Uric Acid—An Emergent Risk Marker for Thrombosis?
Abstract
:1. Introduction
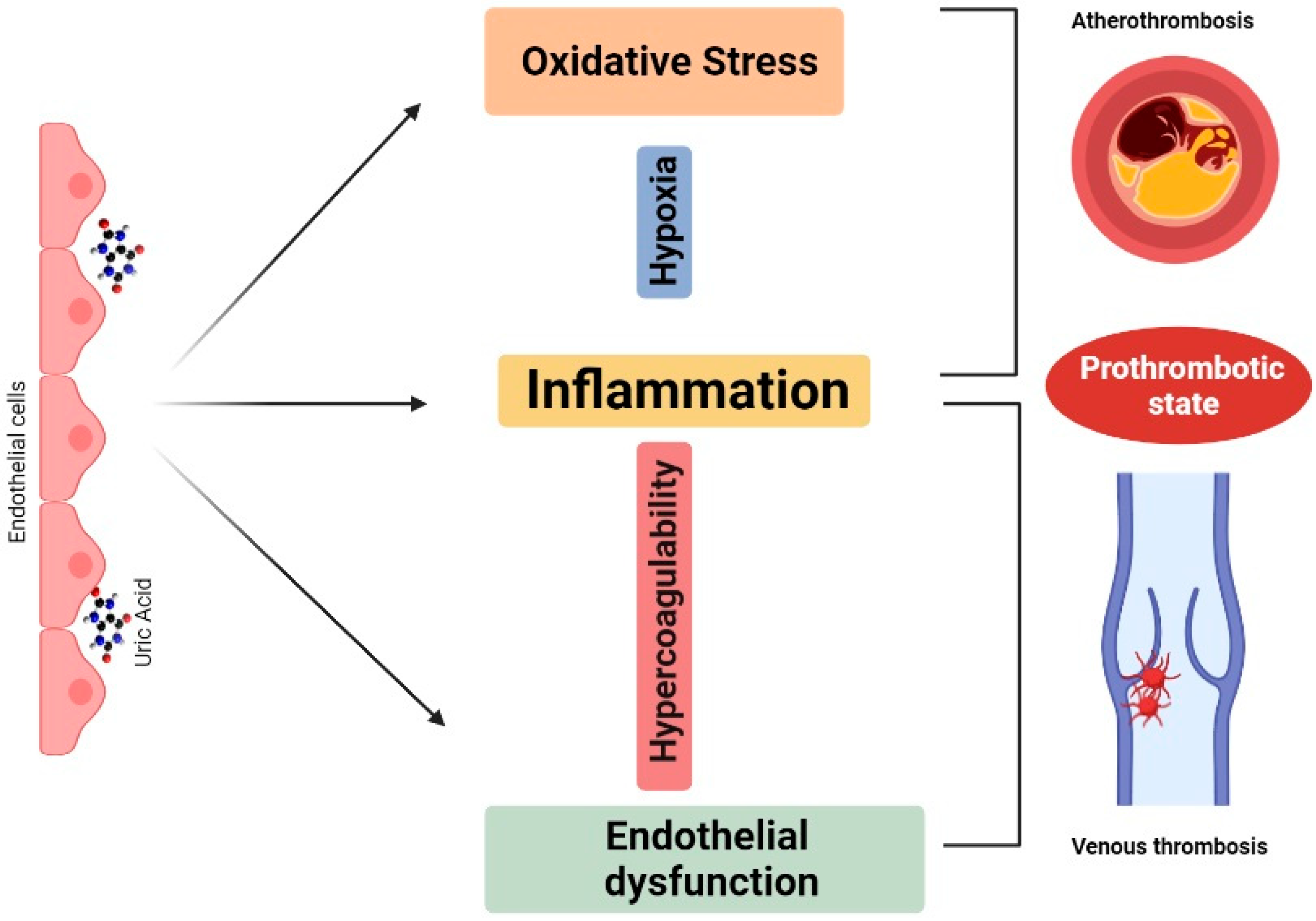
2. Mechanistic Framework
3. Uric Acid as a Risk Marker for Thrombosis in Different Clinical Scenarios
3.1. Uric Acid and VTE
3.2. Uric Acid and Left Atrial Thrombosis
3.3. Uric Acid and MI
3.4. Uric Acid and Thromboembolic Risk in Systemic Diseases
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- White, J.; Sofat, R.; Hemani, G.; Shah, T.; Engmann, J.; Dale, C.; Shah, S.; Kruger, F.A.; Giambartolomei, C.; Swerdlow, D.I.; et al. Plasma urate concentration and risk of coronary heart disease: A Mendelian randomisation analysis. Lancet Diabetes Endocrinol. 2016, 4, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Kleber, M.E.; Delgado, G.; Grammer, T.B.; Silbernagel, G.; Huang, J.; Krämer, B.K.; Ritz, E.; März, W. Uric Acid and Cardiovascular Events: A Mendelian Randomization Study. J. Am. Soc. Nephrol. JASN 2015, 26, 2831–2838. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.; Cameron, A.C.; Burgess, S.; Li, X.; Doherty, D.J.; Karhunen, V.; Abdul-Rahim, A.H.; Taylor-Rowan, M.; Zuber, V.; Tsao, P.S.; et al. Urate, Blood Pressure, and Cardiovascular Disease: Evidence from Mendelian Randomization and Meta-Analysis of Clinical Trials. Hypertension 2021, 77, 383–392. [Google Scholar] [CrossRef]
- Hong, M.; Park, J.W.; Yang, P.S.; Hwang, I.; Kim, T.H.; Yu, H.T.; Uhm, J.S.; Joung, B.; Lee, M.H.; Jee, S.H.; et al. A mendelian randomization analysis: The causal association between serum uric acid and atrial fibrillation. Eur. J. Clin. Investig. 2020, 50, e13300. [Google Scholar] [CrossRef]
- Efstathiadou, A.; Gill, D.; McGrane, F.; Quinn, T.; Dawson, J. Genetically Determined Uric Acid and the Risk of Cardiovascular and Neurovascular Diseases: A Mendelian Randomization Study of Outcomes Investigated in Randomized Trials. J. Am. Heart Assoc. 2019, 8, e012738. [Google Scholar] [CrossRef]
- Keenan, T.; Zhao, W.; Rasheed, A.; Ho, W.K.; Malik, R.; Felix, J.F.; Young, R.; Shah, N.; Samuel, M.; Sheikh, N.; et al. Causal Assessment of Serum Urate Levels in Cardiometabolic Diseases Through a Mendelian Randomization Study. J. Am. Coll. Cardiol. 2016, 67, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Zapolski, T.; Waciński, P.; Kondracki, B.; Rychta, E.; Buraczyńska, M.J.; Wysokiński, A. Uric acid as a link between renal dysfunction and both pro-inflammatory and prothrombotic state in patients with metabolic syndrome and coronary artery disease. Kardiol. Pol. 2011, 69, 319–326. [Google Scholar]
- Maruhashi, T.; Hisatome, I.; Kihara, Y.; Higashi, Y. Hyperuricemia and endothelial function: From molecular background to clinical perspectives. Atherosclerosis 2018, 278, 226–231. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.-M.; Lu, C.-L.; Hung, K.-C.; Wu, P.-C.; Pan, C.-F.; Wu, C.-J.; Syu, R.-S.; Chen, J.-S.; Hsiao, P.-J.; Lu, K.-C. The Paradoxical Role of Uric Acid in Osteoporosis. Nutrients 2019, 11, 2111. [Google Scholar] [CrossRef] [Green Version]
