Right Heart Structure, Geometry and Function Assessed by Echocardiography in 6-Year-Old Children Born Extremely Preterm—A Population-Based Cohort Study
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Ethics
2.3. Clinical and Cardiac Assessments
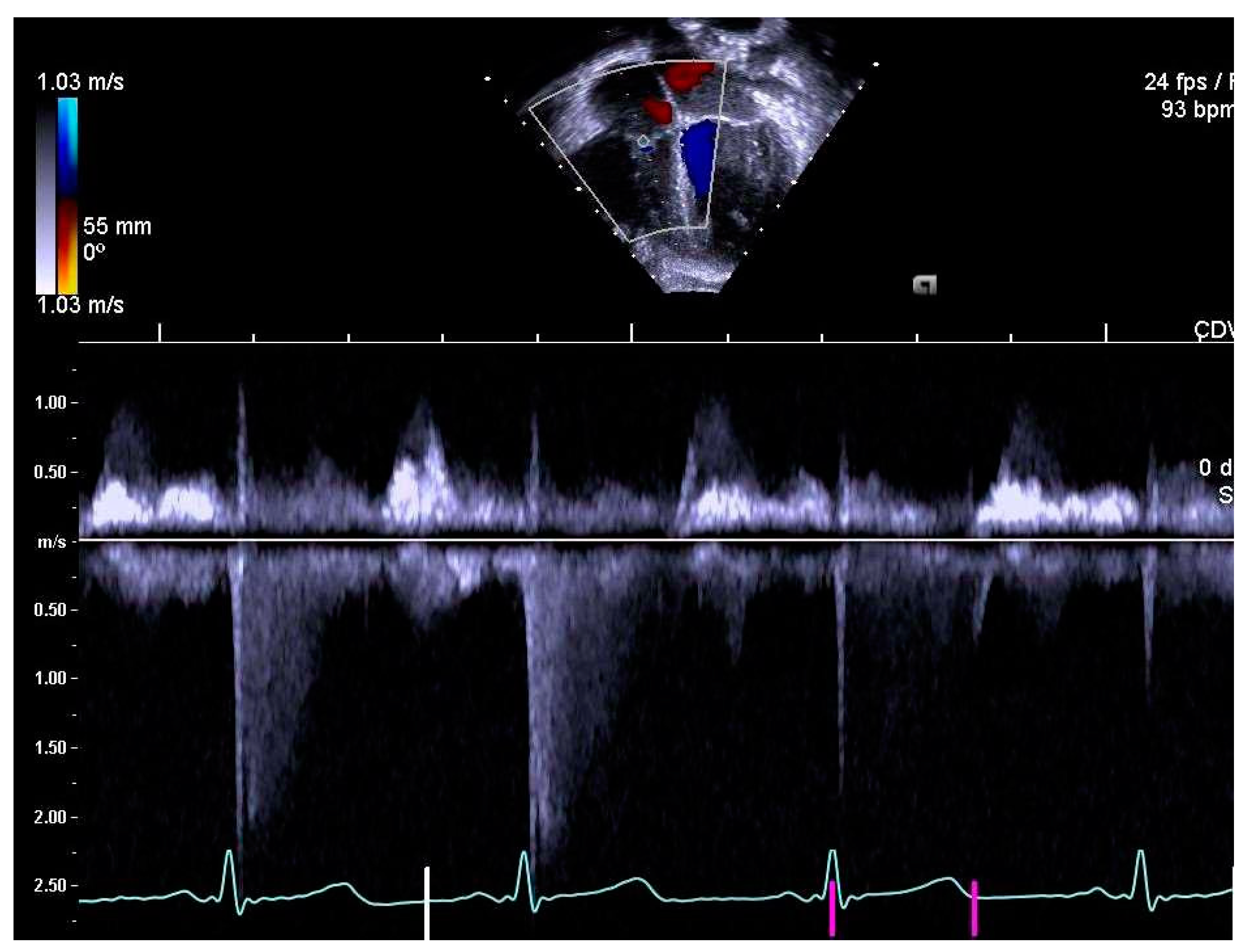
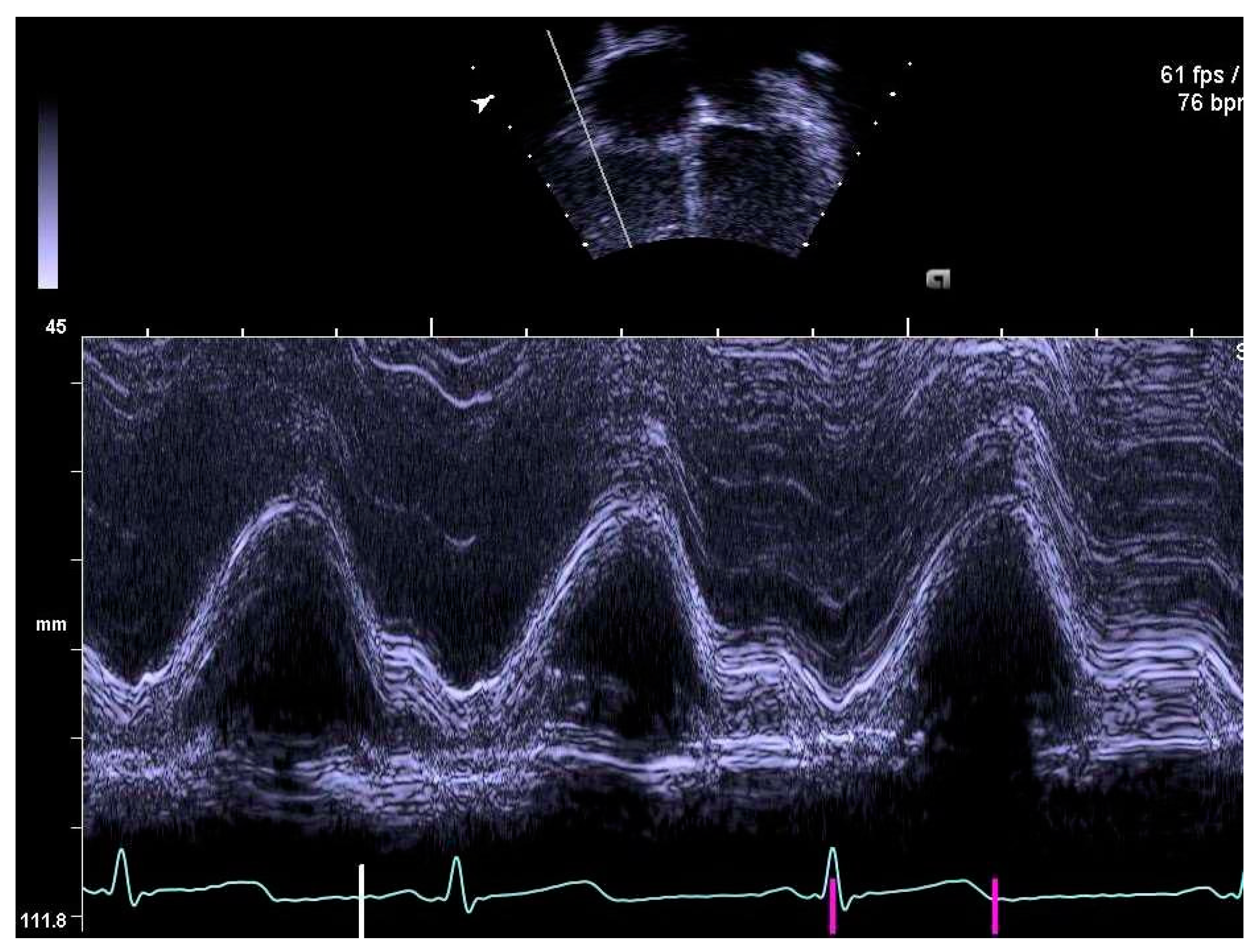
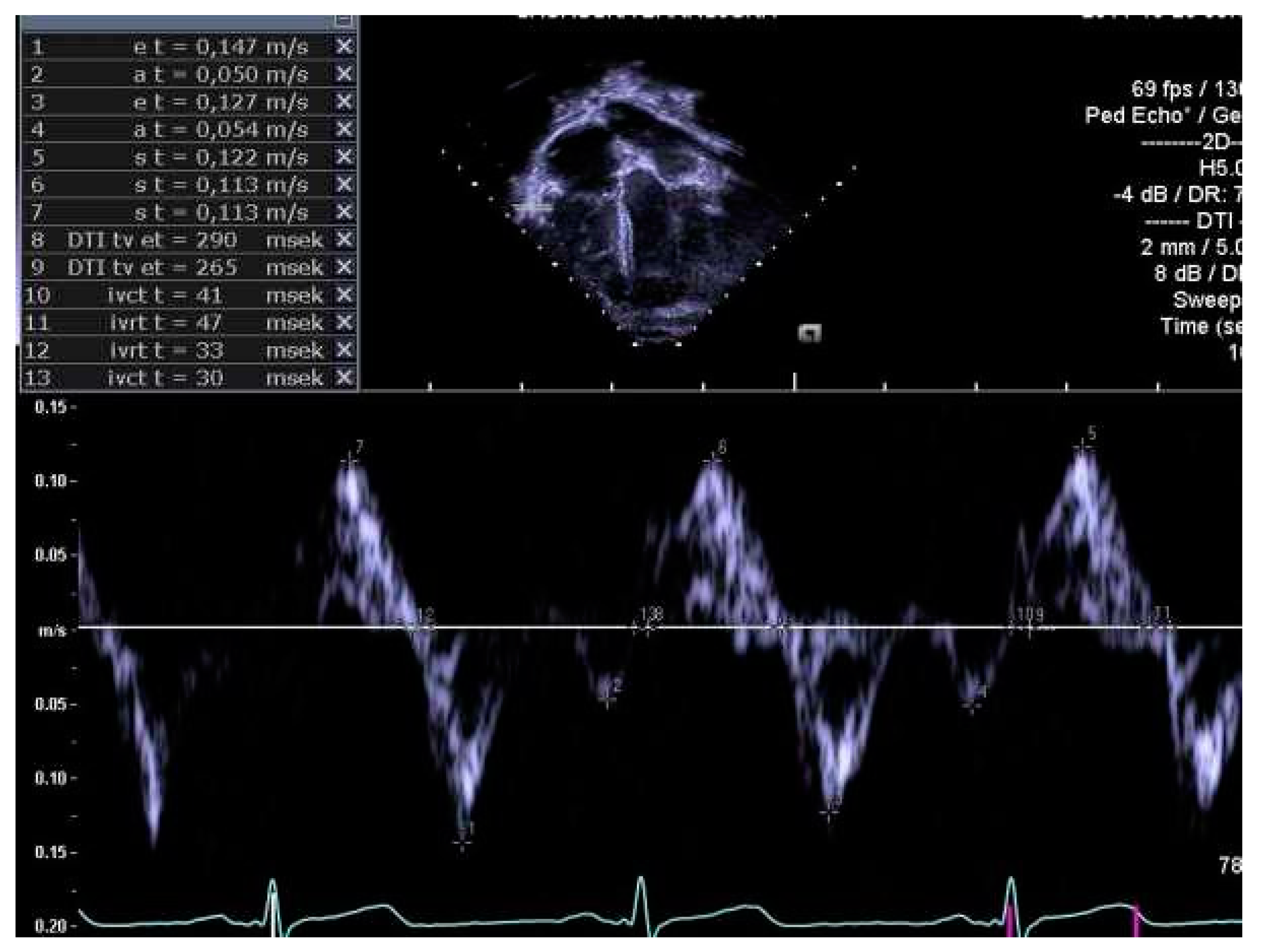
2.4. Determination of Right Heart Outcome Variables
2.5. Statistical Analyses
3. Results
3.1. Cohort Characteristics
3.2. Completeness of Image Analysis
3.3. Right Heart Dimensions, Myocardial Thickness and Volumes
3.4. Pulmonary Artery Diameters
3.5. Right Heart Systolic and Diastolic Functions
3.6. Perinatal Risk Factors and Right Heart Structure and Function in Children Born Extremely Preterm
4. Discussion
Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Glass, H.C.; Costarino, A.T.; Stayer, S.A.; Brett, C.M.; Cladis, F.; Davis, P.J. Outcomes for extremely premature infants. Anesth. Analg. 2015, 120, 1337–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serenius, F.; Sjors, G.; Blennow, M.; Fellman, V.; Holmstrom, G.; Marsal, K.; Lindberg, E.; Olhager, E.; Stigson, L.; Westgren, M.; et al. EXPRESS study shows significant regional differences in 1-year outcome of extremely preterm infants in Sweden. Acta Paediatr. 2014, 103, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Thunqvist, P.; Tufvesson, E.; Bjermer, L.; Winberg, A.; Fellman, V.; Domellof, M.; Melen, E.; Norman, M.; Hallberg, J. Lung function after extremely preterm birth-A population-based cohort study (EXPRESS). Pediatr. Pulmonol. 2018, 53, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Mourani, P.M.; Sontag, M.K.; Younoszai, A.; Miller, J.I.; Kinsella, J.P.; Baker, C.D.; Poindexter, B.B.; Ingram, D.A.; Abman, S.H. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2015, 191, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolton, C.E.; Stocks, J.; Hennessy, E.; Cockcroft, J.R.; Fawke, J.; Lum, S.; McEniery, C.M.; Wilkinson, I.B.; Marlow, N. The EPICure study: Association between hemodynamics and lung function at 11 years after extremely preterm birth. J. Pediatr. 2012, 161, 595–601.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewandowski, A.J.; Bradlow, W.M.; Augustine, D.; Davis, E.F.; Francis, J.; Singhal, A.; Lucas, A.; Neubauer, S.; McCormick, K.; Leeson, P. Right ventricular systolic dysfunction in young adults born preterm. Circulation 2013, 128, 713–720. [Google Scholar] [CrossRef] [Green Version]
- Naumburg, E.; Soderstrom, L. Increased risk of pulmonary hypertension following premature birth. BMC Pediatr 2019, 19, 288. [Google Scholar] [CrossRef]
- Goss, K.N.; Beshish, A.G.; Barton, G.P.; Haraldsdottir, K.; Levin, T.S.; Tetri, L.H.; Battiola, T.J.; Mulchrone, A.M.; Pegelow, D.F.; Palta, M.; et al. Early Pulmonary Vascular Disease in Young Adults Born Preterm. Am. J. Respir. Crit. Care Med. 2018, 198, 1549–1558. [Google Scholar] [CrossRef]
