Prevention of Stricture after Endoscopic Submucosal Dissection for Superficial Esophageal Cancer: A Review of the Literature
Abstract
1. Introduction
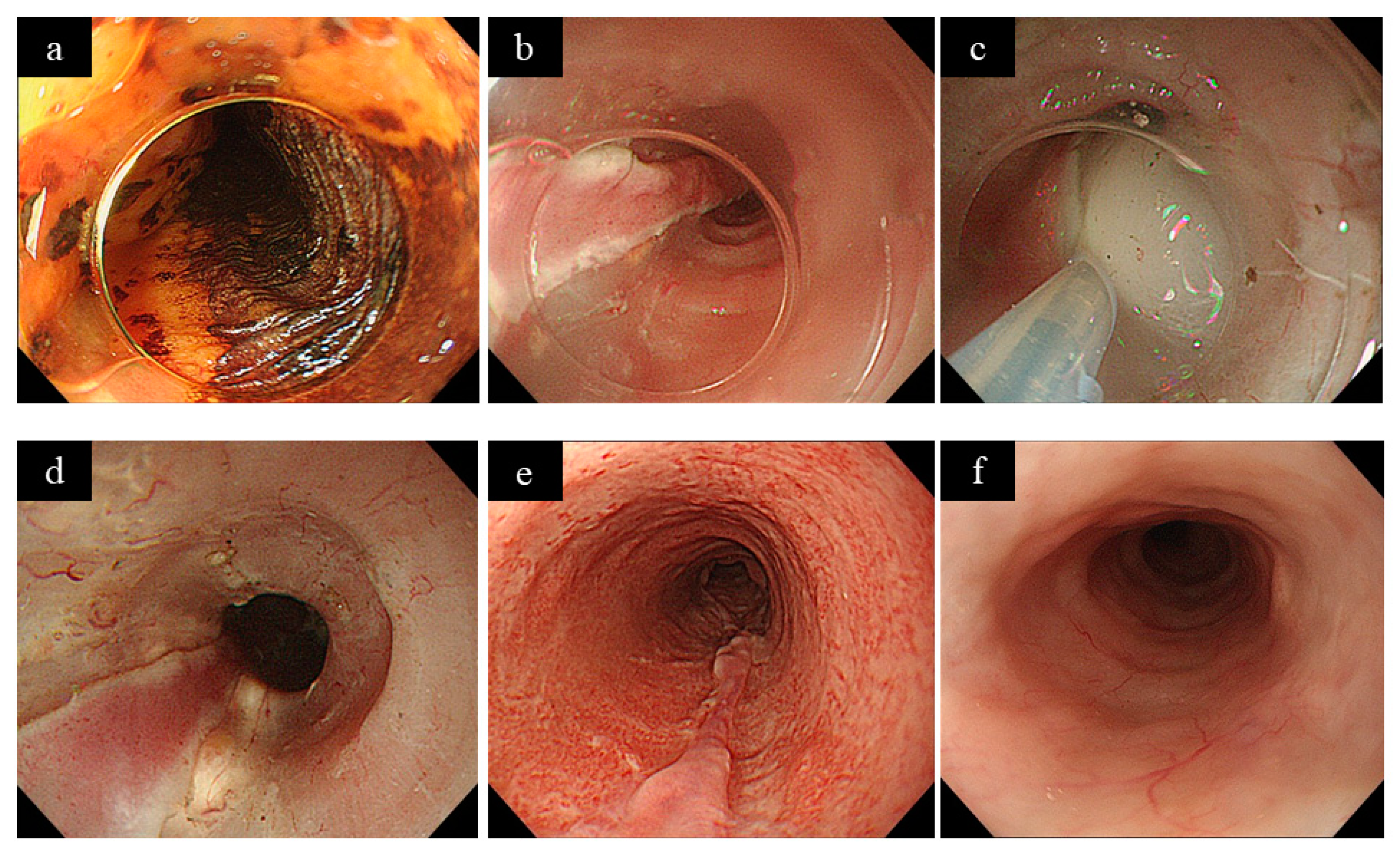
2. Prophylactic EBD
3. Steroid Therapy
4. Steroid Injection Therapy
5. Oral Steroid Administration
6. Other Steroid Administration
7. Comparison among Steroid Therapies
8. Drugs Other Than Steroids
8.1. Botulinum Toxin Injection Therapy
8.2. Oral Tranilast
9. Tissue Shielding Method
9.1. Polyglycolic Acid Sheet
9.2. Carboxymethyl Cellulose Sheet
10. Regenerative Medicine
11. Stent Placement
12. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Ishihara, R.; Iishi, H.; Takeuchi, Y.; Kato, M.; Yamamoto, S.; Yamamoto, S.; Masuda, E.; Tatsumi, K.; Higashino, K.; Uedo, N.; et al. Local recurrence of large squamous-cell carcinoma of the esophageal after endoscopic resection. Gastrointest. Endosc. 2008, 67, 799–804. [Google Scholar] [CrossRef]
- Oyama, T.; Tomori, A.; Hotta, K.; Morita, S.; Kominato, K.; Tanaka, M.; Miyata, Y. Endoscopic submucosal dissection of early esophageal quamous cell neoplasima. Clin. Gastroenterol. Hepatol. 2005, 3, S67–S70. [Google Scholar] [CrossRef]
- Fujishiro, M.; Yahagi, N.; Kakushima, N.; Kodashima, S.; Muraki, Y.; Ono, S. Endoscopic submucosal dissection of sophageal cancer. Clin. Gastroenterol. Hepatol. 2006, 4, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, R.; Iishi, H.; Uedo, N.; Takeushi, Y.; Yamamoto, S.; Yamada, T.; Masuda, E.; Higashino, K.; Kato, M.; Narahara, H.; et al. Comparison of EMR and endoscopic submucosal dis-section for en bloc resection of early esophageal cancers in Japan. Gastrointest. Endosc. 2008, 68, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Fujishiro, M.; Niimi, K.; Goto, O.; Kodashima, S.; Yamamichi, N.; Omata, M. Long-term outcomes of endoscopic submucosal dis-section for superficial esophageal squamous cell neo—Plasms. Gastrointest. Endosc. 2009, 70, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Arimura, Y.; Masao, H.; Okahara, S.; Tanuma, T.; Kodaira, J.; Kagaya, H.; Shimizu, Y.; Hokari, K.; Tsukagoshi, H.; et al. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video). Gastrointest. Endosc. 2010, 72, 255–264.e2. [Google Scholar] [CrossRef] [PubMed]
- Katada, C.; Muto, M.; Manabe, T.; Boku, N.; Ohtsu, A.; Yoshida, S. Esophageal stenosis after endoscopic mucosal resection of super-ficial esophageal lesions. Gastrointest. Endosc. 2003, 57, 165–169. [Google Scholar] [CrossRef]
- Ono, S.; Fujishiro, M.; Niimi, K.; Goto, O.; Kodashima, S.; Yamamichi, N.; Omata, M. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy 2009, 41, 661–665. [Google Scholar] [CrossRef]
- Shi, Q.; Ju, H.; Yao, L.-Q.; Zhou, P.-H.; Xu, M.-D.; Chen, T.; Zhou, J.-M.; Chen, T.-Y.; Zhong, Y.-S. Risk factors for postoperative stricture after endoscopic submucosal dissection for superficial esophageal carcinoma. Endoscopy 2014, 46, 640–644. [Google Scholar] [CrossRef]
- Mizuta, H.; Nishimori, I.; Kuratani, Y.; Higashidani, Y.; Kohsaki, T.; Onishi, S. Predictive factors for esophageal stenosis after endo-scopic submucosal dissection for superficial esophageal cancer. Dis. Esophagus 2009, 22, 626–631. [Google Scholar] [CrossRef]
- Kishida, Y.; Kakushima, N.; Kawata, N.; Tanaka, M.; Takizawa, K.; Imai, K.; Hotta, K.; Matsubayashi, H.; Ono, H. Comparison of endoscopic dilation for esophageal stenosis after endoscopic submucosal dissection of superficial esophageal cancer. Surg. Endosc. 2015, 29, 2953–2959. [Google Scholar] [CrossRef] [PubMed]
- Yoda, Y.; Yano, T.; Kaneko, K.; Tsuruta, S.; Oono, Y.; Kojima, T.; Minashi, K.; Ikematsu, H.; Ohtsu, A. Endoscopic balloon dilatation for benign fibrotic strictures after curative nonsurgical treatment for esophageal cancer. Surg. Endosc. 2012, 26, 2877–2883. [Google Scholar] [CrossRef] [PubMed]
- Kanehara. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus, 4th ed.; The Japan Esophageal Society: Tokyo, Japan, 2017. [Google Scholar]
- Inoue, H.; Minami, H.; Sato, Y.; Kaga, M.; Sugaya, S.; Kudo, S. Technical feasibility of circumferential ESD and prevention balloon dilation. Stomach Intest. 2009, 44, 394–397. [Google Scholar]
- Ezoe, Y.; Muto, M.; Horimatsu, T.; Morita, S.; Miyamoto, S.; Mochizuki, S.; Minashi, K.; Yano, T.; Ohtsu, A.; Chiba, T. Efficacy of preventive endoscopic balloon dilation for esophageal stricture after endoscopic resection. J. Clin. Gastroenterol. 2011, 45, 222–227. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Isomoto, H.; Nakayama, T.; Hayashi, T.; Nishiyama, H.; Ohnita, K.; Takeshima, F.; Shikuwa, S.; Kohno, S.; Nakao, K. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest. Endosc. 2011, 73, 1115–1121. [Google Scholar] [CrossRef]
- Li, L.; Linghu, E.; Chai, N.; Xiang, J.; Wang, Z.; Zou, J.; Linghu, E.; Wang, X. Clinical experience of using a novel self-help inflatable balloon to prevent esophageal stricture after circumferential endoscopic submucosal dissection. Dig. Endosc. 2019, 31, 453–459. [Google Scholar] [CrossRef]