- Saito, Y.; Tanaka, A.; Node, K.; Kobayashi, Y. Uric acid and cardiovascular disease: A clinical review. J. Cardiol. 2020. [Google Scholar] [CrossRef]
- Yu, W.; Cheng, J.-D. Uric Acid and Cardiovascular Disease: An Update from Molecular Mechanism to Clinical Perspective. Front. Pharm. 2020, 11, 582680. [Google Scholar] [CrossRef]
- Ozsu, S.; Çoşar, A.M.; Aksoy, H.B.; Bülbül, Y.; Oztuna, F.; Karahan, S.C.; Ozlu, T. Prognostic Value of Uric Acid for Pulmonary Thromboembolism. Respir. Care 2017, 62, 1091–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasutake, Y.; Tomita, K.; Higashiyama, M.; Furuhashi, H.; Shirakabe, K.; Takajo, T.; Maruta, K.; Sato, H.; Narimatsu, K.; Yoshikawa, K.; et al. Uric acid ameliorates indomethacin-induced enteropathy in mice through its antioxidant activity. J. Gastroenterol. Hepatol. 2017, 32, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: Potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Shen, Y.; Chen, Y.; Zhang, G.; Cheng, J.; Wang, W. High Uric Acid Inhibits Cardiomyocyte Viability Through the ERK/P38 Pathway via Oxidative Stress. Cell. Physiol. Biochem. 2018, 45, 1156–1164. [Google Scholar] [CrossRef]
- Chiu, C.C.; Chen, Y.T.; Hsu, C.Y.; Chang, C.C.; Huang, C.C.; Leu, H.B.; Li, S.Y.; Kuo, S.C.; Huang, P.H.; Chen, J.W.; et al. Association between previous history of gout attack and risk of deep vein thrombosis - a nationwide population-based cohort study. Sci. Rep. 2016, 6, 26541. [Google Scholar] [CrossRef] [Green Version]
- Lyngdoh, T.; Marques-Vidal, P.; Paccaud, F.; Preisig, M.; Waeber, G.; Bochud, M.; Vollenweider, P. Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population-based Colaus study. PLoS ONE 2011, 6, e19901. [Google Scholar] [CrossRef] [Green Version]
- Dalbeth, N.; Merriman, T.R.; Stamp, L.K. Gout. Lancet 2016, 388, 2039–2052. [Google Scholar] [CrossRef]
- Kubota, Y.; McAdams-DeMarco, M.; Folsom, A.R. Serum uric acid, gout, and venous thromboembolism: The atherosclerosis risk in communities study. Thromb. Res. 2016, 144, 144–148. [Google Scholar] [CrossRef] [Green Version]
- Kanbay, M.; Yilmaz, M.I.; Sonmez, A.; Turgut, F.; Saglam, M.; Cakir, E.; Yenicesu, M.; Covic, A.; Jalal, D.; Johnson, R.J. Serum uric acid level and endothelial dysfunction in patients with nondiabetic chronic kidney disease. Am. J. Nephrol. 2011, 33, 298–304. [Google Scholar] [CrossRef] [Green Version]
