The Role of Speckle Strain Echocardiography in the Diagnosis of Early Subclinical Cardiac Injury in Cancer Patients—Is There More Than Just Left Ventricle Global Longitudinal Strain?
Abstract
1. Introduction
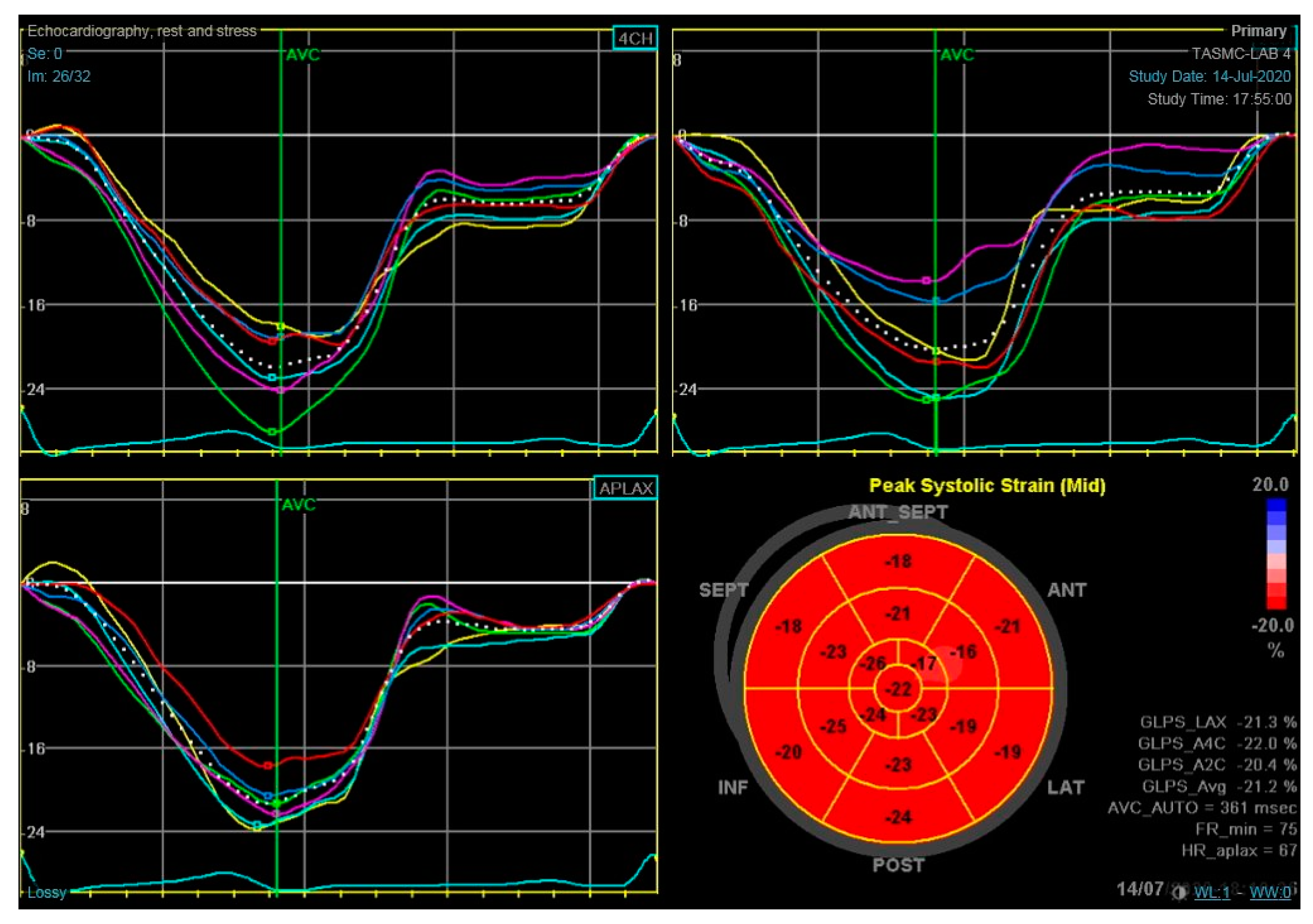
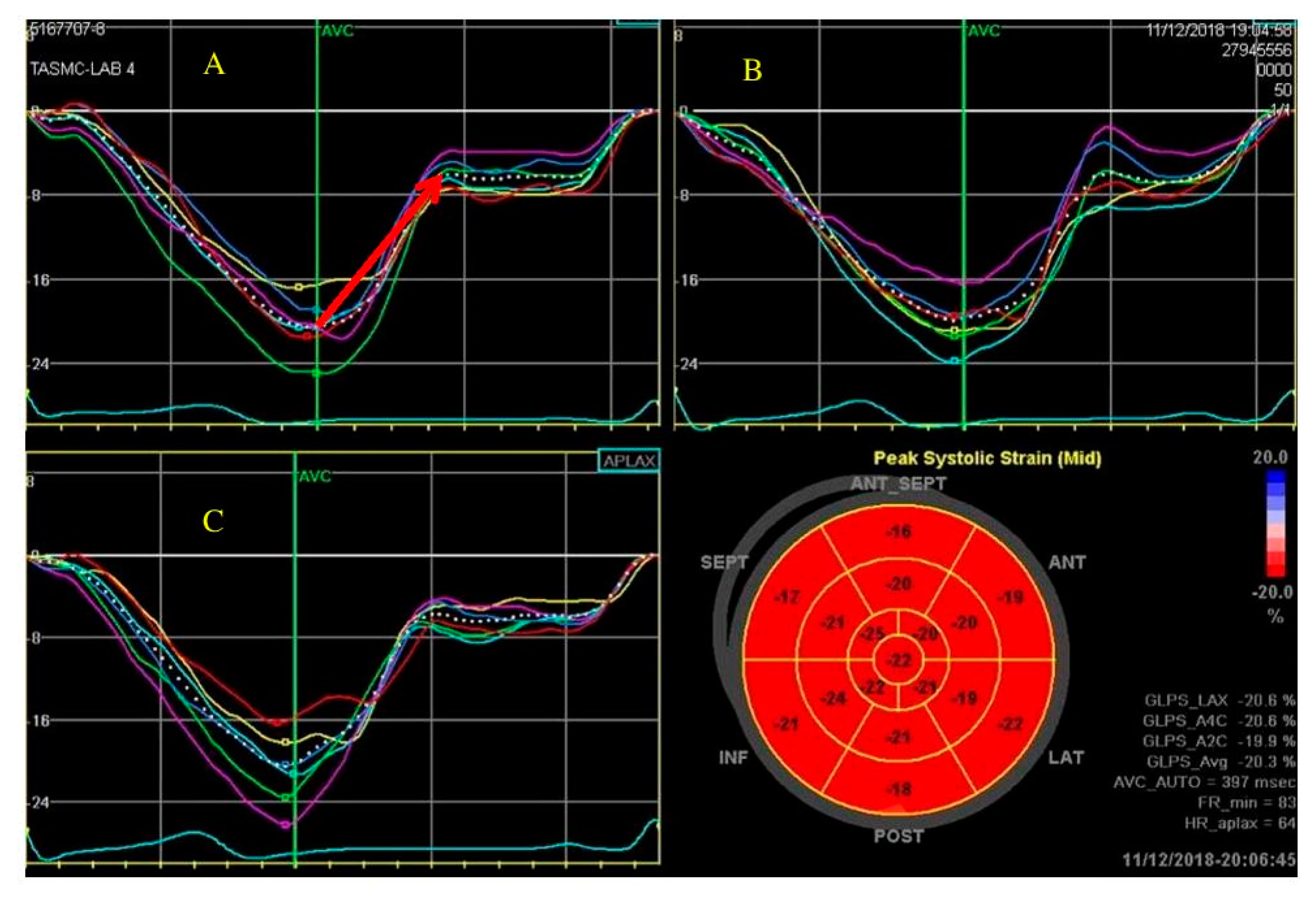
2. LV Global Longitudinal Strain
3. LV Circumferential and Radial Strain
4. Multi-Layers Strain
5. Diastolic Strain
6. Left Atrial Strain
7. Right Ventricle Strain
8. Right Atrial Strain
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Edwards, B.K.; Noone, A.M.; Mariotto, A.B.; Simard, E.P.; Boscoe, F.P.; Henley, S.J.; Jemal, A.; Cho, H.; Anderson, R.N.; Kohler, B.A.; et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 2014, 120, 1290–1314. [Google Scholar] [CrossRef]
- Patnaik, J.L.; Byers, T.; DiGuiseppi, C.; Dabelea, D.; Denberg, T.D. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: A retrospective cohort study. Breast Cancer Res. 2011, 13, R64. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Munoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Yousaf, N.; Battisti, N.M.L.; Moslehi, J.; Larkin, J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018, 19, e447–e458. [Google Scholar] [CrossRef]
- Chang, H.M.; Moudgil, R.; Scarabelli, T.; Okwuosa, T.M.; Yeh, E.T.H. Cardiovascular Complications of Cancer Therapy: Best Practices in Diagnosis, Prevention, and Management: Part 1. J. Am. Coll. Cardiol. 2017, 70, 2536–2551. [Google Scholar] [CrossRef] [PubMed]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2014, 27, 911–939. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.M.; Okwuosa, T.M.; Scarabelli, T.; Okwuosa, T.T.M.; Yeh, E.T.H. Cardiovascular Complications of Cancer Therapy: Best Practices in Diagnosis, Prevention, and Management: Part 2. J. Am. Coll. Cardiol. 2017, 70, 2552–2565. [Google Scholar] [CrossRef] [PubMed]
- Gulati, G.; Heck, S.L.; Ree, A.H.; Hoffmann, P.; Schulz-Menger, J.; Fagerland, M.W.; Gravdehaug, B.; von Knobelsdorff-Brenkenhoff, F.; Bratland, Å.; Storås, T.H.; et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): A 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur. Heart J. 2016, 37, 1671–1680. [Google Scholar] [CrossRef]
- Hardaway, B.W. Adriamycin-associated cardiomyopathy: Where are we now? updates in pathophysiology, dose recommendations, prognosis, and outcomes. Curr. Opin. Cardiol. 2019, 34, 289–295. [Google Scholar] [CrossRef]
- Eidem, B.W. Identification of anthracycline cardiotoxicity: Left ventricular ejection fraction is not enough. J. Am. Soc. Echocardiogr. 2008, 21, 1290–1292. [Google Scholar] [CrossRef]
