Sooriyakum, R.; Miller, D.; Fujiwara, M.; Freeman, B.; Allen, R. Novel thin film composite membrane containing ionizable hydrophobes: pH-dependent reverse osmosis behavior and improved chlorine resistance. J. Mater. Chem. 2010, 20, 4615–4620. [Google Scholar] [CrossRef]
- Abu Seman, M.; Khayet, M.; Hilal, N. Nanofiltration thin-film composite polyester polyethersulfone-based membranes prepared by interfacial polymerization. J. Membr. Sci. 2010, 348, 109–116. [Google Scholar] [CrossRef]
- Abu Seman, M.; Khayet, M.; Hilal, N. Development of antifouling properties and performance of nanofiltration membranes modified by interfacial polymerization. Desalination 2011, 273, 36–47. [Google Scholar] [CrossRef]
- Koo, J.; Petersen, R.; Cadotte, J. ESCA characterization of chlorine-damage polyamide reverse osmosis membrane. ACS Polym. Prepr. 1986, 27, 391–392. [Google Scholar]
- Liu, M.; Zhou, C.; Dong, B.; Wu, Z.; Wang, L.; Yu, S.; Gao, C. Enhancing the permselectivity of thin-film composite poly(vinyl alcohol) (PVA) nanofiltration membrane by incorporating poly(sodium-p-styrene-sulfonate) (PSSNa). J. Membr. Sci. 2014, 463, 173–182. [Google Scholar] [CrossRef]
- Schafer, A.L.; Fane, A.G.; Waite, T.D. Nanofiltration: Principles and Application, 1st ed.; Elservier: New York, NY, USA, 2003. [Google Scholar]
- Petersen, R.J. Composite reverse osmosis and nanofiltration membrane. J. Membr. Sci. 1993, 38, 81–150. [Google Scholar] [CrossRef]
- Hilal, N.; Al-Zoubi, H.; Darwish, N.A.; Mohamma, A.W.; Arabi, M.A. A comprehensive review of nanofiltration membranes: Treatment, pretreatment, modeling and atomic force microscopy. Desalination 2004, 170, 281–308. [Google Scholar] [CrossRef]
- Lau, W.J.; Ismail, A.F. Polymeric nanofiltration membrane for textile dyeing waste treatment: preparation, performance evaluation, transport modeling, and fouling control—A review. Desalination 2010, 245, 4551–4566. [Google Scholar]
- Mickols, W.E. Method of Treating Polyamide Membranes to Increase Flux. U.S. Patent 5,755,964, 26 May 1998. [Google Scholar]
- Kuehne, M.A.; Song, R.Q.; Li, N.N.; Petersen, R.J. Flux enhancement in TFC RO membranes. Environ. Prog. 2001, 20, 23–26. [Google Scholar] [CrossRef]
- Wilf, M.; Alt, S. Application of low fouling RO membrane elements for reclamation of municipal wastewater. Desalination 2000, 132, 11–19. [Google Scholar] [CrossRef]
- Kang, G.D.; Gao, C.J.; Chen, W.D.; Jie, X.M.; Cao, Y.M. Study on hypochlorite degradation of aromatic polyamide reverse osmosis membrane. J. Membr. Sci. 2007, 300, 165–171. [Google Scholar] [CrossRef]
- Sarkar, A.; Carver, P.I.; Zhang, T.; Merrington, A.; Bruza, K.J.; Rousseau, J.L.; Keinath, S.E.; Dvornic, P.R. Dendrimer-based coatings for surface modification of polyamide reverse osmosis membranes. J. Membr. Sci. 2010, 349, 165–171. [Google Scholar] [CrossRef]
- Hong Anh Ngo, T.; Dinh Do, K.; Thi Tran, D. Surface modification of polyamide TFC membranes via redox-initiated graft polymerization of acrylic acid. J. Appl. Polym. Sci. 2017, 134, 45110. [Google Scholar] [CrossRef]
- Wu, S.; Xing, J.; Zheng, C.; Xu, G.; Zheng, G.; Xu, J. Plasma modification of aromatic polyamide reverse osmosis composite membrane surface. J. Appl. Polym. Sci. 1997, 764, 1923–1926. [Google Scholar] [CrossRef]
- Gilman, A.B. Low temperature plasma treatment as an effective method for surface modification of polymeric materials. High Energy Chem. 2003, 37, 17–23. [Google Scholar] [CrossRef]
- Lin, N.H.; Kim, M.M.; Lewis, G.T.; Cohen, Y. Polymer surface nano-structuring of reverse osmosis membrane for fouling resistance and improved flux performance. J. Membr. Sci. 2010, 20, 4642–4652. [Google Scholar] [CrossRef]
- Bing, S.; Wang, J.; Xu, H.; Zhao, Y.; Zhou, Y.; Zhang, L.; Gao, C.; Hou, L.A. Polyamide thin-film composite membrane modified with persulfate for improvement of perm-selectivity and chlorine-resistance. J. Membr. Sci. 2018, 555, 318–326. [Google Scholar] [CrossRef]
- Song, Y.; Sun, P.; Henry, L.L.; Sun, B. Mechanism of structure and performance controlled thin film composite membrane formation via interfacial polymerization process. J. Membr. Sci. 2005, 251, 67–79. [Google Scholar] [CrossRef]
