Graphene Oxide (GO)-Blended Polysulfone (PSf) Ultrafiltration Membranes for Lead Ion Rejection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
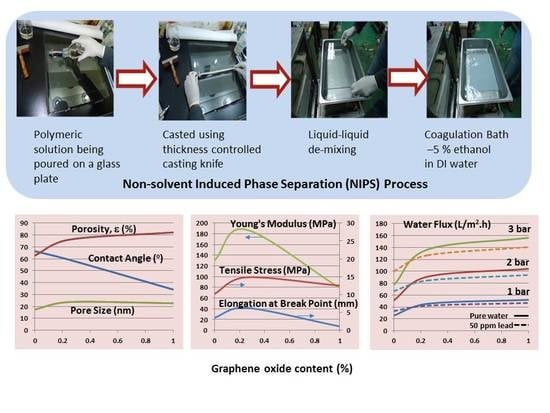
2.2. Preparation of Membrane
2.2.1. Preparation of Casting Solution
2.2.2. Casting of the Membrane
2.3. Characterisation
2.3.1. Hydrophilicity—Water Contact Angle Measurement
2.3.2. Membrane Porosity—Dry Wet Weight Method
2.3.3. Membrane Permeation Test—Flux and Permeability Measurement
- Jv
- permeate flux (L/m2 h)
- A
- effective membrane area (m2)
- Q
- volume flow rate (L/h)
- Jv
- permeate flux (L/m2 h)
- ΔP
- change in pressure (bar)
- Lp
- water permeability (L/(m2 h bar))
2.3.4. Mean Pore Size—Guerout-Elford-Ferry Equation
2.3.5. Mechanical Properties—Tensile Strength
2.3.6. Morphology—Environmental Scanning Electron Microscopy (ESEM)
2.4. Cross-Flow Filtration Cell Setup and Lead Rejection Experiments
3. Results and Discussion
3.1. Characterisation of the Membranes
3.1.1. Hydrophilicity—Water Contact Angle
3.1.2. Membrane Porosity and Pore Size
3.1.3. Membrane Permeation—Pure Water Flux and Permeability
3.1.4. Mechanical Properties—Tensile Strength
3.1.5. Morphology of Membranes
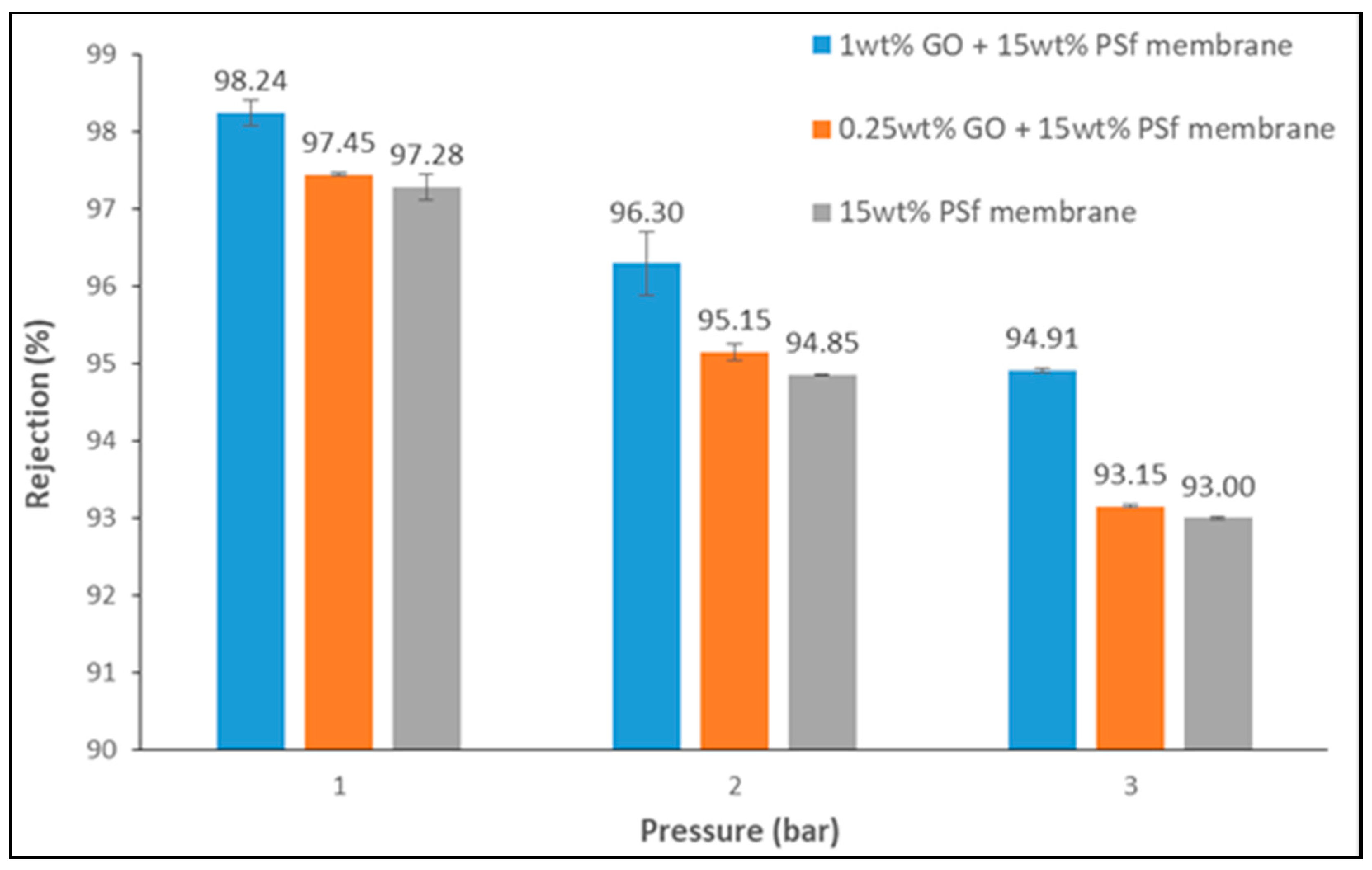
3.2. Lead Rejection
3.3. Performance of Membranes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anderson, J. The environmental benefits of water recycling and reuse. Water Sci. Technol. Water Supply 2003, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Liyanage, C.P.; Yamada, K. Impact of Population Growth on the Water Quality of Natural Water Bodies. Sustainability 2017, 9, 1405. [Google Scholar] [CrossRef]
- Darling, S.B. Perspective: Interfacial materials at the interface of energy and water. J. Appl. Phys. 2018, 124, 030901. [Google Scholar] [CrossRef]
- Azizi, S.; Kamika, I.; Tekere, M. Evaluation of heavy metal removal from wastewater in a modified packed bed biofilm reactor. PLoS ONE 2016, 11, e0155462. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, T.A.; Chan, G.Y.; Lo, W.H.; Babel, S. Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Rezaee, R.; Nasseri, S.; Mahvi, A.H.; Nabizadeh, R.; Mousavi, S.A.; Rashidi, A.; Nazmara, S. Fabrication and characterization of a polysulfone-graphene oxide nanocomposite membrane for arsenate rejection from water. J. Environ. Health Sci. Eng. 2015, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Goh, P.S.; Matsuura, T.; Ismail, A.F.; Hilal, N. Recent trends in membranes and membrane processes for desalination. Desalination 2016, 391, 43–60. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelech, M.; Jiang, Z. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef] [PubMed]
