Real-World Experience with Bezlotoxumab for Prevention of Recurrence of Clostridioides difficile Infection
Abstract
:1. Introduction
2. Materials and Methods
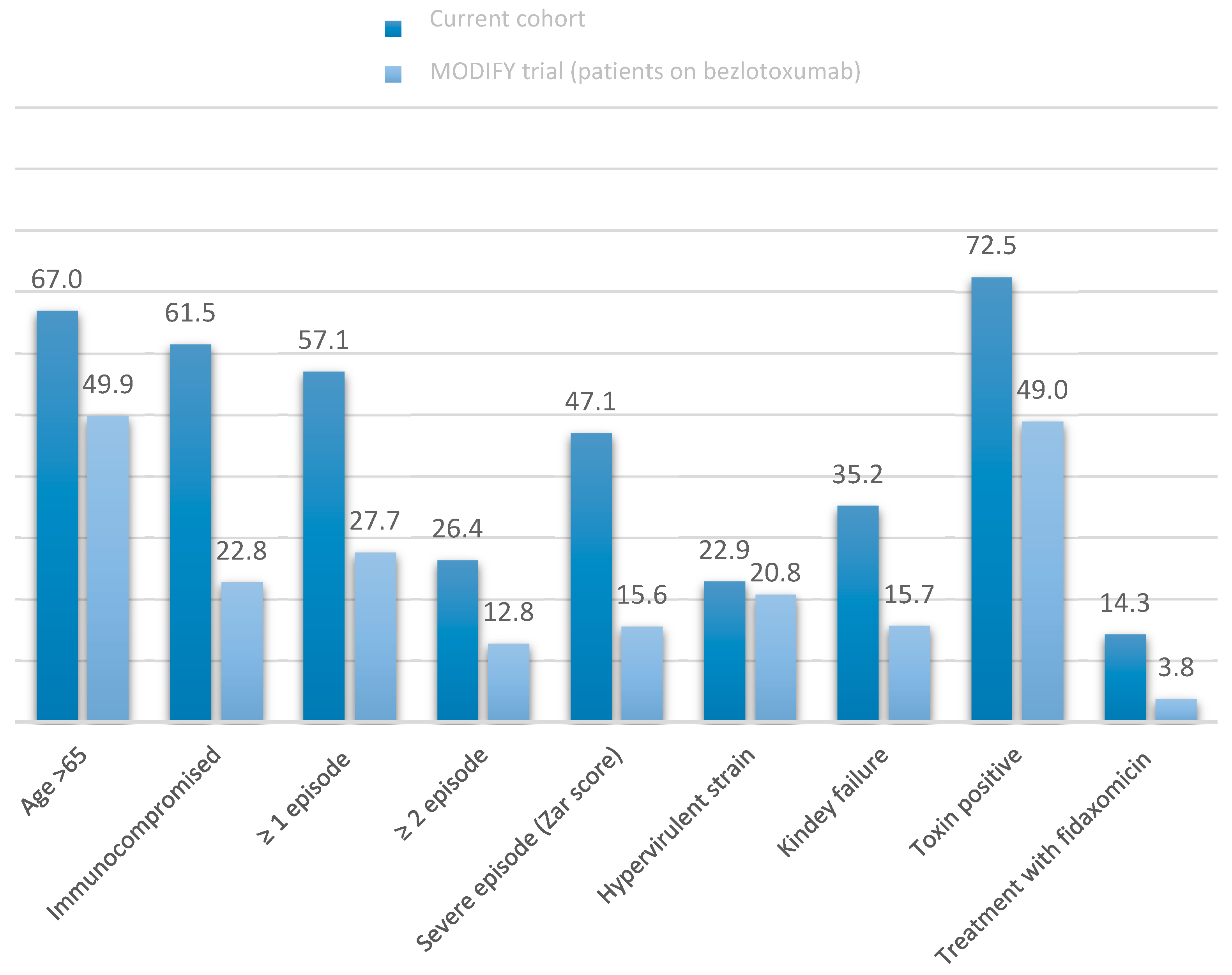
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Olsen, M.A.; Yan, Y.; Reske, K.A.; Zilberberg, M.D.; Dubberke, E.R. Recurrent Clostridium difficile infection is associated with increased mortality. Clin. Microbiol. Infect. 2015, 21, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Eyre, D.W.; Walker, A.S.; Wyllie, D.; Dingle, K.E.; Griffiths, D.; Finney, J.; O’Connor, L.; Vaughan, A.; Crook, D.W.; Wilcox, M.H.; et al. Predictors of First Recurrence of Clostridium difficile Infection: Implications for Initial Management. Clin. Infect. Dis. 2012, 55, S77–S87. [Google Scholar] [CrossRef]
- Sheitoyan-Pesant, C.; Abou Chakra, C.N.; Pepin, J.; Marcil-Héguy, A.; Nault, V.; Valiquette, L. Clinical and Healthcare Burden of Multiple Recurrences of Clostridium difficile Infection. Clin. Infect. Dis. 2016, 62, 574–580. [Google Scholar] [CrossRef] [Green Version]
- Van Dorp, S.M.; Kinross, P.; Gastmeier, P.; Behnke, M.; Kola, A.; Delmée, M.; Pavelkovich, A.; Mentula, S.; Barbut, F.; Hajdu, A.; et al. Standardised surveillance of Clostridium difficile infection in European acute care hospitals: A pilot study, 2013. Eurosurveill. Eur. Cent. Dis. Prev. Control. 2016, 21, 20381. [Google Scholar]
- Louie, T.J.; Miller, M.A.; Mullane, K.M.; Weiss, K.; Lentnek, A.; Golan, Y.; Gorbach, S.; Sears, P.; Shue, Y.-K. Fidaxomicin versus vancomycin for Clostridium difficile infection. N. Engl. J. Med. 2011, 364, 422–431. [Google Scholar] [CrossRef] [Green Version]
- Cornely, O.A.; Crook, D.W.; Esposito, R.; Poirier, A.; Somero, M.S.; Weiss, K.; Sears, P.; Gorbach, S. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: A double-blind, non-inferiority, randomised controlled trial. Lancet Infect. Dis. 2012, 12, 281–289. [Google Scholar] [CrossRef]
- Wilcox, M.H.; Gerding, D.N.; Poxton, I.R.; Kelly, C.; Nathan, R.; Birch, T.; Cornely, O.A.; Rahav, G.; Bouza, E.; Lee, C.; et al. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N. Engl. J. Med. 2017, 376, 305–317. [Google Scholar] [CrossRef]