- Karode, S.K.; Kulkarni, S.S.; Suresh, A.K.; Mashelkar, R.A. New insight into kinetics and thermodynamics of interfacial polymerization. Chem. Eng. Sci. 1998, 53, 2649–2663. [Google Scholar] [CrossRef]
- Dhumal, S.S.; Wagh, S.J.; Suresh, A.K. Interfacial polymerization-modeling of kinetics and film properties. J. Membr. Sci. 2008, 352, 758–771. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Hoek, E.M.V. Impact of reaction and curing condition on polyamide composite reverse osmosis membrane properties. J. Membr. Sci. 2008, 311, 34–45. [Google Scholar] [CrossRef]
- Tomaschke, J.E. Interfacially Synthesized Reverse Osmosis Membrane Containing an Amine Salt and Processes for Preparing the Same. U.S. Patent 4,948,507, 14 August 1990. [Google Scholar]
- Chau, M.M.; Light, W.G.; Chu, H.C. Dry High Flux Semipermeable Membrane. U.S. Patent 4,983,291, 18 January 1991. [Google Scholar]
- Kwak, S.Y.; Jung, S.G.; Kim, S.H. Structure-motion-performance relationship of flux enhanced reverse osmosis (RO) membranes composed of aromatic polyamide thin films. Environ. Sci. Technol. 2001, 35, 4334–4340. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.-H.; Hoek, E.M.V.; Yan, Y.; Subramani, A.; Huang, X.; Hurwitz, G.; Ghosh, A.K.; Jawor, A. Interfacial polymerization of thin film nanocomposites: A new concept for reverse osmosis membranes. J. Membr. Sci. 2007, 294, 1–7. [Google Scholar] [CrossRef]
- Lind, M.L.; Jeong, B.H.; Subramani, A.; Huang, X.; Hoek, E.M.V. Effect of mobile cation on zeolite-polyamide thin film nanocomposite membranes. J. Mater. Res. 2009, 24, 1624–1631. [Google Scholar] [CrossRef]
- Lind, M.L.; Ghosh, A.K.; Jawor, A.; Huang, X.; Hou, W.; Yang, Y.; Hoek, E.M.V. Influence of zeolite crystal size on zeolite-polyamide thin film nanocomposite membranes. Langmuir 2009, 25, 10139–10145. [Google Scholar] [CrossRef] [PubMed]
- Mickols, W.E. Composite Membrane and Method for Making the Same. U.S. Patent 6,878,278, 12 April 2005. [Google Scholar]
- Mickols, W.E. Composite Membrane and Method for Making the Same. U.S. Patent 6,337,018, 8 January 2002. [Google Scholar]
- Khayet, M. Membrane surface modification and characterization by X-ray Photo electron spectroscopy, atomic force microscopy and contact angle measurement. Appl. Surf. Sci. 2004, 238, 269–272. [Google Scholar] [CrossRef]
- Khayet, M.; Suk, D.E.; Narbaitz, R.M.; Santerre, J.P. study on surface modification by surface modifying macromolecules and its application in membrane separation process. Appl. Polym. 2003, 89, 2902–2961. [Google Scholar] [CrossRef]
- Tarboush, B.J.A.; Ranan, D.; Matsuura, T.; Nabratiz, H.A. Preparation of thin film composite polyamide membrane for water desalination using novel hydrophilic surface modifying macromolecules. J. Membr. Sci. 2008, 325, 166–175. [Google Scholar] [CrossRef]
- Jadav, G.L.; Singh, P.S. Synthesis of novel silica-polyamide nanocomposite membrane with enhanced properties. J. Membr. Sci. 2009, 328, 257–267. [Google Scholar] [CrossRef]
- Park, J.; Choi, W.; Kim, S.H.; Chun, B.H.; Bang, J.; Lee, K.B. Enhancement of chlorine resistance in carbon nanotube-based nanocomposite reverse osmosis membranes. Desalin. Water Treat. 2010, 15, 198–204. [Google Scholar] [CrossRef]
- Lind, M.L.; Suk, D.E.; Nguyen, T.V.; Hoek, E.M.V. Tailoring the structure of thin film nanocomposite membranes to achieve seawater RO membrane performance. Environ. Sci. Technol. 2010, 44, 8230–8235. [Google Scholar] [CrossRef] [PubMed]
- Jadav, G.L.; Aswal, V.K.; Singh, P.S. SANS study to probe nanoparticle dispersion in nanocomposite membranes of aromatic polyamide and functionalized silica nanoparticles. J. Colloid. Interface Sci. 2010, 351, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Ntim, S.A.; Mitra, S.; Sirkar, K.K. Facile fabrication of superior nanofiltration membranes from interfacially polymerized CNT-polymer composites. J. Membr. Sci. 2011, 375, 81–87. [Google Scholar] [CrossRef]