- Abe, S.; Iyer, P.G.; Oda, I.; Kanai, N.; Saito, Y. Approaches for stricture prevention after esophageal endoscopic resection. Gastrointest. Endosc. 2017, 86, 779–791. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kikuchi, D.; Nagami, Y.; Nonaka, K.; Tsuji, Y.; Fujimoto, A.; Sanomura, Y.; Tanaka, K.; Abe, S.; Zhang, S.; et al. Management of adverse events related to endo-scopic resection of upper gastrointestinal neoplasms: Review of the literature and recommendations from experts. Dig. Endosc. 2019, 31 (Suppl. 1), 4–20. [Google Scholar] [CrossRef]
- Kanetaka, K.; Kobayashi, S.; Yamaguchi, N.; Yamato, M.; Eguchi, S. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue—Engineered autologous oral mucosal epithelial cell sheets. Nihon Rinsho. Jpn. J. Clin. Med. 2015, 31, 457–462. [Google Scholar]
- Hashimoto, S.; Kobayashi, M.; Takeuchi, M.; Sato, Y.; Narisawa, R.; Aoyagi, Y. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest. Endosc. 2011, 74, 1389–1393. [Google Scholar] [CrossRef]
- Isomoto, H.; Yamaguchi, N.; Nakayama, T.; Hayashi, T.; Nishiyama, H.; Ohnita, K.; Takeshima, F.; Shikuwa, S.; Kohno, S.; Nakao, K. Management of esophageal stricture after complete circular endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. BMC Gastroenterol. 2011, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, N.; Ishihara, R.; Takeuchi, Y.; Uedo, N.; Higashino, K.; Ohta, T.; Kanzaki, H.; Hanafusa, M.; Nagai, K.; Matsui, F.; et al. Intralesional steroid injection to prevent stricture after endoscopic submucosal dissection for esophageal cancer: A controlled prospective study. Endoscopy 2012, 44, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Arimura, Y.; Okahara, S.; Kodaira, J.; Hokari, K.; Tsukagoshi, H.; Shinomura, Y.; Hosokawa, M. A randomized controlled trial of endoscopic steroid injection for prophylaxis of esophageal stenoses after extensive endoscopic submucosal dissection. BMC Gastroenterol. 2015, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Inoue, H.; Kobayashi, Y.; Miselli, R.; Santi, E.G.R.; Hayee, B.H.; Igarashi, K.; Yoshida, A.; Ikeda, H.; Onimaru, M.; et al. Control of severe strictures after circumferential endo-scopic submucosal dissection for esophageal carcinoma: Oral steroid therapy with balloon dilation or balloon dilation alone. Gastrointest. Endosc. 2013, 78, 250–257. [Google Scholar] [CrossRef]
- Mori, H.; Rafiq, K.; Kobara, H.; Fujihara, S.; Nishiyama, N.; Oryuu, M.; Suzuki, Y.; Masaki, T. Steroid permeation into the artificial ulcer by combined steroid gelapplication and balloon dilatation: Prevention of esophageal stricture. J. Gastroenterol. Hepatol. 2013, 28, 999–1003. [Google Scholar] [CrossRef]
- Kadota, T.; Yano, T.; Kato, T.; Imajoh, M.; Noguchi, M.; Morimoto, H.; Osera, S.; Yoda, Y.; Oono, Y.; Ikematsu, H.; et al. Prophylactic steroid administration for strictures after endoscopic resection of large superficial esophageal squamous cell carcinoma. Endosc. Int. Open 2016, 4, E1267–E1274. [Google Scholar] [CrossRef]
- Nagami, Y.; Shiba, M.; Ominami, M.; Sakai, T.; Minamino, H.; Fukunaga, S.; Sugimori, S.; Tanaka, F.; Kamata, N.; Tanigawa, T.; et al. Single locoregional triamcinolone injection imme-diately after esophageal endoscopic submucosal dissection prevents stricture formation. Clin. Transl. Gastroenterol. 2017, 8, e75. [Google Scholar] [CrossRef]
- Zhou, G.; Yuan, F.; Cai, J.; Tang, X.; Gong, W.; Su, L.; Zhang, Y. Efficacy of prednisone for prevention of esophageal stricture after endo-scopic submucosal dissection for superficial esophageal squamous cell carcinoma. Thorac. Cancer 2017, 8, 489–494. [Google Scholar] [CrossRef]
- Iizuka, T.; Kikuchi, D.; Hotera, S.; Kaise, M. Effectiveness of modified oral steroid administration for preventing esophageal stricture after entire circumferential endoscopic submucosal dissection. Dis. Esophagus 2018, 31, dox140. [Google Scholar] [CrossRef]
- Chu, Y.; Chen, T.; Li, H.; Zhou, P.; Zhang, Y.; Chen, W.; Zhong, Y.; Yao, L.; Xu, M. Long-term efficacy and safety of intralesional steroid injection plus oral steroid administration in preventing stricture after endoscopic submucosal dissection for esophageal epithelial neo-plasms. Surg. Endosc. 2019, 33, 1244–1251. [Google Scholar] [CrossRef]
- Pih, G.Y.; Kim, H.; Gong, E.J.; Na, H.K.; Jung, K.W.; Lee, J.H.; Ahn, J.Y.; Choi, K.D.; Song, H.J.; Lee, G.H.; et al. Preventing esophageal strictures with steroids after endoscopic submucosal dissection in superficial esophageal neoplasm. J. Dig. Dis. 2019, 20, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Isomoto, H.; Fukuda, H. Preventing stenosis after circumferential and semi-circumferential esophageal ESD-effect of oral steroid administration. Stomach Intest. 2013, 48, 1291–1302. [Google Scholar]
- Hanaoka, N.; Ishihara, R.; Uedo, N.; Takeuchi, Y.; Higashino, K.; Akasaka, T.; Kanesaka, T.; Matsuura, N.; Yamasaki, Y.; Hamada, K.; et al. Refractory strictures despite ste—Roid injection after esophageal endoscopic resection. Endosc. Int. Open 2016, 4, E354–E359. [Google Scholar] [PubMed]
- Iizuka, T.; Kikuchi, D.; Hoteya, S.; Kajiyama, Y.; Kaise, M. Polyglycolic acid sheet and fibrin glue for preventing esophageal stric-ture after endoscopic submucosal dissection: A historical control study. Dis. Esophagus 2017, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nagami, Y.; Ominami, M.; Shiba, M.; Sakai, T.; Fukunaga, S.; Sugimori, S.; Otani, K.; Hosomi, S.; Tanaka, F.; Taira, K.; et al. Prediction of esophageal stricture in patients given locoregional triamcin-olone injections immediately after endoscopic submucosal dissection. Dig. Endosc. 2018, 30, 198–205. [Google Scholar] [CrossRef]