- Mohlkert, L.A.; Hallberg, J.; Broberg, O.; Hellstrom, M.; Pegelow Halvorsen, C.; Sjoberg, G.; Edstedt Bonamy, A.K.; Liuba, P.; Fellman, V.; Domellof, M.; et al. Preterm arteries in childhood: Dimensions, intima-media thickness, and elasticity of the aorta, coronaries, and carotids in 6-y-old children born extremely preterm. Pediatr. Res. 2017, 81, 299–306. [Google Scholar] [CrossRef]
- Mohlkert, L.A.; Hallberg, J.; Broberg, O.; Rydberg, A.; Pegelow Halvorsen, C.; Liuba, P.; Fellman, V.; Domellöf, M.; Sjöberg, G.; Norman, M. The Preterm Heart in Childhood: Left Ventricular Structure, Geometry and Function Assessed by Echocardiography in 6-Year-Old Survivors of Periviable Births. JAHA 2018, 7, e007742. [Google Scholar] [CrossRef] [Green Version]
- Group, E.; Fellman, V.; Hellstrom-Westas, L.; Norman, M.; Westgren, M.; Kallen, K.; Lagercrantz, H.; Marsal, K.; Serenius, F.; Wennergren, M. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA 2009, 301, 2225–2233. [Google Scholar]
- Group, E. Incidence of and risk factors for neonatal morbidity after active perinatal care: Extremely preterm infants study in Sweden (EXPRESS). Acta Paediatr. 2010, 99, 978–992. [Google Scholar] [CrossRef] [PubMed]
- Serenius, F.; Blennow, M.; Marsal, K.; Sjors, G.; Kallen, K.; Group, E.S. Intensity of perinatal care for extremely preterm infants: Outcomes at 2.5 years. Pediatrics 2015, 135, e1163–e1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serenius, F.; Ewald, U.; Farooqi, A.; Fellman, V.; Hafstrom, M.; Hellgren, K.; Marsal, K.; Ohlin, A.; Olhager, E.; Stjernqvist, K.; et al. Neurodevelopmental Outcomes Among Extremely Preterm Infants 6.5 Years After Active Perinatal Care in Sweden. JAMA Pediatr. 2016, 170, 954–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamir, I.; Stoltz Sjostrom, E.; Edstedt Bonamy, A.K.; Mohlkert, L.A.; Norman, M.; Domellof, M. Postnatal nutritional intakes and hyperglycemia as determinants of blood pressure at 6.5 years of age in children born extremely preterm. Pediatr. Res. 2019, 86, 115–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohlkert, L.A.; Sjoberg, G.; Rydberg, A.; Pegelow Halvorsen, C.; Tufvesson, E.; Hallberg, J.; Domellof, M.; Norman, M. Lung function and pulmonary vascular resistance are not associated in 6-year-old children born extremely preterm. Acta Paediatr. 2019, 109, 746–753. [Google Scholar] [CrossRef]
- Edstedt Bonamy, A.K.; Mohlkert, L.A.; Hallberg, J.; Liuba, P.; Fellman, V.; Domellof, M.; Norman, M. Blood Pressure in 6-Year-Old Children Born Extremely Preterm. J. Am. Heart Assoc. 2017, 6, e005858. [Google Scholar] [CrossRef]
- Marsal, K.; Persson, P.H.; Larsen, T.; Lilja, H.; Selbing, A.; Sultan, B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996, 85, 843–848. [Google Scholar] [CrossRef]
- Haycock, G.B.; Schwartz, G.J.; Wisotsky, D.H. Geometric method for measuring body surface area: A height-weight formula validated in infants, children, and adults. J. Pediatr. 1978, 93, 62–66. [Google Scholar] [CrossRef]
- Abbas, A.E.; Fortuin, F.D.; Schiller, N.B.; Appleton, C.P.; Moreno, C.A.; Lester, S.J. A simple method for noninvasive estimation of pulmonary vascular resistance. J. Am. Coll. Cardiol. 2003, 41, 1021–1027. [Google Scholar] [CrossRef] [Green Version]