- Kong, C.; Koushima, A.; Kamada, T.; Shintani, T.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Enhanced performance of inorganic-polyamide nanocomposite membranes prepared by metal-alkoxide-assisted interfacial polymerization. J. Membr. Sci. 2011, 366, 382–388. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Aroujalian, A.; Raisi, A. Effect of added NaX nano-zeolite into polyamide as a top thin layer of membrane onwater flux and salt rejection in a reverse osmosis process. J. Membr. Sci. 2011, 375, 88–95. [Google Scholar] [CrossRef]
- Rana, D.; Kim, Y.; Matsuura, T.; Arafat, H.A. Development of antifouling thin film-composite membranes for seawater desalination. J. Membr. Sci. 2011, 367, 110–118. [Google Scholar] [CrossRef]
- Yin, J.; Kim, E.S.; Yang, J.; Deng, B. Fabrication of a novel thin-film nanocomposite (TFN) membrane containing MCM-41 silica nanoparticles (NPs) for water purification. J. Membr. Sci. 2012, 423, 238–246. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, C.; Li, X.; Vararattanavech, A.; Shen, W.; Torres, J.; Hélix-Nielsen, C.; Wang, R.; Hu, X.; Fane, A.G.; et al. Synthesis of robust and high performance aquaporin-based biomimetic membranes by interfacial polymerization-membrane preparation and RO performance characterization. J. Membr. Sci. 2012, 423, 422–428. [Google Scholar] [CrossRef]
- Chan, W.F.; Chen, H.Y.; Surapathi, A.; Taylor, M.G.; Shao, X.; Marand, E.; Johnson, J.K. Zwitterion functionalized carbon nanotube/polyamide nanocomposite membranes for water desalination. ACS Nano 2013, 7, 5308–5319. [Google Scholar] [CrossRef] [PubMed]
- De Lannoy, C.F.; Jassby, D.; Gloe, K.; Gordon, A.D.; Wiesner, M.R. Aquatic biofouling prevention by electrically charged nanocomposite polymer thin film membranes. Environ. Sci. Technol. 2013, 47, 2760–2768. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Qu, X.; Ji, X.; Gao, X.; Zhang, L.; Chen, H.; Hou, L. Acid and multivalent ion resistance of thin film nanocomposite RO membranes loaded with silicalite-1 nanozeolites. J. Mater. Chem. A 2013, 1, 11343–11349. [Google Scholar] [CrossRef]
- Huang, H.; Qu, X.; Dong, H.; Zhang, L.; Chen, H. Role of NaA zeolites in the interfacial polymerization process towards a polyamide nanocomposite reverse osmosis membrane. RSC Adv. 2013, 3, 8203–8207. [Google Scholar] [CrossRef]
- Kim, S.G.; Hyeon, D.H.; Chun, J.H.; Chun, B.H.; Kim, S.H. Nanocomposite poly (arylene ether sulfone) reverse osmosis membrane containing functional zeolite nanoparticles for seawater desalination. J. Membr. Sci. 2013, 443, 10–18. [Google Scholar] [CrossRef]
- Pendergast, M.M.; Ghosh, A.K.; Hoek, E.M.V. Separation performance and interfacial properties of nanocomposite reverse osmosis membranes. Desalination 2013, 308, 180–185. [Google Scholar] [CrossRef]
- Bao, M.; Zhu, G.; Wang, L.; Wang, M.; Gao, C. Preparation of monodispersed spherical mesoporous nanosilica-polyamide thin film composite reverse osmosis membranes via interfacial polymerization. Desalination 2013, 309, 261–266. [Google Scholar] [CrossRef]
- Kim, S.G.; Chun, J.H.; Chun, B.H.; Kim, S.H. Preparation, characterization and performance of poly(aylene ether sulfone)/modified silica nanocomposite reverse osmosis membrane for seawater desalination. Desalination 2013, 325, 76–83. [Google Scholar] [CrossRef]
- Baroña, G.N.B.; Lim, J.; Choi, M.; Jung, B. Interfacial polymerization of polyamide-aluminosilicate SWNT nanocomposite membranes for reverse osmosis. Desalination 2013, 325, 138–147. [Google Scholar] [CrossRef]
- Zhao, H.; Qiu, S.; Wu, L.; Zhang, L.; Chen, H.; Cao, C. improving the performance of polyamide reverse osmosis membrane by incorporating of modified multi wall carbon nanotubes. J. Membr. Sci. 2014, 450, 249–256. [Google Scholar] [CrossRef]
- Ghanbaria, M.; Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Ismail, A.F. Synthesis and characterization of novel thin film nanocomposite reverse osmosis membranes with improved organic fouling properties for water desalination. RSC Adv. 2015, 5, 21268–21276. [Google Scholar] [CrossRef]