- Hashimoto, S.; Mizuno, K.I.; Takahashi, K.; Sato, H.; Yokoyama, J.; Takeuchi, M.; Sato, Y.; Kobayashi, M.; Terai, S. Evaluating the effect of injecting triamcino-lone acetonide in two sessions for preventing esophageal stricture after endoscopic submucosal dissection. Endosc. Int. Open 2019, 7, E764–E770. [Google Scholar]
- Kataoka, M.; Anzai, S.; Shirasaki, T.; Ikemiyagi, H.; Fujii, T.; Mabuchi, K.; Suzuki, S.; Yoshida, M.; Kawai, T.; Kitajima, M. Efficacy of short period, low dose oral prednisolone for the prevention of stricture after circumferential endoscopic submucosal dissection (ESD) for esophageal cancer. Endosc. Int. Open 2014, 3, E113–E117. [Google Scholar] [CrossRef]
- Nagami, Y.; Shiba, M.; Tominaga, K.; Ominami, M.; Fukunaga, S.; Sugimori, N.; Tanaka, F.; Kamata, N.; Tanigawa, T.; Yamagami, H.; et al. Hybrid therapy with locoregional steroid injec-tion and polyglycolic acid sheets to prevent stricture after esophageal endoscopic submucosal dissection. Endosc. Int. Open 2016, 4, E1017–E1022. [Google Scholar]
- Sakaguchi, Y.; Tsuji, Y.; Fujishiro, M.; Kataoka, Y.; Takeuchi, C.; Yakabi, S.; Saito, I.; Shichijo, S.; Minatsuki, C.; Asada-Hirayama, I.; et al. Triamcinolone injection and shielding with polygly-colic acid sheets and fibrin glue for postoperative stricture prevention after esophageal endoscopic resection: A pilot study. Am. J. Gastroenterol. 2016, 111, 581–583. [Google Scholar] [CrossRef]
- Nakamura, J.; Hikichi, T.; Watanabe, K.; Sato, M.; Obara, K.; Ohira, H. Feasibility of short-periods, high-dose intravenous methylprednisolone for pre-venting stricture after endoscopic submucosal dissection for esophageal cancer: A preliminary study. Gastroenterol. Res. Pract. 2017, 2017, 9312517. [Google Scholar] [CrossRef]
- Shibagaki, K.; Ishimura, N.; Oshima, N.; Mishiro, T.; Fukuba, N.; Tamagawa, Y.; Yamashita, N.; Mikami, H.; Izumi, D.; Taniguchi, H.; et al. Esophageal triamcinolone acetonide-filling method: A novel pro-cedure to prevent stenosis after extensive esophageal endoscopic submucosal dissection (with videos). Gastrointest. Endosc. 2018, 87, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Shibagaki, K.; Yuki, T.; Taniguchi, H.; Aimi, M.; Miyaoka, Y.; Yuki, M.; Ishimura, N.; Oshima, N.; Mishiro, T.; Tamagawa, Y.; et al. Prospective multicenter study of the esophageal triamcinolone acetonide-filling method in patients with subcircumferential esophageal endoscopic submucosal dissection. Dig. Endosc. 2020, 32, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Tsuji, Y.; Shinozaki, T.; Ohki, D.; Muzutani, H.; Minatsuki, C.; Niimi, K.; Yamamichi, N.; Koike, K. Steroid injection and polyglycolic acid to pre-vent stricture after esophageal endoscopic submucosal dissection: A retrospective comparative analysis (with video). Gastrointest. Endosc. 2020, 92, 1176–1386. [Google Scholar] [CrossRef] [PubMed]
- Miwata, T.; Oka, S.; Tanaka, S.; Kagemoto, K.; Sanomura, Y.; Urabe, Y.; Hiyama, T.; Chayama, K. Risk factors for esophageal stenosis after entire circumferential endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Surg. Endosc. 2016, 30, 4049–4056. [Google Scholar] [CrossRef]
- Kadota, T.; Yoda, Y.; Hori, K.; Shinmura, K.; Oono, Y.; Ikematsu, H.; Yano, T. Prophylactic steroid administration against strictures is not enough for mucosal defects involving the entire circumference of the esophageal lumen after esophageal endoscopic submucosal dissection (ESD). Esophagus 2020, 17, 440–447. [Google Scholar] [CrossRef]
- Nonaka, K.; Miyazawa, M.; Ban, S.; Aikawa, M.; Akimoto, N.; Koyama, I.; Kita, H. Different healing process of esophageal large mu-cosal defects by endoscopic mucosal dissection between with and without steroid injection in an animal model. BMC Gastroenterol. 2013, 13, 722. [Google Scholar]
- Nagami, Y.; Shiba, M.; Tominaga, K.; Minamino, H.; Ominami, M.; Fukunaga, S.; Sugimori, S.; Tanigawa, T.; Yamagami, H.; Watanabe, K.; et al. Locoregional steroid injection prevents stricture formation after endoscopic submucosal dissection for esophageal cancer: A propensity score matching analysis. Surg. Endosc. 2015, 30, 1441–1449. [Google Scholar] [CrossRef]
- Ono, S.; Fujishiro, M.; Kodashima, S.; Minatsuki, C.; Hirano, K.; Niimi, K.; Goto, O.; Yamamichi, N.; Fukuda, T.; Seto, Y.; et al. High-dose dexamethasone may prevent esophageal stricture after endo-scopic submucosal dissection. Clin. J. Gastroenterol. 2010, 3, 155–158. [Google Scholar] [CrossRef]
- Funakawa, K.; Uto, H.; Sasaki, F.; Nasu, Y.; Mawatari, S.; Arima, S.; Nakazawa, J.; Taguchi, H.; Hashimoto, S.; Kanmura, S.; et al. Effect of endoscopic submucosal dissection for superficial esophageal neoplasms and risk factors for postoperative stricture. Medicine 2015, 94, e373. [Google Scholar] [CrossRef]
- Wakahara, C.; Morita, Y.; Tanaka, S.; Hoshi, N.; Kawara, F.; Kibi, M.; Ishida, T.; Man-I, M.; Fujita, T.; Toyonaga, T. Optimization of steroid injection intervals for prevention of stricture after esophageal endoscopic submucosal dissection: A randomized controlled trial. Acta Gastroenterol. Belg. 2016, 79, 315–320. [Google Scholar]