- Jone, P.N.; Ivy, D.D. Echocardiography in pediatric pulmonary hypertension. Front. Pediatr. 2014, 2, 124. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.E.; Franey, L.M.; Marwick, T.; Maeder, M.T.; Kaye, D.M.; Vlahos, A.P.; Serra, W.; Al-Azizi, K.; Schiller, N.B.; Lester, S.J. Noninvasive assessment of pulmonary vascular resistance by Doppler echocardiography. J. Am. Soc. Echocardiogr. 2013, 26, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, A.J.; Augustine, D.; Lamata, P.; Davis, E.F.; Lazdam, M.; Francis, J.; McCormick, K.; Wilkinson, A.R.; Singhal, A.; Lucas, A.; et al. Preterm heart in adult life: Cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation 2013, 127, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telles, F.; McNamara, N.; Nanayakkara, S.; Doyle, M.P.; Williams, M.; Yaeger, L.; Marwick, T.H.; Leeson, P.; Levy, P.T.; Lewandowski, A.J. Changes in the Preterm Heart From Birth to Young Adulthood: A Meta-analysis. Pediatrics 2020, 146, e20200146. [Google Scholar] [CrossRef]
- Carr, H.; Cnattingius, S.; Granath, F.; Ludvigsson, J.F.; Edstedt Bonamy, A.K. Reply: Preterm Birth and Risk of Heart Failure in Childhood and Early Adulthood. J. Am. Coll. Cardiol. 2017, 70, 1944–1945. [Google Scholar] [CrossRef]
- Kjellberg, M.; Sanchez-Crespo, A.; Jonsson, B. Ten-year-old children with a history of bronchopulmonary dysplasia have regional abnormalities in ventilation perfusion matching. Pediatr. Pulmonol. 2019, 54, 602–609. [Google Scholar] [CrossRef] [Green Version]
- Mrocki, M.M.; Nguyen, V.B.; Lombardo, P.; Sutherland, M.R.; Bensley, J.G.; Nitsos, I.; Allison, B.J.; Harding, R.; De Matteo, R.; Schneider, M.; et al. Moderate preterm birth affects right ventricular structure and function and pulmonary artery blood flow in adult sheep. J. Physiol. 2018, 596, 5965–5975. [Google Scholar] [CrossRef] [Green Version]
- Sano, H.; Tanaka, H.; Motoji, Y.; Fukuda, Y.; Mochizuki, Y.; Hatani, Y.; Matsuzoe, H.; Hatazawa, K.; Shimoura, H.; Ooka, J.; et al. Right ventricular relative wall thickness as a predictor of outcomes and of right ventricular reverse remodeling for patients with pulmonary hypertension. Int. J. Cardiovasc. Imaging 2017, 33, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Goss, K.N.; Haraldsdottir, K.; Beshish, A.G.; Barton, G.P.; Watson, A.M.; Palta, M.; Chesler, N.C.; Francois, C.J.; Wieben, O.; Eldridge, M.W. Association Between Preterm Birth and Arrested Cardiac Growth in Adolescents and Young Adults. JAMA Cardiol. 2020, 5, 910–919. [Google Scholar] [CrossRef]
- Bonamy, A.K.; Kallen, K.; Norman, M. High blood pressure in 2.5-year-old children born extremely preterm. Pediatrics 2012, 129, e1199–e1204. [Google Scholar] [CrossRef] [Green Version]
- Naeije, R.; Badagliacca, R. The overloaded right heart and ventricular interdependence. Cardiovasc. Res 2017, 113, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Kukulski, T.; Hubbert, L.; Arnold, M.; Wranne, B.; Hatle, L.; Sutherland, G.R. Normal regional right ventricular function and its change with age: A Doppler myocardial imaging study. J. Am. Soc. Echocardiogr. 2000, 13, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Lamata, P.; Williamson, W.; Alsharqi, M.; Tan, C.M.J.; Burchert, H.; Huckstep, O.J.; Suriano, K.; Francis, J.M.; Pelado, J.L.; et al. Multimodality Imaging Demonstrates Reduced Right-Ventricular Function Independent of Pulmonary Physiology in Moderately Preterm-Born Adults. JACC Cardiovasc. Imaging 2020, 13, 2046–2048. [Google Scholar] [CrossRef] [PubMed]