- Rakhshan, N.; Pakizeh, M. The effect of chemical modification of SiO2 nanoparticles on the nanofiltration characteristics of polyamide membrane. J. Membr. Sci. 2015, 32, 2524–2533. [Google Scholar] [CrossRef]
- Dong, H.; Wu, L.; Zhang, L.; Chen, H.; Cao, C. Clay nano-sheet as charged filler materials for highperformance and fouling resistance thin film nanocomposite membrane. J. Membr. Sci. 2015, 494, 92–103. [Google Scholar] [CrossRef]
- Safarpour, M.; Khataee, A.; Vatanpour, V. Thin film nanocomposite reverse osmosis membrane modified by reduced graphene oxide/ TiO2 with improved desalination performance. J. Membr. Sci. 2015, 489, 43–54. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.J.; Rahbara, R.; Danseshfar, A.; Mayahi, A.; Matsuura, T.; Ismail, A.F. A novel thin film nanocomposite reverse osmosis membrane with superior anti organic fouling affinity for water desalination. Desalination 2015, 368, 106–113. [Google Scholar] [CrossRef]
- Bano, S.; Mahmood, A.; Kim, S.; Lee, K. Graphene oxide modified polyamide nanofiltration membrane with improved flux and antifouling properties. J. Mater. Chem. 2015, 3, 2065–2071. [Google Scholar] [CrossRef]
- Al Hobibi, A.S.; Ghoul, J.; Chiloufi, I.; EL Mir, L. Synthesis and characterization of polyamide thin film nanocomposite membrane reached by aluminum doped ZnO. Mater. Sci. Semicond. Process. 2016, 42, 111–114. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, G.; Liu, Z.; Gao, C. effect of MCM-48 nanoparticles on the performance of TFN membrane for reverse osmosis application. Desalination 2016, 394, 72–82. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B. Graphene oxide enhanced polyamide thin-film nanocomposite membrane for water purification. Desalination 2016, 379, 93–101. [Google Scholar] [CrossRef]
- Mayyahi, A.A.; Deng, B. Efficient water desalination using photo-responsive ZnO polyamide thin film nanocomposite membrane. Environ. Chem. Lett. 2018, 1–7. [Google Scholar] [CrossRef]
- Kadhom, M.; Deng, B. Thin film nanocomposite membrane filled with metal-organic frameworks UiO-66 and MIL-125 nanoparticles for water desalination. Membrane 2017, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Zhang, Q.; Zhang, R.; Su, Y.; Jiang, Z. Thin Film Nanocomposite Membranes Incorporated with Graphene Quantum Dots for High Flux and Antifouling Property. J. Membr. Sci. 2018, 553, 17–24. [Google Scholar] [CrossRef]
- Aljundi, I.H. Desalination characteristics of TFN-RO membrane incorporated with ZIF-8 nanoparticles. Desalination 2017, 430, 12–20. [Google Scholar] [CrossRef]
- Khorshidi, B.; Biswas, I.; Ghosh, T.; Thundat, T.; Sadrzadeh, M. Robust fabrication of thin film polyamide-TiO2 nanocomposite membranes with enhanced thermal stability and anti-biofouling propensity. Sci. Rep. 2018, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, P. Tuning the functional groups of carbon quantum dots in thin film nanocomposite membranes for nanofiltration. J. Membr. Sci. 2018, 564, 394–403. [Google Scholar] [CrossRef]
- He, Y.; Zhao, D.L.; Chung, T.S. Na+ functionalized carbon quantum dot incorporated thin-film nanocomposite membranes for selenium and arsenic removal. J. Membr. Sci. 2018, 564, 483–491. [Google Scholar] [CrossRef]
- Peyki, A.; Rahimpour, A.; Jahanshahi, M. Preparation and characterization of thin film composite reverse osmosis membranes incorporated with hydrophilic SiO2 nanoparticles. Desalination 2015, 368, 152–158. [Google Scholar] [CrossRef]
- Ma, D.; Peh, S.P.; Han, G.; Chen, S.B. Thin-Film Nanocomposite (TFN) Membranes Incorporated with Super-Hydrophilic Metal–Organic Framework (MOF) UiO-66: Toward Enhancement of Water Flux and Salt Rejection. ACS Appl. Mater. Interfaces 2017, 9, 7523–7534. [Google Scholar] [CrossRef] [PubMed]
- Mayyahi, A.A. Thin-film composite (TFC) membrane modified by hybrid ZnO-graphene nanoparticles (ZnO-Gr NPs) for water desalination. J. Environ. Chem. Eng. 2018, 6, 1109–1117. [Google Scholar] [CrossRef]
- Rajaeian, B.; Rahimpour, A.; Tade, M.O. Fabrication and characterization of polyamide thin film nanocomposite (TFN) nanofiltration membrane impregnated with TiO2 nanoparticles. Desalination 2013, 313, 176–188. [Google Scholar] [CrossRef]