- Okamoto, K.; Matsui, S.; Watanabe, T.; Asakuma, Y.; Komeda, Y.; Okamoto, A.; Rei, I.; Kono, M.; Yamada, M.; Nagai, T.; et al. Clinical analysis of esophageal stricture in patients treated with intralesional triamcinolone injection after endoscopic submucosal dissection for superficial esophage-al cancer. Oncology 2017, 93 (Suppl. 1), 9–14. [Google Scholar] [CrossRef] [PubMed]
- Yamashina, T.; Uedo, N.; Fujii, M.; Ishihara, R.; Mikamori, M.; Motoori, M.; Yano, M.; Iishi, H. Delayed perforation after intralesional triamcinolone injection for esophageal stricture following endoscopic submucosal dissection. Endoscopy 2013, 45, E92. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Kato, M.; Fujimoto, A.; Maehata, T.; Sasaki, M.; Inoshita, N.; Sato, H.; Suzuki, K.; Yahagi, N. Inadequate steroid injection after esophageal ESD might cause mural necrosis. Endosc. Int. Open 2019, 7, E115–E121. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Nakao, K.; Eguchi, S.; Isomoto, H. Problems and prospects of treatment for prevention of stenosis after endoscopic submucosal dissec-tion of superficial esophageal cancer: Factors associated with resistance to stenosis prevention treatment, and usefulness of steroid oral + local injec-tion combination therapy. Gastroenterol. Endosc. 2017, 59, 2535–2545. [Google Scholar]
- Tang, B.; Bai, J.-Y.; Zhao, X.-Y.; Fan, C.-Q.; Yang, X.; Deng, L.; Yang, S.-M.; Yu, J. Endoscopic submucosal dissection for superficial esophageal cancer with near-circumferential lesions: Our experience with 40 patients. Surg. Endosc. 2014, 29, 2141–2148. [Google Scholar] [CrossRef]
- Ishida, T.; Morita, Y.; Hoshi, N.; Yoshizaki, T.; Ohara, Y.; Kawara, F.; Tanaka, S.; Yamamoto, Y.; Matsuo, H.; Iwata, K.; et al. Disseminated nocardiosis during systemic steroid therapy for the prevention of esophageal stricture after endoscopic submucosal dissection. Dig. Endosc. 2014, 27, 388–391. [Google Scholar] [CrossRef]
- Kato, R.; Yamasaki, Y.; Tanaka, S. Triamcinolone injection and filling method to prevent stricture after esophageal endoscopic submucosal dissection. Dig. Endosc. 2018, 30, 795–796. [Google Scholar] [CrossRef]
- Wang, W.; Ma, Z. Steroid administration is effective to prevent strictures after endoscopic esophageal submucosal dissection: A network meta-analysis. Medicine 2015, 94, e1664. [Google Scholar] [CrossRef]
- Mizutani, T.; Tanaka, M.; Eba, J.; Mizusawa, J.; Fukuda, H.; Hanaoka, N.; Takeuchi, M.; Aoyama, I.; Kojima, T.; Takizawa, K.; et al. A Phase III study of oral steroid administration ver-sus local steroid injection therapy for the prevention of esophageal stricture after endoscopic submucosal dissection (JCOG1217, Steroid EESD P3). Jpn. J. Clin. Oncol. 2015, 45, 1087–1090. [Google Scholar] [CrossRef]
- Wen, J.; Lu, Z.; Linghu, E.; Yang, Y.; Yang, J.; Wang, S.; Yan, B.; Song, J.; Zhou, X.; Wang, X. Prevention of esophageal strictures after endoscopic submucosal dissec-tion with the injection of botulinum toxin type A. Gastrointest. Endosc. 2016, 84, 606–613. [Google Scholar] [CrossRef]
- Neuhaus, H. Prevention of strictures after endoscopic resection of esophageal neoplasia. Gastrointest. Endosc. 2016, 84, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Uno, K.; Iijima, K.; Koike, T.; Abe, Y.; Asano, N.; Ara, N.; Shimosegawa, T. A pilot study of scheduled endoscopic balloon dilation with oral agent tranilast to improve the efficacy of stricture dilation after endoscopic submucosal dissection of the esophagus. J. Clin. Gastroenterol. 2012, 46, e76–e82. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, T.; Kikuchi, D.; Yamada, A.; Hoteya, S.; Kajiyama, Y.; Kaise, M. Polyglycolic acid sheet application to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Endoscopy 2014, 47, 341–344. [Google Scholar] [CrossRef]
- Ono, S.; Tsuji, Y.; Fujishiro, M.; Kodashima, S.; Yamamichi, N.; Koike, K. An effective technique for delivery of polyglycolic acid sheet after esophageal endoscopic submucosal dissection of the esophagus: The clip and pull method. Endoscopy 2014, 46 (Suppl. 1), E44–E456. [Google Scholar]
- Ono, S.; Sakaguchi, Y.; Tsuji, Y.; Kodashima, S.; Yamamichi, N.; Fujishiro, M.; Koike, K. Foam plombage: A novel technique for optimal fixation of polyglycolic acid sheets positioned using “clip and pull” after esophageal endoscopic submucosal dissection. Endoscopy 2015, 47, E435–E436. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Tsuji, Y.; Ono, S.; Saito, I.; Kataoka, Y.; Takahashi, Y.; Nakayama, C.; Shichijo, S.; Matsuda, R.; Minatsuki, C.; et al. Polyglycolic acid sheets with fibrin glue can prevent esophageal stricture after endoscopic submucosal dissection. Endoscopy 2014, 47, 336–340. [Google Scholar] [CrossRef]
- Yamasaki, M.; Kume, K.; Yoshikawa, I.; Otsuki, M. A novel method of endoscopic submucosal dissection with blunt abrasion by submucosal injection of sodium carboxymethylcellulose: An animal preliminary study. Gastrointest. Endosc. 2006, 64, 958–965. [Google Scholar] [CrossRef]
- Hikichi, T.; Yamasaki, M.; Watanabe, K.; Nakamura, J.; Sato, M.; Takagi, T.; Suzuki, R.; Sugimoto, M.; Kikuchi, H.; Konno, N.; et al. Gastric endoscopic submucosal dissection using sodium carboxymethyl-cellulose as a new injection substance. Fukushima J. Med. Sci. 2016, 62, 43–50. [Google Scholar] [CrossRef][Green Version]