- Oksi, J.; Aalto, A.; Saila, P.; Partanen, T.; Anttila, V.-J.; Mattila, E. Real-world efficacy of bezlotoxumab for prevention of recurrent Clostridium difficile infection: A retrospective study of 46 patients in five university hospitals in Finland. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1947–1952. [Google Scholar] [CrossRef] [Green Version]
- Hengel, R.L.; Ritter, T.E.; Nathan, R.V.; Van Anglen, L.J.; Schroeder, C.P.; Dillon, R.J.; Marcella, S.W.; Garey, K.W. Real-world Experience of Bezlotoxumab for Prevention of Clostridioides difficile Infection: A Retrospective Multicenter Cohort Study. Open Forum Infect. Dis. 2020, 7, ofaa097. [Google Scholar] [CrossRef] [Green Version]
- Alonso, C.D.; Mahoney, M.V. Bezlotoxumab for the prevention of Clostridium difficile infection: A review of current evidence and safety profile. IDR 2019, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sherman, R.E.; Anderson, S.A.; Dal Pan, G.J.; Gray, G.W.; Gross, T.; Hunter, N.L.; LaVange, L.; Marinac-Dabic, D.; Marks, P.W.; Robb, M.A.; et al. Real-World Evidence—What Is It and What Can It Tell Us? N. Engl. J. Med. 2016, 375, 2293–2297. [Google Scholar] [CrossRef] [Green Version]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef] [PubMed]
- Zar, F.A.; Bakkanagari, S.R.; Moorthi, K.M.L.S.T.; Davis, M.B. A Comparison of Vancomycin and Metronidazole for the Treatment of Clostridium difficile Associated Diarrhea, Stratified by Disease Severity. Clin. Infect. Dis. 2007, 45, 302–307. [Google Scholar] [CrossRef]
- Debast, S.B.; Bauer, M.P.; Kuijper, E.J. European Society of Clinical Microbiology and Infectious Diseases: Update of the Treatment Guidance Document for Clostridium difficile Infection. Clin. Microbiol. Infect. 2014, 20, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phatharacharukul, P.; Thongprayoon, C.; Cheungpasitporn, W.; Edmonds, P.J.; Mahaparn, P.; Bruminhent, J. The Risks of Incident and Recurrent Clostridium difficile Associated Diarrhea in Chronic Kidney Disease and End-Stage Kidney Disease Patients: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2015, 60, 2913–2922. [Google Scholar] [CrossRef]
- Deshpande, A.; Pasupuleti, V.; Thota, P.; Pant, C.; Rolston, D.D.K.; Hernandez, A.V.; Donskey, C.J.; Fraser, T.G. Risk Factors for Recurrent Clostridium difficile Infection: A Systematic Review and Meta-Analysis. Infect. Control. Hosp. Epidemiol. 2015, 36, 452–460. [Google Scholar] [CrossRef]