- Glater, J.; Hong, S.; Elimelech, M. The search for a chlorine-resistance reverse osmosis membrane. Desalination 1994, 95, 325–345. [Google Scholar] [CrossRef]
- Xue, S.X.; Ji, C.H.; Xu, Z.L.; Tang, Y.J.; Li, R.H. Chlorine resistant TFN nanofiltration membrane incorporated with octadecylamine-grafted GO and fluorine-containing monomer. J. Membr. Sci. 2018, 545, 185–195. [Google Scholar] [CrossRef]
- Nikolova, J.D.; Islam, M.A. Contribution to adsorbed layer resistance to flux decline in ultrafiltration process. J. Membr. Sci. 1998, 146, 105–111. [Google Scholar] [CrossRef]
- Sadar Ghayeni, S.B.; Beatson, P.J.; Schneider, R.P.; Fane, A.G. Adhesive of water bacteria to reverse osmosis membrane. J. Membr. Sci. 1998, 138, 29–42. [Google Scholar] [CrossRef]
- Kim, E.-S.; Deng, B. Fabrication of polyamide thin-film-nano-composite (PA-TFN) membrane with hydrophilized ordered mesoporous carbon for water purification. J. Membr. Sci. 2011, 375, 46–54. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.; Patel, R.; Im, S.; Kim, J.; Min, B. Silver nanoparticles immobilized on thin film composite polyamide membrane: characterization, nanofiltration, antifouling properties. Polym. Adv. Tech. 2007, 18, 562–568. [Google Scholar] [CrossRef]
- Sondi, I.; Sondi, B. silver nanoparticles as antimicrobial agent: A case study on E. Coil as a model for gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Neigh, A.; Vermeulen, J.; Fonteyne, L.; Verharen, H.; Briede, J.; Loveren, H.; Jong, W. The effect of particle size on the cycotoxicity, inflammation, development of toxicity and genotoxicity of silver nanoparticles. Biomaterials 2011, 36, 9810–9817. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sasson, M.; Zodrow, K.R.; Genggeng, Q.; Kang, Y.; Giannelis, E.P.; Elimelech, M. Surface functionalization of thin-film composite membranes with copper nanoparticles for antimicrobial surface properties. Environ. Sci. Technol. 2014, 48, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kwak, S.; Sohn, B.; Park, T. design of TIO2 nanoparticles self-assembled aromatic polyamide thin-film composite (TFC) membrane as an approach to solve biofouling problem. J. Membr. Sci. 2003, 211, 157–165. [Google Scholar] [CrossRef]
- Choi, W.; Choi, J.; Bang, J.; Lee, J.H. Layer-by-layer assembly of graphene oxide nanosheets on polyamide membranes for durable reverse-osmosis applications. ACS Appl. Mater. Interfaces 2013, 5, 12510–12519. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Mi, B. Enabling graphene oxide nanosheets as water separation membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yang, Y.; Hu, Z.; Deng, B. Attachment of silver nanoparticles (AgNPs) onto thin-film composite (TFC) membranes through covalent bonding to reduce membrane biofouling. J. Membr. Sci. 2013, 441, 73–82. [Google Scholar] [CrossRef]
- Mayyahi, A.A. TiO2 polyamide thin film nanocomposite reverses osmosis membrane for water desalination. Membranes 2018, 8, 66. [Google Scholar] [CrossRef]




| Amine | Chemical Structure | Acid Chloride | Chemical Structure | Membrane Performance | Ref. |
|---|---|---|---|---|---|
| MPD |  | TMC | 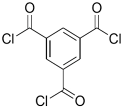 | It is well-known that the interfacial polymerization of MPD and TFC on a porous support layer results in high water flux and salt rejection | [17] |
| BDSA | 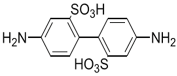 | TMC |  | Water Flux increased by more than 100% by using BDSA in the interfacial polymerization. Simultaneously, salt rejection increased from 89 to 99%. | [24] |
| S-BAPS | 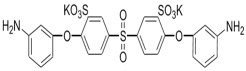 | TMC | 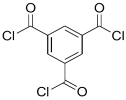 | When compared to the traditional TFC membrane, this membrane showed higher water flux, but lower NaCl rejection and chlorine resistance. | [25] |
| BHDT | 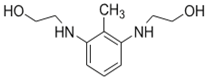 | TMC |  | This membrane demonstrated higher chlorine resistance when compared to the normal TFC membrane. | [26] |