- Lua, G.W.; Tang, J.; Liu, F.; Li, Z.S. Prevention of esophageal strictures after endoscopic submucosal dissection: A promising ther-apy using carboxymethyl cellulose sheets. Dig. Dis. Sci. 2016, 61, 1763–1769. [Google Scholar] [CrossRef][Green Version]
- Tang, J.; Ye, S.; Ji, X.; Liu, F.; Li, Z.-S. Deployment of carboxymethyl cellulose sheets to prevent esophageal stricture after full circumferential endoscopic submucosal dissection: A porcine model. Dig. Endosc. 2018, 30, 608–615. [Google Scholar] [CrossRef]
- Sakurai, T.; Miyazaki, S.; Miyata, G.; Satomi, S.; Hori, Y. Autologous buccal keratinocyte implantation for the prevention of stenosis after EMR of the esophagus. Gastrointest. Endosc. 2007, 66, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Hori, Y.; Nakada, A.; Uji, M.; Nishizawa, Y.; Yamamoto, K.; Kobayashi, T.; Shimada, H.; Kida, N.; Sato, T.; et al. Use of adipose tissue-derived stromal cells for prevention of esophageal stricture after circumferential EMR in a canine model. Gastrointest. Endosc. 2011, 73, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Sagara, S.; Nakajima, N.; Akimoto, T.; Suzuki, K.; Yoneyama, H.; Terai, S.; Yahagi, N. Prevention of esophageal stricture after endoscopic submucosal dissection using RNA-based silencing of carbohydrate sulfotransferase 15 in a porcine model. Endoscopy 2017, 49, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shuai, Q.; Tang, J.; Long, D.; Xu, C.; Liu, F.; Li, Z. Local thymosin β4 gel injection prevents esophageal stricture after circumferential endoscopic submucosal dissection in a porcine model. Dig. Dis. 2018, 37, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, T.; Ohnishi, S.; Hosono, H.; Yamahara, K.; Tsuda, M.; Shimizu, Y.; Shimizu, Y.; Kato, M.; Asaka, M.; Sakamoto, N.; et al. Oral administration of conditioned medium obtained from mesen-chymal stem cell culture prevents subsequent stricture formation after esophageal submucosal dissection in pigs. Gastrointest. Endosc. 2017, 86, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Barret, M.; Bordaçahar, B.; Beuvon, F.; Chaussade, S.; Batteux, F.; Prat, F. Self-assembling peptide matrix for the prevention of esophageal stricture after en-doscopic resection: A randomized controlled trial in a porcine model. Dis. Esophagus 2017, 30, 1–7. [Google Scholar] [CrossRef]
- Oumrani, S.; Barret, M.; Bordaçahar, B.; Terris, B.; Camus, M.; Coriat, R.; Batteux, F.; Prat, F. Application of a self-assembling peptide matrix pre-vents esophageal stricture after circumferential endoscopic submucosal dissection in a pig model. PLoS ONE 2019, 14, e0212362. [Google Scholar] [CrossRef]
- Nieponice, A.; McGrath, K.; Qureshi, I.; Beckman, E.J.; Luketich, J.D.; Gilbert, T.W.; Badylak, S.F. An extracellular matrix scaffold for esophageal stricture preven-tion after circumferential EMR. Gastrointest. Endosc. 2009, 69, 289–296. [Google Scholar] [CrossRef]
- Aoki, S.; Sakata, Y.; Shimoda, R.; Takezawa, T.; Oshikata-Mitazaki, A.; Kimura, H.; Yamamoto, M.; Iwakiri, R.; Fujimoto, K.; Toda, S. High-density collagen patch prevents stricture after endoscopic circumferential submucosal dissection of the esophagus: A porcine model. Gastrointest. Endosc. 2017, 85, 1076–1085. [Google Scholar] [CrossRef]
- Perrod, G.; Rahmi, G.; Pidial, L.; Camilleri, S.; Bellucci, A.; Casanova, A.; Viel, T.; Tavitian, B.; Cellier, C.; Clement, O. Cell sheet transplantation for esophageal stricture prevention after endo-scopic submucosal dissection in a porcine model. PLoS ONE 2016, 11, e0148249. [Google Scholar] [CrossRef]
- Barret, M.; Pratico, C.A.; Camus, M.; Beuvon, F.; Jarraya, M.; Nicco, C.; Mangialavori, L.; Chaussade, S.; Batteux, F.; Prat, F. Amniotic membrane grafts for the prevention of esophageal stricture after circumferential endoscopic submucosal dissection. PLoS ONE 2014, 9, e100236. [Google Scholar] [CrossRef] [PubMed]
- Takase, K.; Aikawa, M.; Okada, K.; Watanabe, Y.; Okamoto, K.; Sato, H.; Nonaka, K.; Yamaguchi, S.; Sakuramoto, S.; Koyama, I.; et al. Development of novel treatment with a bioabsorbable esophageal patch for benign esophageal stricture. Dis. Esophagus 2014, 28, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.K.; Ainoedhofer, H.; Hollwarth, M.E. Esophagus tissue engineering: In vitro generation of esophageal epithelium cell sheets and viability on scaffold. J. Pediatr. Surg. 2009, 44, 896–901. [Google Scholar] [CrossRef]
- Han, Y.; Guo, J.; Sun, S.; Wu, W.; Wang, S.; Ge, N.; Liu, X.; Wang, G.; Wang, S. Acellular dermal matrix for esophageal stricture prevention after endo-scopic submucosal dissection in a porcine model. Gastrointest. Endosc. 2017, 86, 1160–1167. [Google Scholar] [CrossRef]
- Perrod, G.; Pidial, L.; Camilleri, S.; Bellucci, A.; Casanova, A.; Viel, T.; Tavitian, B.; Cellier, C.; Clement, O.; Rahmi, G. ADSC-sheet transplantation to prevent stricture after extended esophageal endoscopic submucosal dissection. J. Vis. Exp. 2017, 120, 55018. [Google Scholar] [CrossRef]
- Hochberger, J.; Koehler, P.; Wedi, E.; Gluer, S.; Rothstein, R.I.; Niemann, H.; Hilfiker, A.; Gonzalez, S.; Kruse, E. Transplantation of mucosa from stomach to esophagus to prevent stric-ture after circumferential endoscopic submucosal dissection of early squamous cell. Gastroenterology 2014, 146, 906–909. [Google Scholar] [CrossRef]