- Breatnach, C.R.; Franklin, O.; James, A.T.; McCallion, N.; El-Khuffash, A. The impact of a hyperdynamic left ventricle on right ventricular function measurements in preterm infants with a patent ductus arteriosus. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F446–F450. [Google Scholar] [CrossRef] [PubMed]
- Schubert, U.; Muller, M.; Abdul-Khaliq, H.; Norman, M. Preterm Birth Is Associated with Altered Myocardial Function in Infancy. J. Am. Soc. Echocardiogr. 2016, 29, 670–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friesen, R.M.; Schafer, M.; Burkett, D.A.; Cassidy, C.J.; Ivy, D.D.; Jone, P.N. Right Ventricular Tissue Doppler Myocardial Performance Index in Children with Pulmonary Hypertension: Relation to Invasive Hemodynamics. Pediatr. Cardiol. 2018, 39, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, D.G.; Malouf, P.J.; Gurudevan, S.V.; Auger, W.R.; Madani, M.M.; Thistlethwaite, P.; Waltman, T.J.; Daniels, L.B.; Raisinghani, A.B.; DeMaria, A.N. Utility of right ventricular Tei index in the noninvasive evaluation of chronic thromboembolic pulmonary hypertension before and after pulmonary thromboendarterectomy. JACC Cardiovasc. Imaging 2009, 2, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Nagiub, M.; Kanaan, U.; Simon, D.; Guglani, L. Risk Factors for Development of Pulmonary Hypertension in Infants with Bronchopulmonary Dysplasia: Systematic Review and Meta-Analysis. Paediatr. Respir. Rev. 2017, 23, 27–32. [Google Scholar] [CrossRef]
- Kwon, H.W.; Kim, H.S.; An, H.S.; Kwon, B.S.; Kim, G.B.; Shin, S.H.; Kim, E.K.; Bae, E.J.; Noh, C.I.; Choi, J.H. Long-Term Outcomes of Pulmonary Hypertension in Preterm Infants with Bronchopulmonary Dysplasia. Neonatology 2016, 110, 181–189. [Google Scholar] [CrossRef]
- Zhang, G.; Feenstra, B.; Bacelis, J.; Liu, X.; Muglia, L.M.; Juodakis, J.; Miller, D.E.; Litterman, N.; Jiang, P.P.; Russell, L.; et al. Genetic Associations with Gestational Duration and Spontaneous Preterm Birth. N. Engl. J. Med. 2017, 377, 1156–1167. [Google Scholar] [CrossRef]

| EXPT (n = 176) | CTRL (n = 134) | p-Value | |
|---|---|---|---|
| Maternal data | |||
| Age, years (mean and range) | 31.4 (18–46) | 31.8 (21–43) | 0.47 |
| University education | 82 (47%) | 84 (63%) | 0.004 |
| Family history of CVD a | 128 (74%) | 102 (77%) | 0.65 |
| Pregnancy data | |||
| Maternal smoking | 9 (5%) | 2 (1%) | 0.09 |
| Preeclampsia | 16 (10%) | 0 | NA |
| Multiple birth | 29 (17%) | 0 | NA |
| Neonatal data | |||
| Gestational age, weeks Range | 24.9 (1.0) 22–26 | 39.4 (1.2) 37–41 | NA |
| Boys | 98 (55%) | 79 (60%) | 0.53 |
| Birth weight, g Range | 788 (169) 348–1161 | 3591 (461) 2430–4315 | NA |
| SGA at birth | 27 (16%) | 0 | NA |
| BPD moderate | 131 (74%) | 0 | NA |
| BPD severe | 29 (16%) | 0 | NA |