| PAMAM | 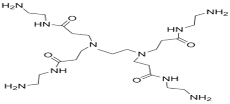 | TMC | 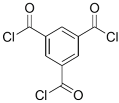 | In this study, the effects of PAMAM content on TFC membrane performance were studied. NaCl rejection was increased when PAMAM concentration was increased from 0.1% to 0.5% (w/v), while water flux was reduced. | [27] |
| DETA, TETA, or TEPA | 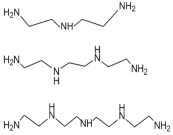 | TMC | 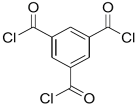 | Under operating pressure of 36.52 psi, water fluxes of TEPA/TMC, TETA/TMC, and DETA/TMC were 51.1 ± 4.5, 43.5 ± 0.5, and 33.5 ± 2 L/m2·h, respectively. On the other hand, Na2SO4 rejection sequence was: DETA/TMC > TEPA/TMC > TETA/TMC. | [28] |
| DPA | 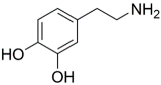 | TMC | 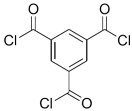 | The polyester bonds of DPA/TMC produced TFC membrane with high chemical stability, while maintaining good performance. | [29] |
| DABA | 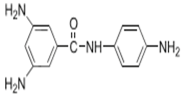 | TMC | 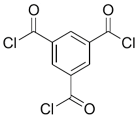 | Results showed that as DABA concentration was increased, the membrane became more hydrophilic and as a result, high water flux (55.4 L/m2·h-250 psi) was achieved. | [23] |
| MPD |  | DMSO | 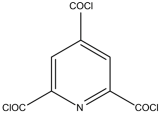 | The developed membrane showed excellent antimicrobial efficiency and high water flux and salt rejection. | [30] |
| MPD | 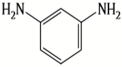 | BTAC |  | Membrane surface was highly negatively charged, smooth, and very thin, which in turn produced high fouling resistance. | [31] |
| SMPD |  | TMC | 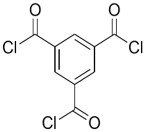 | When SMPS content was increased, the molecular weight of PA was decreased, and it subsequently increased water flux and decreased NaCl rejection. | [32] |
| MPD |  | mm-PETC | 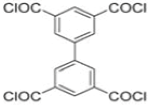 | Under 290 psi, water flux was 37.1 L/m2·h and NaCl rejection was 98.4% | [22] |
| MPD |  | om-PETC | 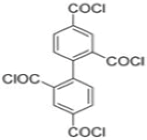 | Under 290 psi, water flux was 50 L/m2·h and NaCl rejection was 97.8% | |
| MPD |  | op-PETC | 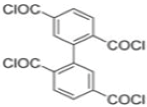 | Under 290 psi, water flux was 45.2 L/m2·h and NaCl rejection was 97.2% | |
| MPD | 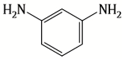 | ICIC | 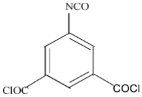 | Under operating pressure of 232 psi, water flux was 63 L/m2·h and NaCl rejection was 98.2%. In addition, the membrane showed significant resistance against chlorine. | [33] |
| MPD | 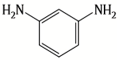 | CFIC |  | Under operating pressure of 232 psi, water flux was around 43.3 L/m2·h and NaCl rejection was around 98.6%. In addition, the membrane showed significant resistance against chlorine. | |
| HFA-MDA | 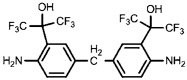 | TMC | 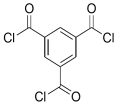 | Under operating pressure of 400 psi, NaCl rejection was 85% at low pH 4, but increased to 96.1% at pH 10. Water flux was 48 L/m2·h and 80 L/m2·h at pH 4 and pH 10, respectively. Besides, the membrane showed significant chlorine resistance. | [34] |
| Bisphenol A |  | TMC | 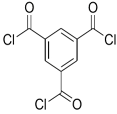 | This membrane showed significant fouling resistance along with high water flux and salt rejection. | [35] |
| TMBPA |  | TMC | 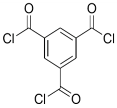 | Under operating pressure of 130 psi, water flux was 66.7 L/m2·h and the membrane showed good antifouling properties. | [36] |
| Nanofiller | PA Layer Monomers | Substrate | Performance of TFN | Ref. |
|---|---|---|---|---|
| Zeolite NaA | MPD-TMC | PSU | Water flux was increased from 2.5 × 1012 to 3.9 × 1012 mPa−1·s−1 without compromising salt rejection (94%) by increasing the concentration of nanoparticles from 0 to 0.4 wt.%. | [60] |
| Zeolite NaAAaA | MPD:TEA-TMC | PSU | Both AgA-TFN and NaA-TFN membranes exhibited higher water flux than that of TFC membrane. No change in salt rejection was observed. Both membranes showed enhanced antimicrobial properties. | [61] |
| Different sized zeolite | MPD:TEA:SLS:IPA-TMC | PSU | The membrane embedded with smaller zeolite NPs produced higher water flux than the membrane with larger zeolite NPs. | [62] |