- Liao, Z.; Liao, G.; Yang, X.; Peng, X.; Zhang, X.; Xie, X.; Zhao, X.; Yang, S.; Fan, C.; Bai, J. Transplantation of autologous esophageal mucosa to prevent stricture after circumferential endoscopic submucosal dissection of early esophageal cancer (with video). Gastrointest. Endosc. 2018, 88, 543–546. [Google Scholar] [CrossRef]
- Ohki, T.; Yamato, M.; Murakami, D.; Takagi, R.; Yang, J.; Namiki, H.; Okano, T.; Takasaki, K. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut 2006, 55, 1704–1710. [Google Scholar] [CrossRef]
- Kanai, N.; Yamato, M.; Ohki, T.; Yamamoto, M.; Okano, T. Fabricated autologous epidermal cell sheets for the prevention of esophageal stricture after circumferential ESD in a porcine model. Gastrointest. Endosc. 2012, 76, 873–881. [Google Scholar] [CrossRef]
- Murakami, D.; Yamato, M.; Nishida, K.; Ohki, T.; Takagi, R.; Yang, J.; Namiki, H.; Okano, T. Fabrication of transplantable human oral mucosal epithe-lial cell sheets using temperature-responsive culture inserts without feeder layer cells. J. Artif. Organs 2006, 9, 185–191. [Google Scholar] [CrossRef]
- Takagi, R.; Murakami, D.; Kondo, M.; Ohki, T.; Sasaki, R.; Mizutani, M.; Yamato, M.; Nishida, K.; Namiki, H.; Yamamoto, M.; et al. Fabrication of human oral mucosal epithelial cell sheets for treatment of esophageal ulceration by endoscopic submucosal dissection. Gastrointest. Endosc. 2010, 72, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Takagi, R.; Yamato, M.; Kanai, N.; Murakami, D.; Kondo, M.; Ishii, T.; Ohki, T.; Namiki, H.; Yamamoto, M.; Okano, T. Cell sheet technology for regeneration of esophageal mucosa. World J. Gastroenterol. 2012, 18, 5145–5150. [Google Scholar] [PubMed]
- Ohki, T.; Yamato, M.; Ota, M.; Takagi, R.; Murakami, D.; Kondo, M.; Sasaki, R.; Namiki, H.; Okano, T.; Yamamoto, M. Prevention of esophageal stricture after endoscopic sub-mucosal dissection using tissue-engineered cell sheets. Gastroenterology 2012, 143, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Ohki, T.; Yamato, M.; Ota, M.; Takagi, R.; Kondo, M.; Kanai, N.; Okano, T.; Yamamoto, M. Application of regenerative medical technology using tis-sue-engineered cell sheets for endoscopic submucosal dissection of esophageal neoplasms. Dig. Endosc. 2015, 27, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Isomoto, H.; Kobayashi, S.; Kanai, N.; Kanetaka, K.; Sakai, Y.; Kasai, Y.; Takagi, R.; Ohki, T.; Fukuda, H.; et al. Oral epithelial cell sheets engraftment for esophageal strictures after endoscopic submucosal dissection of squamous cell carcinoma and airplane transportation. Sci. Rep. 2017, 7, 17460. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Kurumi, H.; Takeda, Y.; Yashima, K.; Isomoto, H. Management of strictures after endoscopic submucosal dissec-tion for superficial esophageal cancer. Ann. Transl. Med. 2017, 5, 184. [Google Scholar] [CrossRef]
- Maeda, M.; Kanai, N.; Kobayashi, S.; Hosoi, T.; Takagi, R.; Ohki, T.; Muragaki, Y.; Yamato, M.; Eguchi, S.; Fukai, F.; et al. Endoscopic cell sheet transplantation device developed by using a 3-dimensional printer and its feasi—Bility evaluation in a porcine model. Gastrointest. Endosc. 2015, 82, 147–152. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kanai, N.; Tanaka, N.; Maeda, M.; Hosoi, T.; Fukai, F.; Eguchi, S.; Yamato, M. Transplantation of epidermal cell sheets by endoscopic balloon dilatation to avoid esophageal re-strictures: Initial experience in a porcine model. Endosc. Int. Open 2016, 4, E1116–E1123. [Google Scholar] [CrossRef]
- Ohmura, K.; Nagashima, R.; Takeda, H.; Takahashi, T. Temporary stenting with metallic endoprosthesis for refractory esophageal stricture secondary to cylindrical resection of carcinoma. Gastrointest. Endosc. 1998, 48, 214–217. [Google Scholar] [CrossRef]
- Yamasaki, T.; Tomita, T.; Takimoto, M.; Ohda, Y.; Oshima, T.; Fukui, H.; Watari, J.; Miwa, H. Esophageal stricture after endoscopic submucosal dissection treated suc-cessfully by temporary stent placement. Clin. J. Gastroenterol. 2016, 9, 337–340. [Google Scholar] [CrossRef]
- Wen, J.; Yang, Y.; Liu, Q.; Yang, J.; Wang, S.; Wang, X.; Du, H.; Meng, J.; Wang, H.; Lu, Z. Preventing stricture formation by covered esophageal stent placement after endoscopic submucosal dissection for early esophageal cancer. Dig. Dis. Sci. 2014, 59, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.P.; Zheng, H.H.; Mao, X.L.; Zang, Y.; Zhou, X.B.; Zhu, L.H. Complete circular endoscopic resection using submucosal tunnel tech-nique combined with esophageal stent placement for circumferential superficial esophageal lesions. Surg. Endosc. 2016, 30, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Chai, N.L.; Feng, J.; Li, L.S.; Liu, S.Z.; Du, C.; Zhang, Q.; Linghu, E.Q. Effect of polyglycolic acid sheet plus esophageal stent placement in pre-venting esophageal stricture after endoscopic submucosal dissection in patients with early-stage esophageal cancer: A ran-domized, controlled trial. World J. Gastroenterol. 2018, 24, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Linghu, E.; Chai, N.; Li, Z.; Zou, J.; Du, C.; Wang, X.; Xiang, J. Efficacy of triamcinolone-soaked polyglycolic acid sheet plus fully covered metal stent for preventing stricture formation after large esophageal endoscopic submucosal dissection. Dis. Esophagus 2019, 32, 1–7. [Google Scholar] [CrossRef]