- Jordan, J.H.; Todd, R.M.; Vasu, S.; Hundley, W.G. Cardiovascular Magnetic Resonance in the Oncology Patient. JACC Cardiovasc. Imaging 2018, 11, 1150–1172. [Google Scholar] [CrossRef] [PubMed]
- Celutkiene, J.; Pudil, R.; Lopez-Fernandez, T.; Grapsa, J.; Nihoyannopoulos, P.; Bergler-Klein, J.; Cohen-Solal, A.; Farmakis, D.; Tocchetti, C.G.; von Haehling, S.; et al. The role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: A Position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2020, 22, 1504–1524. [Google Scholar] [PubMed]
- Voigt, J.U.; Cvijic, M. 2- and 3-Dimensional Myocardial Strain in Cardiac Health and Disease. JACC Cardiovasc. Imaging 2019, 12, 1849–1863. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Poulin, F.; Lim, K.D.; Plana, J.C.; Woo, A.; Marwick, T.H. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: A systematic review. J. Am. Coll. Cardiol. 2014, 63, 2751–2768. [Google Scholar] [CrossRef]
- Mirea, O.; Pagourelias, E.D.; Duchenne, J.; Bogaert, B.; Thomas, J.D.; Badano, L.P.; Voigt, J.U.; EACVI-ASE-Industry Standardization Task Force. Variability and Reproducibility of Segmental Longitudinal Strain Measurement: A Report From the EACVI-ASE Strain Standardization Task Force. JACC Cardiovasc. Imaging 2018, 11, 15–24. [Google Scholar] [CrossRef]
- Nagata, Y.; Takeuchi, M.; Mizukoshi, K.; Wu, V.C.; Lin, F.C.; Negishi, K.; Nakatani, S.; Otsuji, Y. Intervendor variability of two-dimensional strain using vendor-specific and vendor-independent software. J. Am. Soc. Echocardiogr. 2015, 28, 630–641. [Google Scholar] [CrossRef]
- Laufer-Perl, M.; Arnold, J.H.; Mor, L.; Amrami, N.; Derakhshesh, M.; Moshkovits, Y.; Sadeh, B.; Arbel, Y.; Topilsky, Y.; Rozenbaum, Z. The association of reduced global longitudinal strain with cancer therapy-related cardiac dysfunction among patients receiving cancer therapy. Clin. Res. Cardiol. 2020, 109, 255–262. [Google Scholar] [CrossRef]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J. Am. Soc. Echocardiogr. 2015, 28, 183–193. [Google Scholar] [CrossRef]
- Hare, J.L.; Brown, J.K.; Leano, R.; Jenkins, C.; Woodward, N.; Marwick, T.H. Use of myocardial deformation imaging to detect preclinical myocardial dysfunction before conventional measures in patients undergoing breast cancer treatment with trastuzumab. Am. Heart J. 2009, 158, 294–301. [Google Scholar] [CrossRef]