| PDA | 106 (60%) | 0 | NA |
| Mechanical ventilation | 148/171 (87%) | 0 | |
| Duration of mechanical ventilation, days Range | 14.9 (15.5) (0–121) | 0 | NA |
| 6.5 year follow-up | |||
| Age, months | 80.8 (2.3) | 81.1 (2.0) | 0.23 |
| Weight, kg | 20.6 (3.6) | 24.3 (3.9) | <0.001 |
| Height, cm | 118.1 (5.6) | 123.1 (5.0) | <0.001 |
| BMI, kg/m2 | 14.7 (1.6) | 16.0 (2.0) | <0.001 |
| BSA, m2 | 0.82 (0.09) | 0.91 (0.09) | <0.001 |
| HR, bpm | 88 (13) | 85 (9) | 0.016 |
| SBP, mmHg | 98 (8) | 97 (8) | 0.38 |
| DBP, mmHg | 57 (6) | 55 (6) | 0.005 |
| Accepted for Analysis EXPT/CTRL | EXPT a, n = 172 | CTRL a, n = 133 | p-value | Adjusted Mean Difference b, (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| RA dimensions | ||||||
| RA length | 151/126 | 31.9 (3.4) | 35.6 (3.4) | <0.001 | −1.1 (−1.8;−0.3) | 0.006 |
| RA width | 150/126 | 27.9 (3.1) | 31.5 (3.2) | <0.001 | −2.0 (−2.8;−1.2) | <0.001 |
| RA SI | 150/126 | 1.2 (0.1) | 1.1 (0.1) | 0.29 | 0.04 (0.008;0.08) | 0.016 |
| RV dimensions | ||||||
| RV length | 152/128 | 50.7 (4.5) | 53.4 (4.1) | <0.001 | −0.5 (−1.6;0.6) | 0.34 |
| RVwidth | 152/129 | 27.2 (2.8) | 29.4 (2.8) | <0.001 | −1.7 (−2.4;−0.9) | <0.001 |
| RV SI | 152/128 | 1.9 (0.2) | 1.8 (0.2) | 0.04 | 0.09 (0.03;0.1) | 0.002 |
| LV/RV length | 151/127 | 1.08 (0.06) | 1.10 (0.06) | 0.004 | −0.02 (−0.04;−0.008) | 0.004 |
| Wall thickness | ||||||
| RVAW | 100/112 | 2.6 (0.7) | 2.7 (0.8) | 0.59 | −0.007 (−0.2;0.2) | 0.95 |
| IVS | 156/132 | 5.5 (0.9) | 6.1 (0.8) | <0.001 | −0.09 (−0.3;0.1) | 0.39 |
| RWT | 95/109 | 0.32 (0.04) | 0.30 (0.04) | 0.012 | 0.02 (0.002;0.03) | 0.026 |
| PA dimensions | ||||||
| PV ann | 130/90 | 16.2 (2.2) | 17.7 (1.8) | <0.001 | −0.3 (−0.8;0.3) | 0.33 |
| MPA | 141/125 | 17.0 (1.8) | 18.0 (1.8) | 0.001 | −0.008 (−0.5;0.5) | 0.97 |
| LPA | 120/112 | 10.4 (2.3) | 12.0 (1.4) | <0.001 | −0.2 (−0.6;0.2) | 0.34 |
| RPA | 125/118 | 10.3 (2.2) | 11.8 (1.4) | <0.001 | −0.02 (−0.4;0.3) | 0.89 |
| Volumes | ||||||
| SV, ml | 117/85 | 15.2 (3.2) | 17.0 (2.7) | 0.0001 | −1.1 (−2.0;−0.1) | 0.026 |
| CO, l/min | 117/85 | 1.3 (0.3) | 1.4 (0.2) | 0.011 | −0.07 (−0.2;0.02) | 0.11 |
| Accepted for Analysis EXPT/CTRL | EXPT a n = 172 | CTRL a n = 133 | p-Value | Adjusted Mean Difference b, (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| Systolic function | ||||||
| TAPSE, mm | 146/123 | 20.7 (2.9) | 21.2 (2.7) | 0.15 | −0.8 (0.5;1.5) * | 0.037 |
| TVs’septal, cm/s | 143/114 | 6.7 (1.0) | 6.6 (0.7) | 0.53 | 0.1 (−0.4;0.1) | 0.28 |
| TVs’ free wall, cm/s | 115/77 | 11.4 (2.2) | 11.8 (1.7) | 0.15 | −0.5 (−1.1;0.1) | 0.10 |
| mpi’, septal | 114/83 | 0.44 (0.07) | 0.45 (0.07) | 0.16 | −0.01 (−0.04;009) | 0.24 |
| mpi’, free wall | 132/110 | 0.35 (0.1) | 0.34 (0.1) | 0.18 | −0.03 (−0.05;−0.003) | 0.027 |
| PVR | 116/117 | 0.187 (0.04) | 0.174 (0.03) | 0.006 | 0.02 (0.01;0.03) | <0.001 |
| TR, m/s | 125/121 | 2.0 (0.1) | 2.0 (0.3) | 0.31 | 0.1 (0.04;0.2) | 0.001 |
| RVOTvti, cm | 148/126 | 11.9 (2.4) | 11.8 (1.8) | 0.71 | −0.5 (−1.0;−0.07) | 0.024 |
| Diastolic function | ||||||
| TVE, cm/s | 147/129 | 50.5 (11) | 49.1 (10) | 0.28 | −1.0 (−3.4;1.5) | 0.44 |