- Kanbay, M.; Huddam, B.; Azak, A.; Solak, Y.; Kadioglu, G.K.; Kirbas, I.; Duranay, M.; Covic, A.; Johnson, R.J. A randomized study of allopurinol on endothelial function and estimated glomular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin. J. Am. Soc. Nephrol. 2011, 6, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.T.; Liu, F.Z.; Xue, Y.M.; Zhan, X.Z.; Fang, X.H.; Huang, J.; Wei, W.; Rao, F.; Deng, H.; Liu, Y.; et al. Predictive value of serum uric acid on left atrial spontaneous echo contrast in non-valvular atrial fibrillation patients. J. Geriatr. Cardiol. 2015, 12, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Liu, T.; Ma, L.; Liu, Z.; Xin, Y.; Jia, Z.; Chen, Y.; Li, C.; Sun, R. Prothrombotic effects of high uric acid in mice via activation of MEF2C-dependent NF-κB pathway by upregulating let-7c. Aging 2020, 12, 17976–17989. [Google Scholar] [CrossRef]
- Yisireyili, M.; Hayashi, M.; Wu, H.; Uchida, Y.; Yamamoto, K.; Kikuchi, R.; Shoaib Hamrah, M.; Nakayama, T.; Wu Cheng, X.; Matsushita, T.; et al. Xanthine oxidase inhibition by febuxostat attenuates stress-induced hyperuricemia, glucose dysmetabolism, and prothrombotic state in mice. Sci. Rep. 2017, 7, 1266. [Google Scholar] [CrossRef]
- Nishizawa, T.; Taniura, T.; Nomura, S. Effects of febuxostat on platelet-derived microparticles and adiponectin in patients with hyperuricemia. Blood Coagul. Fibrinolysis 2015, 26, 887–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbieri, L.; Verdoia, M.; Pergolini, P.; Nardin, M.; Rolla, R.; Marino, P.; Bellomo, G.; Suryapranata, H.; De Luca, G. Uric acid and high-residual platelet reactivity in patients treated with clopidogrel or ticagrelor. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 352–358. [Google Scholar] [CrossRef]
- Yu, M.; Ling, K.; Teng, Y.; Li, Q.; Mei, F.; Li, Y.; Ouyang, C. Serum uric acid is associated with increased risk of idiopathic venous thromboembolism in high HDL-C population: A case-control study. Exp. Ther. Med. 2016, 11, 2314–2320. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; McCormick, N.; Sayre, E.C.; Esdaile, J.M.; Lacaille, D.; Xie, H.; Choi, H.K.; Aviña-Zubieta, J.A. Trends of venous thromboembolism risk before and after diagnosis of gout: A general population-based study. Rheumatology 2020, 59, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.A.; Muller, S.; Whittle, R.; Roddy, E.; Mallen, C.; Clarson, L. Venous thromboembolism in patients with gout and the impact of hospital admission, disease duration and urate-lowering therapy. Can. Med. Assoc. J. 2019, 191, e597–e603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.C.; Huang, P.H.; Chen, J.H.; Lan, J.L.; Tsay, G.J.; Lin, H.Y.; Tseng, C.H.; Lin, C.L.; Hsu, C.Y. An Independent Risk of Gout on the Development of Deep Vein Thrombosis and Pulmonary Embolism: A Nationwide, Population-Based Cohort Study. Medicine 2015, 94, e2140. [Google Scholar] [CrossRef]
- De Lucchi, L.; Nardin, C.; Sponchiado, A.; Raggi, D.; Faggin, E.; Martini, E.; Pagliara, V.; Callegari, E.; Caberlotto, L.; Plebani, M.; et al. Serum uric acid levels and the risk of recurrent venous thromboembolism. J. Thromb. Haemost. 2021, 19, 194–201. [Google Scholar] [CrossRef]