- Verdonschot, J.A.J.; Merken, J.J.; Brunner-La Rocca, H.P.; Hazebroek, M.R.; Eurlings, C.G.M.J.; Thijssen, E.; Wang, P.; Weerts, J.; van Empel, V.; Schummers, G. Value of Speckle Tracking-Based Deformation Analysis in Screening Relatives of Patients With Asymptomatic Dilated Cardiomyopathy. JACC Cardiovasc. Imaging 2020, 13, 549–558. [Google Scholar] [CrossRef]
- Negishi, K.; Negishi, T.; Haluska, B.A.; Hare, J.L.; Plana, J.C.; Marwick, T.H. Use of speckle strain to assess left ventricular responses to cardiotoxic chemotherapy and cardioprotection. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Charbonnel, C.; Convers-Domart, R.; Rigaudeau, S.; Taksin, A.L.; Baron, N.; Lambert, J.; Ghez, S.; Georges, J.L.; Farhat, H.; Lambert, J.; et al. Assessment of global longitudinal strain at low-dose anthracycline-based chemotherapy, for the prediction of subsequent cardiotoxicity. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Esposito, R.; Lembo, M.; Sorrentino, R.; De Santo, I.; Luciano, F.; Casciano, O.; Giuliano, M.; De Placido, S.; Trimarco, B.; et al. Strain-oriented strategy for guiding cardioprotection initiation of breast cancer patients experiencing cardiac dysfunction. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, H.; Sebag, I.A.; Plana, J.C.; Januzzi, J.L.; Ky, B.; Tan, T.C.; Cohen, V.; Banchs, J.; Carver, J.R.; Wiegers, S.E.; et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ. Cardiovasc. Imaging 2012, 5, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Poterucha, J.T.; Kutty, S.; Lindquist, R.K.; Li, L.; Eidem, B.W. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J. Am. Soc. Echocardiogr. 2012, 25, 733–740. [Google Scholar] [CrossRef]
- Laufer-Perl, M.; Derakhshesh, M.; Milwidsky, A.; Mor, L.; Ravid, D.; Amrami, N.; Sherez, J.; Keren, G.; Topilsky, Y.; Arbel, Y. Usefulness of Global Longitudinal Strain for Early Identification of Subclinical Left Ventricular Dysfunction in Patients With Active Cancer. Am. J. Cardiol. 2018, 122, 1784–1789. [Google Scholar] [CrossRef]
- Peleg Hasson, S.; Salwen, B.; Sivan, A.; Shamai, S.; Geva, R.; Merimsky, O.; Raphael, A.; Shmilovich, H.; Moshkovits, Y.; Kapusta, L.; et al. Re-introducing immunotherapy in patients surviving immune checkpoint inhibitors-mediated myocarditis. Clin. Res. Cardiol. 2020. [Google Scholar] [CrossRef]
- Pourier, M.S.; Mavinkurve-Groothuis, A.M.C.; Dull, M.M.; Weijers, G.; Loonen, J.; Bellersen, L.; de Korte, C.L.; Kapusta, L. Myocardial 2D Strain During Long-Term (>5 Years) Follow-Up of Childhood Survivors of Acute Lymphoblastic Leukemia Treated with Anthracyclines. Am. J. Cardiol. 2020, 127, 163–168. [Google Scholar] [CrossRef]