- Shi, K.-D.; Ji, F. Prophylactic stenting for esophageal stricture prevention after endoscopic submucosal dissection. World J. Gastroenterol. 2017, 23, 931–934. [Google Scholar] [CrossRef]
- Saito, Y.; Tanaka, T.; Andoh, A.; Minematsu, H.; Hata, K.; Tsujikawa, T.; Nitta, N.; Murata, K.; Fujiyama, Y. Novel biodegradable stents for benign esophageal strictures following endoscopic submucosal dissection. Dig. Dis. Sci. 2007, 53, 330–333. [Google Scholar] [CrossRef]
- Yano, T.; Yoda, Y.; Nomura, S.; Toyosaki, K.; Hasegawa, H.; Ono, H.; Tanaka, M.; Morimoto, H.; Horimatsu, T.; Nonaka, S.; et al. Prospective trial of biodegradable stents for refractory benign esophageal stric-tures after curative treatment of esophageal cancer. Gastrointest. Endosc. 2017, 86, 492–499. [Google Scholar] [CrossRef]
- Saito, Y.; Tanaka, T.; Andoh, A.; Minematsu, H.; Hata, K.; Tsujikawa, T.; Nitta, N.; Murata, K.; Fujiyama, Y. Usefulness of biodegradable stents constructed of poly-l-lactic acid monofilaments in patients with benign esophageal stenosis. World J. Gastroenterol. 2007, 13, 3977–3980. [Google Scholar] [CrossRef]


| Prophylactic EBD | |
| Steroid therapy | Steroid injection therapy (ex. TA) |
| Oral steroid administration (ex. PSL) | |
| Other steroid administration: combination of TA injection with oral PSL, TA injection with PGA, TA injection with EBD, TA-filling method | |
| Drugs other than steroids | Botulinum toxin injection therapy |
| Oral tranilast | |
| Tissue shielding method | PGA sheet |
| Carboxymethyl cellulose sheet | |
| Regenerative medicine | Autologous oral mucosal epithelial cell sheet transplantation, et al. |
| Stent placement | Temporary metal stent placement, bioabsorbable stent placement |
| Author | Year | Study Design | Protocol Therapy | Mucosal Deffect Circumference | Case Numbers (Protocol: Control) | Incidence of Stricture (Protocol vs. Control) | p-Value *1 |
|---|---|---|---|---|---|---|---|
| Hashimoto [21] | 2011 | Retrospective, historical control | TA injection | >3/4 | 21:20 (untreated) | 19% vs. 75% | <0.001 |
| Yamaguchi [16] *2 | 2011 | Retrospective, historical control | Oral PSL for eight weeks | >3/4 | 19:22 (prophylactic EBD) *3 | 5.3% vs. 31.8% | 0.03 |
| Isomoto [22] *2 | 2011 | Retrospective, historical control | Oral PSL for eight weeks | Total circumference | 4:3 (prophylactic EBD) | 50% vs. 100% | N.S. |
| Hanaoka [23] | 2012 | Prospective, historical control | TA injection | >3/4 | 30:29 (untreated) | 10% vs. 66% | <0.001 |
| Takahashi [24] | 2012 | Prospective, randomized | TA injection | Lesion > 2/3 | 16:16 (untreated) *4 | 62.5% vs. 87.5% | 0.22 |
| Sato [25] | 2013 | Prospective, historical control | Oral PSL for eight weeks + prophylactic EBD | Total circumference | 10:13 (prophylactic EBD) *5 | 100% vs. 100% | N.S. |
| Mori [26] | 2013 | Prospective, randomized | ① TA gel + prophylactic EBD ② TA injection + prophylactic EBD | >2/3 | 20:21 (①:②) | N/A *6 | N/A |
| Kadota [27] | 2016 | Retrospective | ① TA injection + Oral PSL for eight weeks ② TA injection | >3/4 | 29:53:33 (①:②: untreated) | 41% vs. 43% Vs. 67% (①:②: untreated) | 0.073 (① vs. untreated) 0.046 (② vs. untreated) |
| Nagami [28] | 2017 | Retrospective, matched | TA injection | >2/3 | 37:37 (untreated) | 18.9% vs. 45.9% | 0.016 |
| Zhou [29] | 2017 | Retrospective | Oral PSL for 12 weeks | >3/4 *7 | 13:10 (untreated) | 23.1% vs. 80% | <0.05 |
| Iizuka [30] | 2018 | Retrospective, historical control | ① Oral PSL for 18 weeks (±TA injection) *8 ② Oral PSL for eight weeks (±TA injection) *8 | Total circumference | 11:11 (①:②) | 36.4% vs. 82% | 0.04 |
| Chu [31] | 2019 | Retrospective | TA injection + Oral PSL for eight weeks | >2/3 | 34:36 (untreated) | 14.7% vs. 52.8% | 0.001 |
| Pih [32] | 2019 | Retrospective | ① Oral PSL ② TA injection | >3/4 | 25:6:22 (①: ②: untreated) | 20% vs. 33.3% vs. 50% (①:②: untreated) | 0.037 (① vs. untreated) 0.046 (①+② vs. untreated) |
| Author | Year | Study Design | Drugs | Dose | Timing of Intervention | Mucosal Defect Circumference | Incidence of Stricture |
|---|---|---|---|---|---|---|---|
| Steroid injection | |||||||
| Hashimoto [21] | 2011 | Retrospective | TA | 18–62 mg | Day 3, 7, 10 (3 times) | >3/4 | 19% (4/21) |
| Hanaoka [23] | 2012 | Prospective | TA | 100 mg | Day 0 | >3/4 | 10% (3/30) |
| Yamaguchi [33] | 2013 | Retrospective | TA | 40 mg (<3 cm in longitudinal mucosal defect), 80 mg (≥ 3 cm) | Day 0 (>9/10 in circumference or ≥5 cm in longitudinal mucosal defect: additionally Day 21) | >3/4 | 4.3% (1/23) |
| Takahashi [24] | 2015 | Prospective, randomized | TA | 40 mg | Day 0 | >2/3 (lesion *) | 45.5% (5/11) |
| Hanaoka [34] | 2016 | Retrospective | TA | 50–100 mg | Day 0 | >3/4 | 11.3% (13/115) |
| Kadota [27] | 2016 | Retrospective | TA | 50 mg | Day 3, 7, 10 (three times) →Day 1 or Day 0 (once) | >3/4 | 36.2% (17/47) |
| Nagami [28] | 2017 | Retrospective | TA | 80 mg | Day 0 | >2/3 | 20.7% (12/58) |
| Iizuka [35] | 2017 | Retrospective | TA | 40 mg | Day 0 | >1/2 | 10.3% (3/29) |
| Nagami [36] | 2018 | Retrospective | TA | 80 mg | Day 0 | >2/3 | 16.8% (17/101) |