- Origüen, J.; Corbella, L.; Orellana, M.Á.; Fernández-Ruiz, M.; Lopez-Medrano, F.; San Juan, R.; Lizasoain, M.; Ruiz-Merlo, T.; Morales-Cartagena, A.; Maestro1et, G.; et al. Comparison of the clinical course of Clostridium difficile infection in glutamate dehydrogenase-positive toxin-negative patients diagnosed by PCR to those with a positive toxin test. Clin. Microbiol. Infect. 2018, 24, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Guh, A.Y.; Hatfield, K.M.; Winston, L.G.; Martin, B.; Johnston, H.; Brousseau, G.; Farley, M.M.; Wilson, L.; Perlmutter, R.; Phipps, E.C.; et al. Toxin Enzyme Immunoassays Detect Clostridioides difficile Infection with Greater Severity and Higher Recurrence Rates. Clin. Infect. Dis. 2019, 69, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, C.M.; Brandt, L.J.; Binion, D.G.; Ananthakrishnan, A.N.; Curry, S.R.; Gilligan, P.H.; McFarland, L.V.; Mellow, M.; Zuckerbraun, B.S. Guidelines for Diagnosis, Treatment, and Prevention of Clostridium difficile Infections. Am. J. Gastroenterol. 2013, 108, 478–498. [Google Scholar] [CrossRef] [PubMed]

| Anti-C. difficile Treatment | Patients | Duration of Treatment | Follow-up after the End of Anti-C. difficile Treatment | Bezlotoxumab Infusion Time from the Start of Treatment |
|---|---|---|---|---|
| Vancomycin + metronidazole | 5 | 10 (10–10) | 76 (75–79) | 2 (1–5) |
| Vancomycin | 40 | 11 (10–14) | 82 (77.5–86) | 6.5 (3–10) |
| Vancomycin (tapered) | 32 | 42 (35.5–55.5) | 62 (45–73) | 14 (3.5–29.5) |
| Fidaxomicin | 9 | 11 (10–13) | 79 (70.5–82) | 5 (2–8) |
| Fidaxomicin (extend regimen) | 4 | 24.5 (23–26.2) | 79 (71–88) | 12.5 (1.5–22) |
| FMT (after vancomycin) | 1 | 9 | 79 | 12 |
| Cohort | Recurrence | No Recurrence | p | 95% CI | |
|---|---|---|---|---|---|
| Number of patients | 91 | 13 | 78 | ||
| Men | 46 (50.5) | 5 (38.5) | 41 (52.6) | 0.35 | 0.53–5.9 |
| Age (years) * | 71 (59–82) | 68 (57–80) | 72 (60–82) | 0.96 | 0.96–1.04 |
| Age > 65 | 61 (66.3) | 8 (61.5) | 53 (68.0) | 0.65 | 0.22–2.54 |
| Age > 85 | 17 (18.7) | 3 (23.1) | 14 (18.0) | 0.66 | 0.33–5.64 |
| Charlson index * | 4 (2–6) | 3 (2–5) | 4 (2–6) | 0.22 | 0.64–1.11 |