- Negishi, K.; Negishi, T.; Hare, J.L.; Haluska, B.A.; Plana, J.C.; Marwick, T.H. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J. Am. Soc. Echocardiogr. 2013, 26, 493–498. [Google Scholar] [CrossRef]
- Liu, J.; Banchs, J.; Mousavi, N.; Plana, J.C.; Scherrer-Crosbie, M.; Thavendiranathan, P.; Barac, A. Contemporary Role of Echocardiography for Clinical Decision Making in Patients During and After Cancer Therapy. JACC Cardiovasc. Imaging 2018, 11, 1122–1131. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Negishi, T.; Somerset, E.; Negishi, K.; Penicka, M.; Lemieux, J.; Aakhus, S.; Miyazaki, S.; Shirazi, M.; Galderisi, M.; et al. Strain-Guided Management of Potentially Cardiotoxic Cancer Therapy. J. Am. Coll. Cardiol. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Yingchoncharoen, T.; Agarwal, S.; Popovic, Z.B.; Marwick, T.H. Normal ranges of left ventricular strain: A meta-analysis. J. Am. Soc. Echocardiogr. 2013, 26, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Mavinkurve-Groothuis, A.M.; Marcus, K.A.; Pourier, M.; Loonen, J.; Feuth, T.; Hoogerbrugge, P.M.; de Korte, C.L.; Kapusta, L. Myocardial 2D strain echocardiography and cardiac biomarkers in children during and shortly after anthracycline therapy for acute lymphoblastic leukaemia (ALL): A prospective study. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 562–569. [Google Scholar] [CrossRef]
- Stoodley, P.W.; Richards, D.A.; Hui, R.; Boyd, A.; Harnett, P.R.; Meikle, S.R.; Clarke, J.; Thomas, L. Two-dimensional myocardial strain imaging detects changes in left ventricular systolic function immediately after anthracycline chemotherapy. Eur. J. Echocardiogr. 2011, 12, 945–952. [Google Scholar] [CrossRef]
- Kang, Y.; Xiao, F.; Chen, H.; Wang, W.; Shen, L.; Zhao, H.; Shen, X.; Chen, F.; He, B. Subclinical Anthracycline-Induced Cardiotoxicity in the Long-Term Follow-Up of Lymphoma Survivors: A Multi-Layer Speckle Tracking Analysis. Arq. Bras. Cardiol. 2018, 110, 219–228. [Google Scholar] [CrossRef]
- Altiok, E.; Neizel, M.; Tiemann, S.; Krass, V.; Becker, M.; Zwicker, C.; Koos, R.; Kelm, M.; Kraemer, N.; Schoth, F.; et al. Layer-specific analysis of myocardial deformation for assessment of infarct transmurality: Comparison of strain-encoded cardiovascular magnetic resonance with 2D speckle tracking echocardiography. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Hamada, S.; Schroeder, J.; Hoffmann, R.; Altiok, E.; Keszei, A.; Almalla, M.; Napp, A.; Marx, N.; Becker, M. Prediction of Outcomes in Patients with Chronic Ischemic Cardiomyopathy by Layer-Specific Strain Echocardiography: A Proof of Concept. J. Am. Soc. Echocardiogr. 2016, 29, 412–420. [Google Scholar] [CrossRef]