| Silica | MPD-TMC | PSU | By increasing silica concentration, the thermal properties of the membrane were considerably enhanced. | [68] |
| MWCNTs | MPD-TMC | PSU | Under filtration pressure of 225 psi, both water flux and salt rejection were decreased from 18 to 12 L/m2·h and 98 to 92.2 wt.%, respectively, by increasing the concentration of MWCNTs from 0 to 1 wt.%. On the other hand, the membrane demonstrated significant chlorine resistance. | [69] |
| Zeolite -LTA | MPD-TMC-post Treatment | PSU | NaCl rejection and water flux were 99.4 wt.% and 42 L/m·h, respectively, and had a filtration pressure of 300 psi. | [70] |
| F-Silica | MPD-TMC | PSU | When NPs concentration was 0.4 wt.%, the membrane showed high thermal stability. | [71] |
| F-MWCNTs | MPD-TMC | PSU | The membrane showed high dyes and brilliant blue rejection (91%) | [72] |
| Metal alkokxide | MPD: SLS-TMC | PSU | Water flux was encreased by approximately 2-fold when compared with the virgin membrane. | [73] |
| Zeolite NaX | MPD-TMC | PES | Under filtration pressure of 175 psi, the water flux was increased from 8.01 to 29.76 L/m2·h by increasing the content of NPs from 0 to 0.2 wt.% without jeopordizing NaCl rejection (above 90%). Also, the membrane showed good thermal stability. | [74] |
| iLSMM | MPD-TMC | PSU | Under filtration presure of 300 psi, the optimized water flux was 42 L/m2·h and the NaCl rejection was 97%. Besides, the membrane showed good antifouling properties. | [75] |
| MCM-41 | MPD-TMC | PSU | Under filtration pressure of 300 psi, Water flux was increased from 28 to 46 L/m2·h by increasing the concentration of NPs from 0 to 0.1 wt.%, while NaCl rejection was maintained (97 wt.%). | [76] |
| APQZ | MPD-TMC | PSU | Water flux was increased from 16 to 40 L/m2·h by increasing the concentration of NP from 0 to 0.1 wt.%. In addition, the membrane showed good mechanical stability. | [77] |
| Zwitterion-CNT | MPD-TMC | PES | Under 530 psi, the optimized water flux was 48.46 L/m2·h, and NaCl rejection was 98.6%. | [78] |
| Carboxylic MWNTs | MPD-TMC | PES | Under 100 psi, the optimized water flux was 40 L/m2·h. Moreover, the membrane showed good mechanical stability. | [79] |
| Zeolite (Silicate-1) | MPD-TMC | PSU | The membrane showed higher chemical stability than the one with NaX-Zeolite NPs. | [80] |
| Zeolite-NaA | MPD-TMC | PSU | Under 232 psi, good water flux was achieved (46.5 L/m2·h) by adding the NPs in organic phase and high salt rejection (97%) by adding the NPs in aqueous phase. | [81] |
| Aminated Zeolite | MPD:aPES:TEA-TMC | PSU | Under 797 psi, adding PES and TEA to MPD-nanoparticle solution increased water flux from 23.2 to 37.8 L/m2·h without compromising salt rejection (98%). Moreover, the membrane showed good chlorine resistance. | [82] |
| Zeolite-A | MPD-TMC | PSU | The membrnae showed significant fouling resistance. | [83] |
| Mesoporous SiO2 | MPD-TMC | PSU | Under 232 psi, water flux was increased from 19 to 53 L/m2·h by increasing the concentration of NPs from 0 to 0.1 wt.%, while NaCl rejection remained (97%). | [84] |
| HBP-g-silica | MPD: aPES-TMC | PSU | Under 797.7 psi, the optimized water flux was 34.4 L/m2·h, while the salt rejection was 97.7%. And, the membrane showed better chlorine resistance. | [85] |
| Aluminosilicate CNTs | MPD-TMC | PSU | Under 232 psi, the optimized water flux was 23 L/m2·h, while NaCl rejection was 97.5%. | [86] |
| F-MWCNTs | MPD-TMC | PSU | Under 232 psi, the optimized water flux was 28.05 L/m2·h, while salt rejection was 90%. In addition, the membrane showed better antifouling and antioxidant properties. | [87] |
| HNTs | MPD-TMC | PSU | Under 217.5 psi, water flux was increased from 18 to 36.1 L/m2·h by increasing the concentration of NPs from 0 to 0.1% without sacrificing NaCl rejection (93%). Besides, the membrane had enhanced fouling properties. | [88] |
| OA-SiO2 | MPD-TMC | PSU | The OA modified-silica PA membrane produced higher salt rejection (98%) when compared to the unmodified silica PA membrane (95%). | [89] |