- Lee, J.H.; Huh, J.W.; Hong, S.-B.; Oh, Y.-M.; Shim, T.S.; Lim, C.-M.; Lee, S.-D.; Koh, Y.; Kim, W.S.; Lee, J.S. Prognostic value of blood biomarkers in patients with unprovoked acute pulmonary embolism. Ann. Thorac. Med. 2019, 14, 248–253. [Google Scholar] [CrossRef]
- Xu, J.J.; Jiang, L.; Song, Y.; Yao, Y.; Jia, S.D.; Liu, Y.; Yuan, D.S.; Li, T.Y.; Chen, J.; Wu, Y.; et al. Related factors and the long-term outcome after percutaneous coronary intervention of premature acute myocardial infarction. Zhonghua Xin Xue Guan Bing Za Zhi 2020, 48, 655–660. [Google Scholar] [CrossRef]
- Tamariz, L.; Hernandez, F.; Bush, A.; Palacio, A.; Hare, J.M. Association between serum uric acid and atrial fibrillation: A systematic review and meta-analysis. Heart Rhythm 2014, 11, 1102–1108. [Google Scholar] [CrossRef]
- Ning, W.; Li, Y.; Ma, C.; Qiu, L.; Yu, B. The Refinement of Risk Stratification for Atrial Thrombus or Spontaneous Echo Contrast in Nonvalvular Atrial Fibrillation. Int. Heart J. 2017, 58, 885–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Numa, S.; Hirai, T.; Nakagawa, K.; Ohara, K.; Fukuda, N.; Nozawa, T.; Inoue, H. Hyperuricemia and transesophageal echocardiographic thromboembolic risk in patients with atrial fibrillation at clinically low-intermediate risk. Circ. J. 2014, 78, 1600–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, R.B.; Dong, J.Z.; Yan, X.L.; Du, X.; Kang, J.P.; Wu, J.H.; Yu, R.H.; Long, D.Y.; Ning, M.; Sang, C.H.; et al. Serum uric acid and risk of left atrial thrombus in patients with nonvalvular atrial fibrillation. Can. J. Cardiol. 2014, 30, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Celik, M.; Yalcinkaya, E.; Yuksel, U.C.; Gokoglan, Y.; Bugan, B.; Kabul, H.K.; Barcin, C. Increased serum uric acid levels are correlated with decreased left atrial appendage peak flow velocity in patients with atrial fibrillation. Med. Princ. Pract. 2015, 24, 263–268. [Google Scholar] [CrossRef] [Green Version]
- Ozturk, D.; Celık, O.; Akın, F.; Akturk, F.; Aslan, S.; Ozyılmaz, S.O.; Bırand, A.; Yıldırım, A. Usefulness of the uric acid and CHA₂DS₂-VASc score in prediction of left atrial thrombosis in patients with mitral stenosis and sinus rhythm. Cardiol. J. 2015, 22, 336–342. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Hu, M.; Wang, X.; Zhang, C.; Chen, W.; Chen, S.; Zhou, J.; Chen, Y.; Lou, L.; Chen, G.; et al. New perspective on the risk markers for left atrial thrombosis in patients with atrial fibrillation. Eur. J. Prev. Cardiol. 2020, 2047487320912084. [Google Scholar] [CrossRef]
- Pugliese, N.R.; Mengozzi, A.; Virdis, A.; Casiglia, E.; Tikhonoff, V.; Cicero, A.F.G.; Ungar, A.; Rivasi, G.; Salvetti, M.; Barbagallo, C.M.; et al. The importance of including uric acid in the definition of metabolic syndrome when assessing the mortality risk. Clin. Res. Cardiol. 2021. [Google Scholar] [CrossRef]