| Hashimoto [37] | 2019 | Retrospective | TA | 40–100 mg (2nd session: 16–50 mg) | Day 0, 14 (two times) | >3/4 | 45.7% (16/35) |
| Oral steroid administration | |||||||
| Yamaguchi [16] | 2011 | Retrospective | PSL | 30 mg | Tapering gradually for eight weeks | >3/4 | 6.3% (1/16) |
| Yamaguchi [33] | 2013 | Retrospective | PSL | 30 mg | Tapering gradually for 6–12 weeks | >3/4 | 10% (4/40) |
| Kataoka [38] | 2015 | Retrospective | PSL | 30 mg | Tapering gradually for three weeks | >3/4 | 14.3% (2/14) |
| Modified or hybrid steroid therapy | |||||||
| Kadota [27] | 2016 | Retrospective | TA + Oral PSL | TA: 50 mg PSL: 30 mg | TA: Day 3, 7, 10 (three times) →Day 1 or Day 0 (once) PSL: tapering gradually for eight weeks | >3/4 | 13.3% (2/15) |
| Nagami [39] | 2016 | Retrospective | TA injection + PGA | TA: 80 mg | Day 0 | >5/6 | 25% (1/4) |
| Sakaguchi [40] | 2016 | Retrospective | TA injection + PGA | TA: 40 mg | Day 0 | >3/4 | 11.1% (1/9) |
| Nakamura [41] | 2017 | Prospective | Pulse therapy | mPSL: 500 mg (intravenous administration) | Day 1, 2, 3 (three consecutive days) | >3/4 | 66.7% (6/9) |
| Shibagaki [42] | 2018 | Retrospective | TA filling method | TA: 80 mg | Day 1 and Day 7 and when mild stricture was found | >3/4 | 6.7% (1/15) |
| Shibagaki [43] | 2020 | Prospective | TA filling method | TA: 80 mg | Day 1 and Day 7 and when mild stricture was found | >3/4 | 5% (1/20) |
| Sakaguchi [44] | 2020 | Retrospective | TA injection + PGA | TA: 40 mg | Day 0 | >3/4 | 18.9% (7/37) |
| Author | Year | Study Design | Drugs | Dose | Timing of Intervention | Incidence of Stricture |
|---|---|---|---|---|---|---|
| Steroid injection | ||||||
| Yamaguchi [33] | 2013 | Retrospective | TA | 80 mg | Day 0, 21 | 100% (4/4) |
| Takahashi [24] | 2015 | Prospective, randomized | TA | 40 mg | Day 0 | 100% (5/5) |
| Hanaoka [34] | 2016 | Retrospective | TA | 100 mg | Day 0 | 91.7% (11/12) |
| Miwata [45] | 2016 | Retrospective | PSL | N/A | Day 1 | 100% (6/6) |
| Hashimoto [37] | 2019 | Retrospective | TA | 40–100 mg (second: 16–50 mg) | Day 0, 14 (two times) | 80% (4/5) |
| Oral steroid administration | ||||||
| Yamaguchi [16] | 2011 | Retrospective | PSL | 30 mg | Tapering gradually for eight weeks | 0% (0/3) |
| Isomoto [22] | 2011 | Retrospective | PSL | 30 mg | Tapering gradually for eight weeks | 50% (2/4) |
| Sato [25] | 2013 | Prospective | PSL | 30 mg | Tapering gradually for eight weeks | 100% (10/10) |
| Yamaguchi [33] | 2013 | Retrospective | PSL | 30 mg | Tapering gradually for 8–18 weeks | 27.3% (3/11) |
| Kataoka [38] | 2015 | Retrospective | PSL | 30 mg | Tapering gradually for three weeks | 33.3% (1/3) |
| Miwata [45] | 2016 | Retrospective | PSL | 0.5 mg/kg | Tapering gradually 5 mg/week | 100% (13/13) |
| Modified or hybrid steroid therapy | ||||||
| Kadota [27] | 2016 | Retrospective | TA + Oral PSL | TA: 50 mg PSL: 30 mg | TA: Day 3, 7, 10 (three times) →Day 1 or Day 0 (once) PSL: tapering gradually for eight weeks | 71% (10/14) |
| Nagami [39] | 2016 | Retrospective | TA injection + PGA | TA: 80 mg | Day 0 | 66.7% (4/6) |
| Sakaguchi [40] | 2016 | Retrospective | TA injection + PGA | TA: 40 mg | Day 0 | 50% (1/2) |
| Iizuka [30] | 2018 | Retrospective | Oral PSL ±TA injection | PSL: 30 mg TA: 80–120 mg | PSL: tapering gradually for eight weeks (TA injection: Day 0) | 81.8% (9/11) |
| Oral PSL ±TA injection | PSL: 30 mg TA: 80–120 mg | PSL: tapering gradually for 18 weeks (TA injection: Day 0) | 36.4% (4/11) | |||
| Shibagaki [42] | 2018 | Retrospective | TA filling method | TA: 80 mg | Day 1 and Day 7 and when mild stricture was found | 0% (0/7) |
| Kadota [46] | 2020 | Retrospective | TA + Oral PSL | TA: 50 or 100 mg PSL: 30 mg | TA: Day 0 PSL: tapering gradually for eight weeks | 61.5% (16/26) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hikichi, T.; Nakamura, J.; Takasumi, M.; Hashimoto, M.; Kato, T.; Kobashi, R.; Takagi, T.; Suzuki, R.; Sugimoto, M.; Sato, Y.; et al. Prevention of Stricture after Endoscopic Submucosal Dissection for Superficial Esophageal Cancer: A Review of the Literature. J. Clin. Med. 2021, 10, 20. https://doi.org/10.3390/jcm10010020
Hikichi T, Nakamura J, Takasumi M, Hashimoto M, Kato T, Kobashi R, Takagi T, Suzuki R, Sugimoto M, Sato Y, et al. Prevention of Stricture after Endoscopic Submucosal Dissection for Superficial Esophageal Cancer: A Review of the Literature. Journal of Clinical Medicine. 2021; 10(1):20. https://doi.org/10.3390/jcm10010020
Chicago/Turabian StyleHikichi, Takuto, Jun Nakamura, Mika Takasumi, Minami Hashimoto, Tsunetaka Kato, Ryoichiro Kobashi, Tadayuki Takagi, Rei Suzuki, Mitsuru Sugimoto, Yuki Sato, and et al. 2021. "Prevention of Stricture after Endoscopic Submucosal Dissection for Superficial Esophageal Cancer: A Review of the Literature" Journal of Clinical Medicine 10, no. 1: 20. https://doi.org/10.3390/jcm10010020
APA StyleHikichi, T., Nakamura, J., Takasumi, M., Hashimoto, M., Kato, T., Kobashi, R., Takagi, T., Suzuki, R., Sugimoto, M., Sato, Y., Irie, H., Okubo, Y., Kobayakawa, M., & Ohira, H. (2021). Prevention of Stricture after Endoscopic Submucosal Dissection for Superficial Esophageal Cancer: A Review of the Literature. Journal of Clinical Medicine, 10(1), 20. https://doi.org/10.3390/jcm10010020