| TVA, cm/s | 139/126 | 32.6 (7.8) | 32.0 (7.9) | 0.56 | −0.4 (−2.4;1.6) | 0.68 |
| Septal | ||||||
| Annular e’, cm/s | 143/113 | 12.4 (1.4) | 12.1 (1.3) | 0.13 | 0.09 (−0.3;0.5) | 0.63 |
| Annular a’, cm/s | 143/114 | 4.5 (1.1) | 4.5 (0.9) | 0.45 | −0.02 (−0.3;0.2) | 0.91 |
| E/e’ | 132/112 | 4.1 (0.9) | 4.0 (1.0) | 0.38 | −0.09 (−0.3;0.2) | 0.46 |
| ivct, msec | 143/113 | 60 (12) | 65 (13) | 0.001 | −4.6 (−8.0;−1.3) | 0.006 |
| ivrt, msec | 143/112 | 57 (10) | 56 (8) | 0.65 | −0.7 (−2.9;1.6) | 0.57 |
| Free wall | ||||||
| TVe’, cm/s | 134/113 | 15.0 (2.7) | 14.1 (2.1) | 0.003 | 0.5 (−0.1;1.2) | 0.13 |
| TVa’, cm/s | 132/113 | 7.6 (2.0) | 6.8 (1.7) | 0.003 | 0.5 (−0.01;1.0) | 0.056 |
| E/e’ | 124/112 | 3.4 (0.8) | 3.5 (0.9) | 0.23 | −0.2 (−0.4;−0.002) | 0.059 |
| ivct, msec | 133/113 | 59 (14) | 65 (16) | 0.002 | −5.9 (−9.8;−2.0) | 0.003 |
| ivrt, msec | 133/111 | 25 (13) | 35 (21) | <0.001 | −0.6 (−3.9;3.1) | 0.72 |
| Accepted for Analysis PDA/No PDA | PDA a (n = 106) | No PDA a (n = 66) | p-Value | Adjusted Mean Difference b, (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| RA dimensions | ||||||
| RA length | 87/57 | 31.5 (3.1) | 32.6 (3.7) | 0.054 | −0.6 (−1.4;0.3) | 0.18 |
| RA width | 86/57 | 27.6 (3.3) | 28.4 (2.8) | 0.12 | −0.7 (−1.7;0.3) | 0.18 |
| RA SI | 86/57 | 1.2 (0.1) | 1.2 (0.1) | 0.94 | 0.01 (−0.03;0.06) | 0.54 |
| RV dimensions | ||||||
| RV length | 88/57 | 50.3 (4.7) | 51.2 (4.2) | 0.20 | −0.4 (−1.6;0.9) | 0.57 |
| RV width | 88/57 | 27.0 (2.9) | 27.4 (2.6) | 0.35 | −0.2 (−1.1;0.7) | 0.68 |
| RV SI | 88/57 | 1.9 (0.2) | 1.9 (0.2) | 0.98 | 0.005 (−0.6;0.07) | 0.86 |
| LV/RV length | 88/56 | 1.1 (0.06) | 1.1 (0.06) | 0.99 | 0.008 (−0.01;0.03) | 0.41 |
| PA dimensions | ||||||
| PV ann | 75/50 | 16.0 (2.3) | 16.4 (1.9) | 0.44 | −0.2 (−0.9;0.4) | 0.50 |
| MPA | 80/54 | 16.9 (1.9) | 17.2 (1.8) | 0.30 | −0.2 (−0.8;0.4) | 0.47 |
| LPA | 70/44 | 10.2 (2.4) | 10.5 (2.0) | 0.51 | −0.01 (−0.6;0.5) | 0.96 |
| RPA | 73/44 | 10.0 (2.2) | 10.5 (2.0) | 0.30 | 0.008 (−0.4;0.4) | 0.97 |
| Wall thickness | ||||||
| RVAW | 57/38 | 2.7 (0.7) | 2.6 (0.8) | 0.33 | 0.09 (−0.2;0.4) | 0.55 |
| IVS | 90/54 | 5.5 (0.9) | 5.6 (0.9) | 0.21 | 0.007 (−0.3;0.3) | 0.96 |
| RWT | 55/35 | 0.32 (0.04) | 0.32 (0.05) | 0.78 | 0.003 (−0.02;0.02) | 0.77 |
| Volume | ||||||
| SV, ml | 66/46 | 14.8 (2.9) | 16.0 (3.6) | 0.040 | −0.9 (−2.1;0.3) | 0.14 |
| CO, l/min | 66/46 | 1.3 (0.3) | 1.3 (0.3) | 0.68 | 0.006 (−0.1;0.1) | 0.92 |
| Accepted for Analysis PDA/No PDA | PDA a (n = 103) | No PDA a (n = 66) | p-Value | Adjusted Mean Difference b, (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| Systolic function | ||||||
| TAPSE, mm | 84/55 | 20.2 (2.7) | 21.4 (2.8) | 0.010 | −0.8 (−1.7;0.1) * | 0.08 |
| TV s’ septal, cm/s | 87/49 | 6.7 (1.1) | 6.6 (0.8) | 0.36 | 0.2 (−0.2;0.5) | 0.27 |
| TV s’ free wall, cm/s | 67/42 | 11.3 (2.1) | 11.6 (2.1) | 0.58 | −0.2 (−1.0;0.6) | 0.69 |
| mpi’, septal | 70/37 | 0.44 (0.07) | 0.44 (0.06) | 0.49 | 0.01 (−0.02;0.04) | 0.49 |
| mpi’, free wall | 79/46 | 0.37 (0.1) | 0.33 (0.1) | 0.082 | 0.03 (0.002;0.06) | 0.036 |
| PVR | 64/44 | 0.189 (0.04) | 0.170 (0.04) | 0.023 | 0.01 (0.001;0.03) | 0.032 |
