Design of a Hollow Fiber Supported Liquid Membrane System for Zn Speciation in Natural Waters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Instrumentation
2.3. Hollow Fiber Supported Liquid Membrane (HFSLM) Experiments
2.4. Chemical Speciation Software
2.5. Effect of Ligands on Zn Transport with the HFSLM System
2.6. Selectivity of the Membrane System
2.7. Performance of the HFSLM System in Natural Waters
3. Results and Discussion
3.1. Kinetics of the Zn(II) Transport through the HFSLM
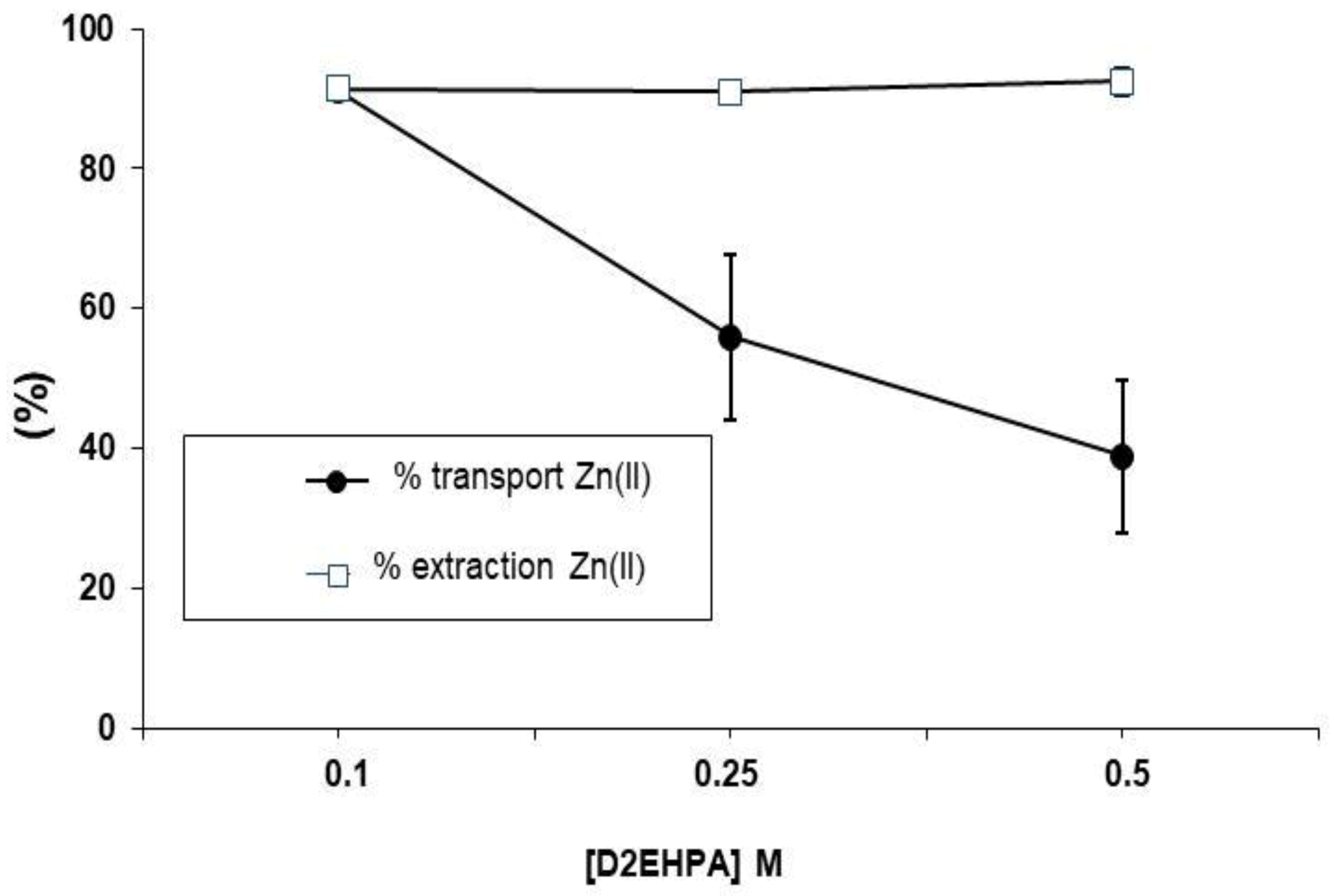
3.2. Influence of D2EHPA Concentration on Zn Transport
3.3. Influence of the Stripping Solution Composition on Zn Transport
3.4. Effect of the Presence of Zn Ligands on the HFSLM System
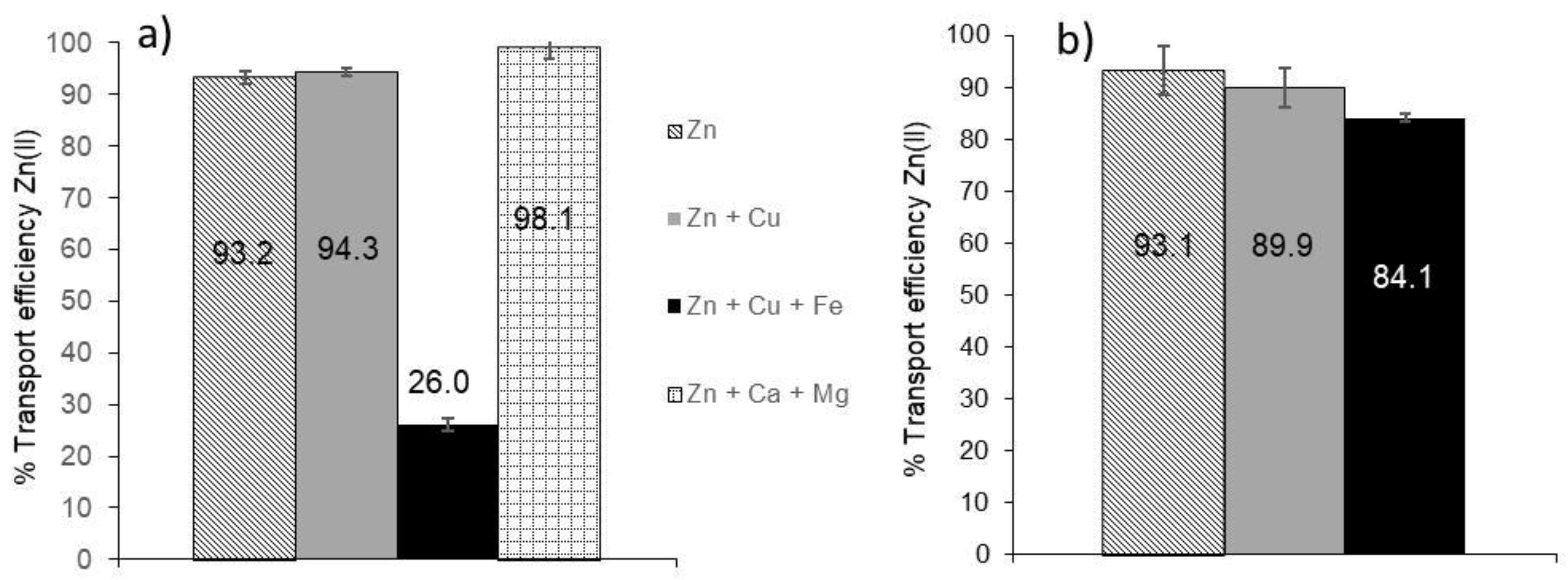
3.5. Selectivity of the HFSLM System towards Different Metals
3.6. Preconcentration of Zinc
3.7. Performance of the HFSLM System in Natural Waters
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Worms, I.; Simon, D.F.; Hassler, C.S.; Wilkinson, K.J. Bioavailability of trace metals to aquatic microorganisms: Importance of chemical, biological and physical processes on biouptake. Biochimie 2006, 88, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.G.C. Interaccions between trace metals and aquatic organisms: A critique of the free-ion activity model. In Metal Speciation and Bioavailability in Aquatic Systems; Tessier, A., Turner, D.R., Eds.; John Wiley & Sons: Chichester, UK, 1995; pp. 45–102. ISBN 978-0-471-95830-7. [Google Scholar]
- Namieśnik, J.; Rabajczyk, A. The speciation and physicochemical forms of metals in surface waters and sediments. Chem. Spec. Bioavailab. 2010, 22, 1–24. [Google Scholar] [CrossRef]
- Nordberg, M.; Templeton, D.M.; Andersen, O.; Duffus, J.H. Glossary of terms used in ecotoxicology (IUPAC recommendations 2009). Pure Appl. Chem. 2009, 81, 829–870. [Google Scholar] [CrossRef]
- Humayan Kabir, A.; Swaraz, A.M.; Stangoulis, J. Zinc-deficiency resistance and biofortification in plants. J. Plant Nutr. Soil Sci. 2014, 177, 311–319. [Google Scholar] [CrossRef]
- Bodar, C.W.M. Environmental Risk Limits for Zinc; RIVM Letter Report 11235; National Institute for Public Health and the Environment: Bilthoven, The Netherlands, 2007; Available online: https://www.rivm.nl/bibliotheek/rapporten/601782004.pdf (accessed on 26 September 2018).