- Mokkapati, V.R.S.S.; Koseoglu-Imer, D.Y.; Yilmaz-Deveci, N.; Mijakovic, I.; Koyuncu, I. Membrane properties and anti-bacterial/anti-biofouling activity of polysulfone-graphene oxide composite membranes phase inversed in graphene oxide non-solvent. RSC Adv. 2017, 7, 4378–4386. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Alam, J.; Alhoshan, M.; Dass, L.A.; Muthumareeswaran, M.R. Development of a nanocomposite ultrafiltration membrane based on polyphenylsulfone blended with graphene oxide. Sci. Rep. 2017, 7, 41976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhang, S.; Chung, T.S. Nanometric graphene oxide framework membranes with enhanced heavy metal removal via nanofiltration. Environ. Sci. Technol. 2015, 49, 10235–10242. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.; Bhunia, P.; De, S. Impact of graphene oxide on removal of heavy metals using mixed matrix membrane. Chem. Eng. J. 2016, 292, 284–297. [Google Scholar] [CrossRef]
- Ganesh, B.M.; Isloor, A.M.; Ismail, A.F. Enhanced hydrophilicity and salt rejection study of graphene oxide-polysulfone mixed matrix membrane. Desalination 2013, 313, 199–207. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Xia, J.; Zhang, F.; Li, F.; Xia, Y.; Li, Y. Novel GO-blended PVDF ultrafiltration membranes. Desalination 2012, 299, 50–54. [Google Scholar] [CrossRef]
- Al-Rashdi, B.A.M.; Johnson, D.J.; Hilal, N. Removal of heavy metal ions by nanofiltration. Desalination 2013, 315, 2–17. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Rahimpour, A. Effect of type of solvent and non-solvents on morphology and performance of polysulfone and polyethersulfone ultrafiltration membranes for milk concentration. Polym. Adv. Technol. 2005, 16, 717–724. [Google Scholar] [CrossRef]
- Winecker, R.E.; Ropero-Miller, J.D.; Broussard, L.A.; Hammett-Stabler, C.A. The toxicology of heavy metals: Getting the lead out. Lab. Med. 2002, 33, 934–947. [Google Scholar] [CrossRef]
- Assi, M.A.; Hezmee, M.N.M.; Haron, A.W.; Sabri, M.Y.M.; Rajion, M.A. The detrimental effects of lead on human and animal health. Vet. World 2016, 9, 660–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravishankar, H.; Wang, J.; Shu, L.; Jegatheesan, V. Removal of Pb(II) ions using polymer based graphene oxide magnetic nano-sorbent. Process Saf. Environ. 2016, 104, 472–480. [Google Scholar] [CrossRef]