| Kidney failure | 32 (35.2) | 4 (30.8) | 28 (35.9) | 0.72 | 0.22–2.81 |
| Cancer | 20 (22.0) | 3 (23.1) | 17 (21.8) | 0.92 | 0.27–4.36 |
| Leukaemia/Lymphoma | 17 (18.7) | 1 (7.7) | 16 (20.5) | 0.29 | 0.04–2.67 |
| Any neoplasm | 33 (36.3) | 3 (23.1) | 30 (38.5) | 0.29 | 0.12–1.89 |
| Liver disease | 9 (9.9) | 2 (15.4) | 7 (9.0) | 0.71 | 0.34–10.04 |
| Intestinal inflammatory disease | 6 (6.6) | 1 (7.7) | 5 (6.4) | 0.86 | 0.13–11.34 |
| Immunosuppression: | 56 (61.5) | 7 (53.9) | 48 (62.8) | 0.54 | 0.21–2.25 |
| Chemotherapy | 13 (14.3) | 2 (15.4) | 11 (14.1) | 0.90 | 0.22–5.68 |
| Steroids | 14 (15.4) | 1 (7.7) | 13 (16.7) | 0.42 | 0.05–3.49 |
| Immunosuppressive drugs (not steroids) | 16 (17.6) | 1 (7.7) | 15 (19.2) | 0.33 | 0.04–2.91 |
| Solid organ transplant | 20 (22.0) | 3 (23.1) | 17 (21.8) | 0.92 | 0.27–4.36 |
| Previous CDI episodes: | |||||
| 0 | 39 (42.9) | 5 (38.5) | 35 (44.9) | 0.73 | 0.24–2.70 |
| 1 | 28 (30.8) | 2 (15.4) | 26 (33.3) | 0.21 | 0.08–1.76 |
| ≥2 | 24 (26.4) | 6 (46.2) | 18 (23.1) | 0.09 | 0.85–9.59 |
| Proton pump inhibitor use | 59 (64.8) | 8 (61.5) | 51 (65.4) | 0.79 | 0.25–2.84 |
| Previous antibiotic treatment | 79 (86.8) | 10 (76.9) | 69 (88.5) | 0.27 | 0.10–1.88 |
| Classification of CDI episodes: | |||||
| CA | 11 (12.1) | 1 (7.7) | 10 (12.8) | 0.60 | 0.07–4.84 |
| CO-HCFA | 35 (38.5) | 3 (23.1) | 32 (41.0) | 0.23 | 0.11–1.69 |
| HO-HCFA | 39 (42.9) | 7 (53.9) | 32 (41.0) | 0.39 | 0.52–5.46 |
| Indeterminate | 6 (6.6) | 2 (15.4) | 4 (5.1) | 0.19 | 0.55–20.59 |
| Toxin positive | 66 (72.5) | 8 (61.5) | 58 (74.4) | 0.34 | 0.16–1.88 |
| NAAT positive/toxin negative | 25 (27.5) | 5 (38.5)) | 20 (25.6) | 0.34 | 0.16–1.88 |
| IDSA severe or fulminant colitis | 35 (38.5) | 5 (38.5) | 30 (38.5) | 1.00 | 0.30–3.34 |
| Severe (Zar) | 41 (45.1) | 7 (53.9) | 34 (43.6) | 0.49 | 0.46–4.91 |
| Admitted to ICU | 11 (12.1) | 1 (7.7) | 10 (12.8) | 0.60 | 0.07–4.84 |
| 027 ribotype (based on 48 patients) | 10 (20.8) | 4 (44.4) | 6 (15.4) | 0.07 | 0.91–21.29 |
| Concomitant antibiotics | 25 (27.5) | 1 (7.7) | 24 (30.8) | 0.12 | 0.02–1.52 |
| Anti-C. difficile treatment: | |||||
| Vancomycin | 40 (44.0) | 5 (38.5) | 35 (44.9) | 0.67 | 0.23–2.56 |
| Fidaxomicin | 9 (9.9) | 2 (15.4) | 7 (9.0) | 0.48 | 0.34–10.04 |