| TR, m/s | 72/47 | 2.0 (0.3) | 2.0 (0.4) | 0.82 | 0.003 (−0.09;0.1) | 0.95 |
| RVOTvti, m | 83/58 | 0.12 (0.02) | 0.13 (0.03) | 0.034 | −0.008 (−0.02;−0.001) | 0.020 |
| Diastolic function | ||||||
| TVE, cm/s | 85/55 | 49.9 (11.8) | 51.9 (10.6) | 0.30 | −2.0 (−5.5;1.4) | 0.25 |
| TVA, cm/s | 82/50 | 32.9 (8.2) | 31.7 (7.5) | 0.41 | 0.7 (−2.1;3.5) | 0.62 |
| Septal | ||||||
| Annular e’, cm/s | 87/49 | 12.3 (1.5) | 12.6 (1.4) | 0.15 | −0.4 (−0.9;0.1) | 0.13 |
| Annular a’, cm/s | 87/49 | 4.7 (1.1) | 4.3 (1.0) | 0.06 | 0.4 (0.01;0.8) | 0.042 |
| E/e’ | 80/45 | 4.1 (1.0) | 4.2 (0.9) | 0.65 | −0.08 (−0.4;0.2) | 0.64 |
| ivct, ms | 87/49 | 60 (13) | 59 (10) | 0.59 | 1.1 (−3.3;5.5) | 0.62 |
| ivrt, ms | 87/49 | 56 (10) | 58 (10) | 0.29 | −1.6 (−4.9;1.8) | 0.35 |
| Free wall | ||||||
| TVe’, cm/s | 80/47 | 14.9 (2.8) | 15.5 (2.6) | 0.27 | −0.5 (−1.5;0.4) | 0.28 |
| TVa’, cm/s | 78/47 | 7.6 (2.0) | 7.3 (1.7) | 0.38 | 0.4 (−0.3;1.0) | 0.31 |
| E/e’ | 74/43 | 3.4 (0.9) | 3.3 (0.7) | 0.40 | 0.1 (−0.2;0.4) | 0.39 |
| ivct, ms | 79/47 | 61 (15) | 57 (11) | 0.058 | 4.8 (−0.2;9.8) | 0.061 |
| ivrt, ms | 79/47 | 35 (21) | 35 (22) | 0.92 | −0.2 (−4.4;4.0) | 0.94 |
| Accepted for Analysis BPD No or Moderate/Severe | BPD a No or Moderate n = 131 | BPD a Severe n = 29 | p-Value | Adjusted Mean Difference b, (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| RA dimensions | ||||||
| RA length | 113/24 | 32.2 (3.3) | 30.3 (3.4) | 0.012 | 0.18 (−1.33;0.98) | 0.76 |
| RA width | 112/24 | 28.0 (3.0) | 26.6 (3.2) | 0.046 | 0.49 (−1.83;0.85) | 0.47 |
| RA SI | 112/24 | 1.16 (0.12) | 1.15 (0.14) | 0.72 | 0.01 (−0.04;0.07) | 0.65 |
| RV dimensions | ||||||
| RV length | 113/24 | 50.7 (4.3) | 50.3 (5.7) | 0.70 | 1.15 (−0.56;2.86) | 0.19 |
| RV width | 113/24 | 27.3 (2.7) | 26.2 (3.3) | 0.07 | 1.22 (−2.4;−0.03) | 0.044 |
| RV SI | 113/24 | 1.87 (0.19) | 1.93 (0.17) | 0.13 | −0.12 (0.041;0.20) | 0.004 |
| LV/RV length | 112/24 | 1.08 (0.06) | 1.08 (0.06) | 0.81 | 0.01 (−0.04;0.02) | 0.46 |
| PA dimensions | ||||||
| PAV ann | 96/24 | 16.3 (2.1) | 15.3 (2.0) | 0.024 | 0.44 (−1.25;0.37) | 0.28 |
| MPA | 104/23 | 17.2 (1.8) | 16.0 (1.5) | 0.005 | 0.6 (−1.38;0.17) | 0.13 |
| LPA | 90/17 | 10.4 (2.3) | 9.9 (2.3) | 0.44 | 0.49 (−0.23;1.20) | 0.18 |
| RPA | 95/18 | 10.2 (2.1) | 9.5 (2.4) | 0.20 | 0.37 (−0.22;0.96) | 0.22 |
| Wall thickness | ||||||
| RVAW | 75/13 | 2.7 (0.7) | 2.4 (0.6) | 0.22 | 0.2 (−0.7;0.2) | 0.26 |
| IVS | 116/26 | 5.6 (0.9) | 5.3 (1.0) | 0.20 | 0.002 (−0.4;0.4) | 0.99 |
| RWT | 72/12 | 0.32 (0.04) | 0.31 (0.05) | 0.51 | 0.01 (−0.04;0.02) | 0.49 |
| Volume | ||||||
| SV, ml | 85/22 | 15.4 (3.3) | 15.0 (2.8) | 0.55 | 0.02 (−1.6;1.3) | 0.80 |
| CO, l/min | 84/24 | 1.33 (0.30) | 1.26 (0.23) | 0.33 | 0.05 (−0.19;0.091) | 0.49 |
| Accepted for Analysis BPD No or Moderate/Severe | BPD a No or Moderate n = 131 | BPD a Severe n = 29 | p-Value | Adjusted Mean Difference b, (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| Systolic function | ||||||
| TAPSE, mm | 107/24 | 20.7 (2.7) | 19.8 (3.0) | 0.15 | 0.3 (−1.5;0.8) * | 0.59 |
| TVs’ septal, cm/s | 106/23 | 6.6 (1.1) | 6.8 (1.0) | 0.57 | −0.1 (−0.6;0.4) | 0.68 |