- Pesavento, M.; Alberti, G.; Biesuz, R. Analytical methods for determination of free metal ion concentration, labile species fraction and metal complexation capacity of environmental waters: A review. Anal. Chim. Acta 2009, 631, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Mathiasson, L.; Jönsson, J.Å.; Mathiasson, L. Membrane extraction in analytical chemistry. J. Sep. Sci. 2001, 24, 495–507. [Google Scholar] [CrossRef]
- López-López, J.A.; Mendiguchía, C.; Pinto, J.J.; Moreno, C. Liquid membranes for quantification and speciation of trace metals in natural waters. TRAC Trends Anal. Chem. 2010, 29, 645–653. [Google Scholar] [CrossRef]
- Parthasarathy, N.; Buffle, J. Capabilities of supported liquid membranes for metal speciation in natural waters: Application to copper speciation. Anal. Chim. Acta 1994, 284, 649–659. [Google Scholar] [CrossRef]
- Yang, X.J.; Fane, A.G.; Soldenhoff, K. Comparison of Liquid Membrane Processes for Metal Separations: Permeability, Stability, and Selectivity. Ind. Eng. Chem. Res. 2003, 42, 392–403. [Google Scholar] [CrossRef]
- Parhi, P.K. Supported Liquid Membrane Principle and Its Practices: A Short Review. J. Chem. 2012, 2013, e618236. [Google Scholar] [CrossRef]
- Singh, R.; Mehta, R.; Kumar, V. Simultaneous removal of copper, nickel and zinc metals ions using bulk liquid membrane system. Desalination 2011, 272, 170–173. [Google Scholar] [CrossRef]
- Sánchez, J.M.; Hidalgo, M.; Salvadó, V.; Valiente, M. Extraction of neodymium(III) at trace level with di(2-ethyl-hexyl)phosphoric acid in hexane. Solvent Extr. Ion Exch. 1999, 17, 455–474. [Google Scholar] [CrossRef]
- de Gyves, J.; Rodríguez de San Miguel, E. Metal ions separations by supported liquid membranes. Ind. Eng. Chem. Res. 1999, 38, 2182–2202. [Google Scholar] [CrossRef]
- Resina, M.; Macanás, J.; de Gyves, J.; Muñoz, M. Zn(II), Cd(II) and Cu(II) separation through organic–inorganic Hybrid Membranes containing di-(2-ethylhexyl) phosphoric acid or di-(2-ethylhexyl) dithiophosphoric acid as a carrier. J. Membr. Sci. 2006, 268, 57–64. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Chan, C.; Pettigrove, V.J.; Cattrall, R.W.; Kolev, S.D. Development of a passive sampler for Zinc(II) in urban pond waters using a polymer inclusion membrane. Environ. Pollut. 2014, 193, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Vera, R.; Fontàs, C.; Galceran, J.; Serra, O.; Anticó, E. Polymer inclusion membrane to access Zn speciation: Comparison with root uptake. Sci. Total Environ. 2018, 622–623, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Pont, N.; Salvadó, V.; Fontàs, C. Selective transport and removal of Cd from chloride solutions by polymer inclusion membranes. J. Membr. Sci. 2008, 318, 340–345. [Google Scholar] [CrossRef]
- Bautista-Flores, A.N.; Rodríguez de San Miguel, E.; de Gyves, J.; Jönsson, J.A. Optimization, evaluation, and characterization of a hollow fiber supported líquid membrane for sampling and speciation of lead(II) from aqueous solutions. J. Membr. Sci. 2010, 363, 180–187. [Google Scholar] [CrossRef]
- Rodríguez-Morales, E.A.; Rodríguez de San Miguel, E.; de Gyves, J. Evaluation of the measurement of Cu(II) bioavailability in complex aqueousmedia using a hollow-fiber supported liquid membrane device (HFSLM) and two microalgae species (Pseudokirchneriella subcapitata and Scenedesmus acutus). Environ. Pollut. 2015, 206, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morales, E.A.; Rodríguez de San Miguel, E.; de Gyves, J. Evaluation of a hollow fiber supported líquid membrane device as a chemical suurogate for the measurements of Zn(II) bioavailability using two microalgae strains as biological references. Chemosphere 2017, 171, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Flores, A.N.; Rodríguez de San Miguel, E.; de Gyves, J.; Jönsson, J.A. Nickel(II)preconcentration and speciation analysis during transport from aqueous solutions using a hollow fiber permeation líquid membrane (HFPLM) device. Membranes 2011, 1, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Gramlich, A.; Tandy, S.; Slaveykova, V.I.; Duffner, A.; Schulin, R. The use of permeation liquid membranes for free zinc measurements in aqueous solution. Environ. Chem. 2012, 9, 429–437. [Google Scholar] [CrossRef]
- Rodríguez de San Miguel, E.; Liu, J.; Mayer, P.; Jönsson, J.A. Multivariate analysis of selected metal ion transport through a hollow fiber supported líquid membrane device for passive sampling monitoring. Solvent Extr. Ion Exch. 2008, 26, 602–623. [Google Scholar] [CrossRef]
- Puigdomenech, I. MEDUSA (Make Equilibrium Diagrams Using Sophisticated Algorithms); School of Chemical Science and Engineering, Royal Institute of Technology: Stockholm, Sweden, 2013; Available online: http://www.kth.se/en/che/medusa/ (accessed on 26 September 2018).