- Chang, W.T.; Feng, Y.H.; Kuo, Y.H.; Chen, W.Y.; Wu, H.C.; Huang, C.T.; Huang, T.L.; Chen, Z.C. Layer-specific distribution of myocardial deformation from anthracycline-induced cardiotoxicity in patients with breast cancer-From bedside to bench. Int. J. Cardiol. 2020, 311, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Lassen, M.C.H.; Biering-Sorensen, S.R.; Olsen, F.J.; Skaarup, K.G.; Tolstrup, K.; Qasim, A.N.; Møgelvang, R.; Jensen, J.S.; Biering-Sørensen, T. Ratio of transmitral early filling velocity to early diastolic strain rate predicts long-term risk of cardiovascular morbidity and mortality in the general population. Eur. Heart J. 2019, 40, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.A.; Boldt, L.H.; Eichstadt, H.; Ozcelik, C.; Haverkamp, W. Myocardial systolic and diastolic performance derived by 2-dimensional speckle tracking echocardiography in heart failure with normal left ventricular ejection fraction. Circ. Heart Fail. 2012, 5, 610–620. [Google Scholar] [CrossRef]
- Stoodley, P.W.; Richards, D.A.; Boyd, A.; Hui, R.; Harnett, P.R.; Meikle, S.R.; Clarke, J.L.; Thomas, L. Altered left ventricular longitudinal diastolic function correlates with reduced systolic function immediately after anthracycline chemotherapy. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Hochstadt, A.; Arnold, J.; Rosen, R.; Sherez, C.; Sherez, J.; Mor, L.; Derakhshesh, M.; Moshkovits, Y.; Merdler, I.; Arbel, Y.; et al. Diastolic strain time as predictor for systolic dysfunction among patients with active breast cancer. Echocardiography 2020, 37. [Google Scholar] [CrossRef] [PubMed]
- Hochstadt, A.; Arnold, J.; Rosen, R.; Sherez, C.; Sherez, J.; Mor, L.; Moshkovits, Y.; Merdler, I.; Szekely, Y.; Arbel, Y.; et al. Longitudinal diastolic strain slope as an early sign for systolic dysfunction among patients with active cancer. Clin. Res. Cardiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.A.; Belyavskiy, E.; Aravind-Kumar, R.; Kropf, M.; Frydas, A.; Braunauer, K.; Marquez, E.; Krisper, M.; Lindhorst, R.; Osmanoglou, E.; et al. Potential Usefulness and Clinical Relevance of Adding Left Atrial Strain to Left Atrial Volume Index in the Detection of Left Ventricular Diastolic Dysfunction. JACC Cardiovasc. Imaging 2018, 11, 1405–1415. [Google Scholar] [CrossRef]