| TVs’ free wall, cm/s | 83/20 | 11.4 (2.1) | 11.7 (2.3) | 0.50 | 0.2 (−0.8;1.3) | 0.64 |
| mpi’, septal | 81/19 | 0.44 (0.07) | 0.44 (0.05) | 0.97 | 0.003 (−0.03;0.04) | 0.88 |
| mpi’, free wall | 97/20 | 0.35 (0.11) | 0.41 (0.14) | 0.025 | 0.02 (−0.03;0.06) | 0.45 |
| PVR | 83/17 | 0.182 (0.04) | 0.171 (0.04) | 0.33 | 0.003 (−0.01;0.02) | 0.76 |
| TR, m/s | 92/19 | 2.0 (0.3) | 2.0 (0.3) | 0.75 | 0.1 (−0.004;0.3) | 0.84 |
| RVOTvti, m | 109/24 | 11.8 (2.4) | 12.6 (2.3) | 0.20 | 0.04 (−0.9;0.9) | 0.94 |
| Diastolic function | ||||||
| TVE, cm/s | 108/24 | 50.9 (11.9) | 50.8 (9.3) | 0.95 | −3.6 (−8.3;1.0) | 0.12 |
| TVA, cm/s | 103/22 | 32.5 (7.9) | 33.9 (8.6) | 0.46 | −0.7 (−3.1;4.4) | 0.72 |
| Septal | ||||||
| TVe’, cm/s | 106/23 | 12.4 (1.5) | 12.3 (1.3) | 0.63 | −0.4 (−1.1;0.3) | 0.25 |
| TVa’, cm/s | 106/23 | 4.6 (1.0) | 4.6 (1.4) | 0.99 | −0.1 (−0.6;0.4) | 0.65 |
| E/e’ | 97/21 | 4.2 (0.8) | 4.1 (1.0) | 0.93 | −0.3 (−0.7;0.2) | 0.21 |
| ivct, ms | 106/23 | 60 (13) | 56 (10) | 0.14 | −3.7 (−9.6;2.1) | 0.21 |
| ivrt, ms | 106/23 | 57 (10) | 60 (7) | 0.13 | 2.2 (−2.2;6.6) | 0.32 |
| Free wall | ||||||
| TVe’, cm/s | 99/20 | 15.1 (2.6) | 15.5 (3.6) | 0.62 | −0.1 (−1.4;1.2) | 0.87 |
| TVa’, cm/s | 97/20 | 7.5 (1.8) | 7.6 (2.4) | 0.87 | −0.1 (−1.1;0.8) | 0.78 |
| E/e’ | 91/18 | 3.4 (0.8) | 3.3 (0.6) | 0.60 | 0.3 (−0.7;0.2) | 0.21 |
| ivct, ms | 98/20 | 59 (14) | 62 (14) | 0.38 | 2.9 (−4.1;9.8) | 0.42 |
| ivrt, ms | 98/20 | 34 (21) | 46 (23) | 0.017 | −1.9 (−3.9;7.8) | 0.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohlkert, L.-A.; Hallberg, J.; Broberg, O.; Sjöberg, G.; Rydberg, A.; Liuba, P.; Fellman, V.; Domellöf, M.; Norman, M.; Pegelow Halvorsen, C. Right Heart Structure, Geometry and Function Assessed by Echocardiography in 6-Year-Old Children Born Extremely Preterm—A Population-Based Cohort Study. J. Clin. Med. 2021, 10, 122. https://doi.org/10.3390/jcm10010122
Mohlkert L-A, Hallberg J, Broberg O, Sjöberg G, Rydberg A, Liuba P, Fellman V, Domellöf M, Norman M, Pegelow Halvorsen C. Right Heart Structure, Geometry and Function Assessed by Echocardiography in 6-Year-Old Children Born Extremely Preterm—A Population-Based Cohort Study. Journal of Clinical Medicine. 2021; 10(1):122. https://doi.org/10.3390/jcm10010122
Chicago/Turabian StyleMohlkert, Lilly-Ann, Jenny Hallberg, Olof Broberg, Gunnar Sjöberg, Annika Rydberg, Petru Liuba, Vineta Fellman, Magnus Domellöf, Mikael Norman, and Cecilia Pegelow Halvorsen. 2021. "Right Heart Structure, Geometry and Function Assessed by Echocardiography in 6-Year-Old Children Born Extremely Preterm—A Population-Based Cohort Study" Journal of Clinical Medicine 10, no. 1: 122. https://doi.org/10.3390/jcm10010122
APA StyleMohlkert, L.-A., Hallberg, J., Broberg, O., Sjöberg, G., Rydberg, A., Liuba, P., Fellman, V., Domellöf, M., Norman, M., & Pegelow Halvorsen, C. (2021). Right Heart Structure, Geometry and Function Assessed by Echocardiography in 6-Year-Old Children Born Extremely Preterm—A Population-Based Cohort Study. Journal of Clinical Medicine, 10(1), 122. https://doi.org/10.3390/jcm10010122