- Kuźma, Ł.; Kulikowska, A.; Kurasz, A.; Niwińska, M.M.; Zalewska-Adamiec, M.; Dobrzycki, S.; Bachórzewska-Gajewska, H. The effect of serum uric acid levels on the long-term prognosis of patients with non-ST-elevation myocardial infarction. Adv. Clin. Exp. Med. 2020, 29, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Centola, M.; Maloberti, A.; Castini, D.; Persampieri, S.; Sabatelli, L.; Ferrante, G.; Lucreziotti, S.; Morici, N.; Sacco, A.; Oliva, F.; et al. Impact of admission serum acid uric levels on in-hospital outcomes in patients with acute coronary syndrome. Eur. J. Intern. Med. 2020, 82, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Yang, D.; Wu, D.; Liu, H.; Chen, S.; Liu, J.; Lei, L.; Liu, Y.; Rao, L.; Zhang, L. Hyperuricemia and long-term mortality in patients with acute myocardial infarction undergoing percutaneous coronary intervention. Ann. Transl. Med. 2019, 7, 636. [Google Scholar] [CrossRef]
- Casiglia, E.; Tikhonoff, V.; Virdis, A.; Masi, S.; Barbagallo, C.M.; Bombelli, M.; Bruno, B.; Cicero, A.F.G.; Cirillo, M.; Cirillo, P.; et al. Serum uric acid and fatal myocardial infarction: Detection of prognostic cut-off values: The URRAH (Uric Acid Right for Heart Health) study. J. Hypertens. 2020, 38, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Nakayama, T.; Sugimoto, K.; Fujimoto, Y.; Kobayashi, Y. Relation of Lipid Content of Coronary Plaque to Level of Serum Uric Acid. Am. J. Cardiol. 2015, 116, 1346–1350. [Google Scholar] [CrossRef]
- Akpek, M.; Kaya, M.G.; Uyarel, H.; Yarlioglues, M.; Kalay, N.; Gunebakmaz, O.; Dogdu, O.; Ardic, I.; Elcik, D.; Sahin, O.; et al. The association of serum uric acid levels on coronary flow in patients with STEMI undergoing primary PCI. Atherosclerosis 2011, 219, 334–341. [Google Scholar] [CrossRef]
- Verdoia, M.; Schaffer, A.; Barbieri, L.; Di Giovine, G.; Marino, P.; De Luca, G. Uric acid and risk of periprocedural myocardial infarction in patients undergoing percutaneous coronary intervention. Diabetes Metab. Res. Rev. 2014, 30, 297–304. [Google Scholar] [CrossRef]
- Tian, X.; Zuo, Y.; Chen, S.; Wang, A.; Li, H.; He, Y.; Zhang, L.; An, J.; Wu, S.; Luo, Y. Associations between changes in serum uric acid and the risk of myocardial infarction. Int. J. Cardiol. 2020, 314, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Lucijanic, M.; Krecak, I.; Galusic, D.; Sedinic, M.; Holik, H.; Perisa, V.; Moric Peric, M.; Zekanovic, I.; Stoos-Veic, T.; Pejsa, V.; et al. Higher serum uric acid is associated with higher risks of thrombosis and death in patients with primary myelofibrosis. Wien. Klin. Wochenschr. 2021, 1–7. [Google Scholar] [CrossRef]
- Krečak, I.; Lucijanić, M.; Gverić-Krečak, V.; Duraković, N. Hyperuricemia might promote thrombosis in essential thrombocythemia and polycythemia vera. Leuk. Lymphoma 2020, 61, 1744–1747. [Google Scholar] [CrossRef] [PubMed]