- Gustafsson, J.P. Visual MINTEQ (Version 3.1). 2014. Available online: https://vminteq.lwr.kth.se/ (accessed on 26 September 2018).
- Corcoll, N.; Bonet, B.; Morin, S.; Tlili, A.; Leira, M.; Guasch, H. The effect of metals on photosynthesis processes and diatom metrics of biofilm from a metal-contaminated river: A translocation experiment. Ecol. Indic. 2012, 18, 620–631. [Google Scholar] [CrossRef]
- Mansur, M.B.; Slater, M.J.; Biscaia, E.C., Jr. Equilibrium analysis of the reactive liquid–liquid test System ZnSO4/D2EHPA/n-heptane. Hydrometallurgy 2002, 63, 117–126. [Google Scholar] [CrossRef]
- Zhang, B.; Gozzelino, G. Facilitated transport of FeIII and CuII ions through supported liquid membranes. Colloids Surf. A Physicochem. Eng. Asp. 2003, 215, 67–76. [Google Scholar] [CrossRef]
- Venkateswaran, P.; Gopalakrishnan, A.N.; Palanivelu, P. Di(2-ethylhexyl)phosphoric acid-coconut oil supported liquid membrane for the separation of copper ions from copper plating wastewater. J. Environ. Sci. 2007, 19, 1446–1453. [Google Scholar] [CrossRef]
- Ciceri, D.; Mason, L.R.; Harvie, D.J.E.; Perera, J.M.; Stevens, G.W. Extraction kinetics of Fe(III) by di-(2-ethylhexyl) phosphoric acid using a Y–Y shaped microfluidic device. Chem. Eng. Res. Des. 2014, 92, 571–580. [Google Scholar] [CrossRef]
- Rose, A.L.; Waite, D.T. Reconciling kinetic and equilibrium observations of iron(III) solubility in aqueous solutions with a polymer-based model. Geochim. Cosmochim. Acta 2007, 71, 5605–5619. [Google Scholar] [CrossRef]
- Vergel, C.; Mendiguchia, C.; Moreno, C. Liquid Membranes as a Tool for Chemical Speciation of Metals in Natural Waters: Organic and Inorganic Complexes of Nickel. Membranes 2018, 8, 19. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontàs, C.; Anticó, E.; Salvadó, V. Design of a Hollow Fiber Supported Liquid Membrane System for Zn Speciation in Natural Waters. Membranes 2018, 8, 88. https://doi.org/10.3390/membranes8040088
Fontàs C, Anticó E, Salvadó V. Design of a Hollow Fiber Supported Liquid Membrane System for Zn Speciation in Natural Waters. Membranes. 2018; 8(4):88. https://doi.org/10.3390/membranes8040088
Chicago/Turabian StyleFontàs, Clàudia, Enriqueta Anticó, and Victòria Salvadó. 2018. "Design of a Hollow Fiber Supported Liquid Membrane System for Zn Speciation in Natural Waters" Membranes 8, no. 4: 88. https://doi.org/10.3390/membranes8040088
APA StyleFontàs, C., Anticó, E., & Salvadó, V. (2018). Design of a Hollow Fiber Supported Liquid Membrane System for Zn Speciation in Natural Waters. Membranes, 8(4), 88. https://doi.org/10.3390/membranes8040088