| Vancomycin/metronidazole | 5 (5.5) | 1 (7.7) | 4 (5.1) | 0.71 | 0.16–14.99 |
| Vancomycin (tapered) | 32 (35.2) | 4 (30.8) | 28 (35.9) | 0.72 | 0.22–2.81 |
| Fidaxomicin extended–pulsed | 4 (4.4) | 0 | 4 (5.1) | 0.40 | - |
| Faecal microbiota transplant | 1 (6.0) | 1 (7.7) | 0 | 0.01 | - |
| Extended/pulsed–tapered treatments | 36 (39.6) | 4 (30.8) | 32 (41.0) | 0.49 | 0.18–2.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escudero-Sánchez, R.; Ruiz-Ruigómez, M.; Fernández-Fradejas, J.; García Fernández, S.; Olmedo Samperio, M.; Cano Yuste, A.; Valencia Alijo, A.; Díaz-Pollán, B.; Rodríguez Hernández, M.J.; Merino De Lucas, E.; et al. Real-World Experience with Bezlotoxumab for Prevention of Recurrence of Clostridioides difficile Infection. J. Clin. Med. 2021, 10, 2. https://doi.org/10.3390/jcm10010002
Escudero-Sánchez R, Ruiz-Ruigómez M, Fernández-Fradejas J, García Fernández S, Olmedo Samperio M, Cano Yuste A, Valencia Alijo A, Díaz-Pollán B, Rodríguez Hernández MJ, Merino De Lucas E, et al. Real-World Experience with Bezlotoxumab for Prevention of Recurrence of Clostridioides difficile Infection. Journal of Clinical Medicine. 2021; 10(1):2. https://doi.org/10.3390/jcm10010002
Chicago/Turabian StyleEscudero-Sánchez, Rosa, María Ruiz-Ruigómez, Jorge Fernández-Fradejas, Sergio García Fernández, María Olmedo Samperio, Angela Cano Yuste, Angela Valencia Alijo, Beatriz Díaz-Pollán, María Jesús Rodríguez Hernández, Esperanza Merino De Lucas, and et al. 2021. "Real-World Experience with Bezlotoxumab for Prevention of Recurrence of Clostridioides difficile Infection" Journal of Clinical Medicine 10, no. 1: 2. https://doi.org/10.3390/jcm10010002
APA StyleEscudero-Sánchez, R., Ruiz-Ruigómez, M., Fernández-Fradejas, J., García Fernández, S., Olmedo Samperio, M., Cano Yuste, A., Valencia Alijo, A., Díaz-Pollán, B., Rodríguez Hernández, M. J., Merino De Lucas, E., Martín Segarra, O., Sáez Bejar, C., Armiñanzas Castillo, C., Gutiérrez-Gutiérrez, B., Rodríguez-Pardo, D., Ramos-Martínez, A., Torre-Cisneros, J., López-Medrano, F., & Cobo Reinoso, J. (2021). Real-World Experience with Bezlotoxumab for Prevention of Recurrence of Clostridioides difficile Infection. Journal of Clinical Medicine, 10(1), 2. https://doi.org/10.3390/jcm10010002