- Atıl, A.; Deniz, A. Could be serum uric acid a risk factor for thrombosis and/or uveitis in Behcet’s disease? Vascular 2018, 26, 378–386. [Google Scholar] [CrossRef] [PubMed]
| Study, Year | Study Design | Participants | Outcome, Mean Period of Follow-Up | Results | Conclusions |
|---|---|---|---|---|---|
| Kubota Y. et al. [19], 2016 | Prospective | 14,126 participants aged 45–64, without a history of VTE or gout and not using anticoagulants/gout medications. | VTE occurrence, 22.5 years | 632 incident cases of VTE (236 unprovoked and 396 provoked). | Elevated SUA was associated with increased risk of VTE. |
| Chiu C.C. et al. [16], 2016 | Prospective, nationwide longitudinal cohort study | 35,959 patients with history of gout attack and 35,959 matched controls without gout. | DVT incidence, 7.4 years | Patients with gout were found to have a 1.38-fold (95% CI, 1.18 to 1.62, p < 0.001) higher risk of developing DVT. | The incidence rate of DVT was significantly higher in patients with gout than that in control group. |
| Li, L. et al. [28], 2020 | Prospective, 1:1 matched cohort study | 130,708 incident gout patients, and 131,349 non-gout individuals. | First VTE event (either DVT or PE), 15 years | 2071 incident VTE, 1377 DVT, and 1012 PE events occurred in the gout cohort, compared with 1629, 1032, and 854 in the non-gout cohort, respectively. | The overall risks of VTE, DVT, and PE were significantly increased both before and after gout diagnosis when compared with the general population. |
| Sultan, A.A. et al. [29], 2019 | Prospective, 1:1 matched cohort study | 62,234 patients with incident gout matched to 62,234 controls. | Incident gout cases, 19 years | Gout was associated with higher risk of venous thromboembolism compared with controls (absolute rate 37.3 vs. 27.0) | Gout was associated with higher risk of VTE, particularly when the patient was not in hospital and regardless of exposure to urate-lowering therapy. |
| Huang, C.C. et al. [30], 2015 | Prospective, 2:1 matched cohort study | 57,981 patients with gout and 115,961 controls. | Occurrence of VTE (DVT or PE), 12 years | The incidence of DVT was 5.26 per 104 person-years in the gout cohort, which was 2-fold higher than the incidence of 2.63 per 104 person-years in the reference cohort. | Gout increased the risk of DVT and PE. |
| De Lucchi, L. et al. [31], 2021 | Monocenter, prospective study | 280 patients with a previous episode of VTE that completed the oral anticoagulant period. | VTE recurrence, 71.1 ± 29.2 months | Patients with SUA levels ≥4.38 mg/dL showed a 3-fold increase in the risk of VTE recurrence. | Elevated SUA levels are associated with increased risk of recurrent VTE independently from traditional risk factors. |