- Cameli, M.; Sparla, S.; Losito, M.; Righini, F.M.; Menci, D.; Lisi, M.; D’Ascenzi, F.; Focardi, M.; Favilli, R.; Pierli, C.; et al. Correlation of Left Atrial Strain and Doppler Measurements with Invasive Measurement of Left Ventricular End-Diastolic Pressure in Patients Stratified for Different Values of Ejection Fraction. Echocardiography 2016, 33, 398–405. [Google Scholar] [CrossRef]
- Kebed, K.Y.; Addetia, K.; Lang, R.M. Importance of the Left Atrium: More Than a Bystander? Heart Fail. Clin. 2019, 15, 191–204. [Google Scholar] [CrossRef]
- Freed, B.H.; Daruwalla, V.; Cheng, J.Y.; Aguilar, F.G.; Beussink, L.; Choi, A.; Klein, D.A.; Dixon, D.; Baldridge, A.; Rasmussen-Torvik, L.J.; et al. Prognostic Utility and Clinical Significance of Cardiac Mechanics in Heart Failure With Preserved Ejection Fraction: Importance of Left Atrial Strain. Circ. Cardiovasc. Imaging 2016, 9. [Google Scholar] [CrossRef]
- Singh, A.; Addetia, K.; Maffessanti, F.; Mor-Avi, V.; Lang, R.M. LA Strain for Categorization of LV Diastolic Dysfunction. JACC Cardiovasc. Imaging 2017, 10, 735–743. [Google Scholar] [CrossRef]
- Patel, N.R.; Chyu, C.K.; Satou, G.M.; Halnon, N.J.; Nguyen, K.L. Left atrial function in children and young adult cancer survivors treated with anthracyclines. Echocardiography 2018, 35, 1649–1656. [Google Scholar] [CrossRef]
- Santoro, C.; Arpino, G.; Esposito, R.; Lembo, M.; Paciolla, I.; Cardalesi, C.; de Simone, G.; Trimarco, B.; De Placido, S.; Galderisi, M. 2D and 3D strain for detection of subclinical anthracycline cardiotoxicity in breast cancer patients: A balance with feasibility. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 930–936. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Liu, K.; Zhang, J.; Ma, X.; Li, L.; Li, M.; Liu, J. Evaluation of changes in right ventricular myocardial mechanical properties in breast cancer patients receiving pirarubicin using three-dimensional speckle tracking imaging. Nan Fang Yi Ke Da Xue Xue Bao 2018, 38, 1032–1038. [Google Scholar] [PubMed]
- Calleja, A.; Poulin, F.; Khorolsky, C.; Shariat, M.; Bedard, P.L.; Amir, E.; Rakowski, H.; McDonald, M.; Delgado, D.; Thavendiranathan, P. Right Ventricular Dysfunction in Patients Experiencing Cardiotoxicity during Breast Cancer Therapy. J. Oncol. 2015, 2015, 609194. [Google Scholar] [CrossRef] [PubMed]
- Keramida, K.; Farmakis, D.; Bingcang, J.; Sulemane, S.; Sutherland, S.; Bingcang, R.A.; Ramachandran, K.; Tzavara, C.; Charalampopoulos, G.; Filippiadis, D.; et al. Longitudinal changes of right ventricular deformation mechanics during trastuzumab therapy in breast cancer patients. Eur. J. Heart Fail. 2019, 21, 529–535. [Google Scholar] [CrossRef]