- Zhao, G.; Ren, X.; Gao, X.; Tan, X.; Li, J.; Chen, C.; Wang, X. Removal of Pb(II) ions from aqueous solutions on few-layered graphene oxide nanosheets. Dalton Trans. 2011, 40, 10945–10952. [Google Scholar] [CrossRef] [PubMed]
- Ionita, M.; Pandele, A.M.; Crica, L.; Pilan, L. Improving the thermal and mechanical properties of polysulfone by incorporation of graphene oxide. Compos. Part B Eng. 2014, 59, 133–139. [Google Scholar] [CrossRef]
- Ravishankar, H.; Roddick, F.; Navaratna, D.; Jegatheesan, V. Preparation, characterisation and critical flux determination of graphene oxide blended polysulfone (PSf) membranes in an MBR system. J. Environ. Manag. 2018, 213, 168–179. [Google Scholar] [CrossRef] [PubMed]






| Sample | GO (wt/wt, %) | PSf (wt/wt, %) | NMP (wt/wt, %) | Total (g) |
|---|---|---|---|---|
| 1 | 0.00 | 15% | 85.00 | 100 |
| 2 | 0.25 | 15% | 84.75 | 100 |
| 3 | 1.00 | 15% | 84.00 | 100 |
| Membrane | Young’s Modulus (MPa) (Mean ± 3 S.D.) | Tensile Stress (MPa) (Mean ± 3 S.D.) | Elongation at Break (mm) (Mean ± 3 S.D.) | Pore Size (nm) (Mean ± 3 S.D.) |
|---|---|---|---|---|
| 15 wt % PSf | 130.73 ± 15.84 | 3.54 ± 0.23 | 10.23 ± 4.36 | 17.36 ± 5.4 |
| 15 wt % PSf–0.25 wt % GO | 188.13 ± 15.36 | 6.31 ± 0.47 | 14.83 ± 3.75 | 23.72 ± 6.9 |
| 15 wt % PSf–1 wt % GO | 79.46 ± 28.57 | 1.10 ± 0.83 | 12.48 ± 6.5 | 22.73 ± 9.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravishankar, H.; Christy, J.; Jegatheesan, V. Graphene Oxide (GO)-Blended Polysulfone (PSf) Ultrafiltration Membranes for Lead Ion Rejection. Membranes 2018, 8, 77. https://doi.org/10.3390/membranes8030077
Ravishankar H, Christy J, Jegatheesan V. Graphene Oxide (GO)-Blended Polysulfone (PSf) Ultrafiltration Membranes for Lead Ion Rejection. Membranes. 2018; 8(3):77. https://doi.org/10.3390/membranes8030077
Chicago/Turabian StyleRavishankar, Harish, Jens Christy, and Veeriah Jegatheesan. 2018. "Graphene Oxide (GO)-Blended Polysulfone (PSf) Ultrafiltration Membranes for Lead Ion Rejection" Membranes 8, no. 3: 77. https://doi.org/10.3390/membranes8030077
APA StyleRavishankar, H., Christy, J., & Jegatheesan, V. (2018). Graphene Oxide (GO)-Blended Polysulfone (PSf) Ultrafiltration Membranes for Lead Ion Rejection. Membranes, 8(3), 77. https://doi.org/10.3390/membranes8030077