| Clay | MPD-TMC | PSU | Under 232 psi, water flux was increased from 36.6 to 51 L/m2·h by adding 0.1 wt.% NPs without compromising NaCl rejection (around 99%). Also, the membrane exhibited significant antifouling properties. | [90] |
| GO-TiO2 | MPD-TMC | PSU | Under 217.5 psi, both water flux and salt rejection were increased from 34 to 51 L/m2·h and 97 to 99%, respectively, by adding 0.02 wt.% NPs. Besides, the membrane demonstrated robust chlorine resistance. | [91] |
| HN2-TNTs | MPD-TMC | PSU | Under 217.5 psi, both water flux and NaCl rejection were increased from 19 to 36 L/m2·h and 94 to 96%, respectively, by adding 0.05 wt.% NPs. Moreover, the membrane showed good fouling resistance. | [92] |
| GO | MPD-TMC | PSU | Under 217 psi, the optimized water flux was 22 L/m2·h, while NaCl rejection was above 80%. Moreover, the modified membrane exhibited excellent fouling resistance against BSA and HA. | [93] |
| Al-ZnO | MPD-TMC | PSU | Under 225 psi, the optimized water flux was 32 L/m2·h, while NaCl rejection was 98%. | [94] |
| MCM-48-SiO2 | MPD-TMC | PSU | Under 232 psi, the optimized water flux was 68 L/m2·h. And, NaCl rejection was around 97%. | [95] |
| GO | MPD-TMC | PSU | Under 300 psi, water flux was increased from 39 to 60 L/m2·h by increasing NPs concentrations from 0 to 0.015 wt.%, while NaCl rejection was above 93%. | [96] |
| ZnO | MPD-TMC | PSU | Under 300 psi, water flux was increased from 60 to 85 L/m2·h by increasing the concentration of ZnO from 0 to 0.1 wt.%. Under UV irradiation the membrane showed super water flux (120 L/m2·h). In addition, the membrane showed excellent fouling resistance. | [97] |
| MOFs | MPD-TMC | PSU | Under operation pressure of 300 psi, water flux and NaCl rejection were 85 L/m2·h and 98.5%, respectively. | [98] |
| Graphene quantum dots | PIP-TMC | PES | Under operation pressure of 0.2 Mpa, water flux was 120 L/m2·h, 6.8-times higher than that of the virgin membrane. Moreover, the membrane showed excellent fouling resistance. | [99] |
| ZIF-8 | MPD-TMC | PSU | 53% enhancement in water flux was achieved. NaCl rejection was 99.4%. | [100] |
| TiO2 | MPD-TMC | PES | The addition of TiO2 resulted in higher water flux (24.3 L/m2·h) as compasred with the virgin TFC (21.5 L/m2·h), while membrane selectivity was preserved (97%). Additionally, by increasing feed solution temeprature from 25 to 65 °C, further enhancement in water flux was achieved. | [101] |
| CQDs | PIP-TMC | PSU | The addition of carbon quantum dots led to significant incerease in permeate flux (from 18 to 42.1 L/m2·h) without jeopordizing Na2SO4 rejection (93%). Moreover, the fouling capacity of membrane was enhanced. | [102] |
| Na+ functionalized CQDs | MPD-TMC | PES | Impresive water flux (104 L/m2·h), high rejection of SeO32 (97.5%), and excellent fouling resistance were achieved when quantum dots concentration was 0.05 wt.%. | [103] |
| SiO2 | MPD-TMC | PSU | Water flux was increased from 30 to 50 L/m2·h by increassing NPs concentration from 0 to 0.1 wt.% along with slight increase in salt rejection (from 92 to 95%). | [104] |
| Ziconiumv (IV)-carboxylate MOFs | MPD-TMC | PSU-PVP-LiCl | 52% increase in water flux was achieved without comprimising NaCl rejection (95.5%). | [105] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Mayyahi, A. Important Approaches to Enhance Reverse Osmosis (RO) Thin Film Composite (TFC) Membranes Performance. Membranes 2018, 8, 68. https://doi.org/10.3390/membranes8030068
Al Mayyahi A. Important Approaches to Enhance Reverse Osmosis (RO) Thin Film Composite (TFC) Membranes Performance. Membranes. 2018; 8(3):68. https://doi.org/10.3390/membranes8030068
Chicago/Turabian StyleAl Mayyahi, Ahmed. 2018. "Important Approaches to Enhance Reverse Osmosis (RO) Thin Film Composite (TFC) Membranes Performance" Membranes 8, no. 3: 68. https://doi.org/10.3390/membranes8030068
APA StyleAl Mayyahi, A. (2018). Important Approaches to Enhance Reverse Osmosis (RO) Thin Film Composite (TFC) Membranes Performance. Membranes, 8(3), 68. https://doi.org/10.3390/membranes8030068