- Chang, W.T.; Shih, J.Y.; Feng, Y.H.; Chiang, C.Y.; Kuo, Y.H.; Chen, W.Y.; Wu, H.C.; Cheng, J.T.; Wang, J.J.; Chen, Z.C. The Early Predictive Value of Right Ventricular Strain in Epirubicin-Induced Cardiotoxicity in Patients with Breast Cancer. Acta Cardiol. Sin. 2016, 32, 550–559. [Google Scholar] [PubMed]
- Chen, L.; Huang, J.; Wu, W.; Ta, S.; Xie, X. The impact of right ventricular function on prognosis in patients with stage III non-small cell lung cancer after concurrent chemoradiotherapy. Int. J. Cardiovasc. Imaging 2019, 35, 1009–1017. [Google Scholar] [CrossRef]
- Wright, L.M.; Dwyer, N.; Wahi, S.; Marwick, T.H. Association with right atrial strain with right atrial pressure: An invasive validation study. Int. J. Cardiovasc. Imaging 2018, 34, 1541–1548. [Google Scholar] [CrossRef]
- Jain, S.; Kuriakose, D.; Edelstein, I.; Ansari, B.; Oldland, G.; Gaddam, S.; Javaid, K.; Manaktala, P.; Lee, J.; Miller, R.; et al. Right Atrial Phasic Function in Heart Failure with Preserved and Reduced Ejection Fraction. JACC Cardiovasc. Imaging 2019, 12, 1460–1470. [Google Scholar] [CrossRef]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laufer-Perl, M.; Gilon, D.; Kapusta, L.; Iakobishvili, Z. The Role of Speckle Strain Echocardiography in the Diagnosis of Early Subclinical Cardiac Injury in Cancer Patients—Is There More Than Just Left Ventricle Global Longitudinal Strain? J. Clin. Med. 2021, 10, 154. https://doi.org/10.3390/jcm10010154
Laufer-Perl M, Gilon D, Kapusta L, Iakobishvili Z. The Role of Speckle Strain Echocardiography in the Diagnosis of Early Subclinical Cardiac Injury in Cancer Patients—Is There More Than Just Left Ventricle Global Longitudinal Strain? Journal of Clinical Medicine. 2021; 10(1):154. https://doi.org/10.3390/jcm10010154
Chicago/Turabian StyleLaufer-Perl, Michal, Dan Gilon, Livia Kapusta, and Zaza Iakobishvili. 2021. "The Role of Speckle Strain Echocardiography in the Diagnosis of Early Subclinical Cardiac Injury in Cancer Patients—Is There More Than Just Left Ventricle Global Longitudinal Strain?" Journal of Clinical Medicine 10, no. 1: 154. https://doi.org/10.3390/jcm10010154
APA StyleLaufer-Perl, M., Gilon, D., Kapusta, L., & Iakobishvili, Z. (2021). The Role of Speckle Strain Echocardiography in the Diagnosis of Early Subclinical Cardiac Injury in Cancer Patients—Is There More Than Just Left Ventricle Global Longitudinal Strain? Journal of Clinical Medicine, 10(1), 154. https://doi.org/10.3390/jcm10010154