| Study, Year | Study Design | Participants | Outcome | Results | Conclusions |
|---|---|---|---|---|---|
| Ning, W. et al. [35], 2017 | Retrospective | 284 non-valvular AF patients without prior oral anticoagulation. | Additional predictive value of SUA and LAD for CHADS2 and CHA2DS2-VASc. | In 61 patients (21.48%) with LAT/SEC, SUA and LAD were independent risk factors of LAT/SEC and increased the predictive value of CHADS2 and CHA2DS2-VASc. | SUA and LAD enhance the predictive ability of CHADS2 and CHA2DS2-VASc for LAT/SEC. |
| Numa, S. et al. [36], 2014 | Retrospective | 470 patients with nonvalvular AF. | Relationship between SUA levels and thromboembolic risk in patients with AF. | SUA level was associated with thromboembolic risk in patients with nonvalvular AF at low-intermediate risk stratified by CHA2DS2-VASc score. | SUA level is an independent predictor of thromboembolic risk on TEE in AF patients at low-intermediate risk. |
| Liao, H.T. et al. [22], 2015 | Retrospective | 1476 consecutive hospitalized patients with AF. | Relationship between SUA and LA-SEC in non-valvular AF patients. | SUA level is significantly higher in non-valvular AF patients with LA-SEC. | SUA level is an independent risk factor and has a moderate predictive value for LA-SEC. |
| Tang, R.B. et al. [37], 2014 | Retrospective | 1359 consecutive patients undergoing TEE. | Relationship between SUA and the risk of LA thrombus in patients with nonvalvular AF. | SUA levels in patients with LA thrombus were significantly higher. | Hyperuricemia is a risk factor for LA thrombus. |
| Celik, M. et al. [38], 2015 | Retrospective | 153 patients with AF who underwent TEE. | Relationship between SUA levels and LAA peak flow velocity. | SUA levels were significant predictors of the LAA peak flow velocity. | High SUA levels are associated with a low contractile function of the LAA. |
| Ozturk, D. et al. [39], 2015 | Retrospective | 207 consecutive patients with mitral stenosis who underwent both TTE and TEE. | Risk factors for LA trombus in patients with mitral stenosis in sinus rhythm. | Uric acid was higher in patients with LA thrombus. | A larger LAD and an elevated SUA level are independent predictors of LA thrombosis in patients with mitral stenosis in sinus rhythm. |
| Zhang, X. et al. [40], 2020 | Retrospective | 2246 patients who underwent TEE | Risk markers for LA thrombosis. | In 30 patients (1.33%) with LAT, high SUA levels and obesity were risk markers for LAT. | High SUA level is an independent risk marker for LAT. After considering SUA, the CHA2DS2-VASc score for LAT is more accurate. |
| Study, Year | Study Design | Participants | Outcome | Results | Conclusions |
|---|---|---|---|---|---|
| Kuźma, Ł. et al. [42], 2020 | Retrospective | 549 patients diagnosed with NSTEMI. | Relationship between SUA levels and the long-term prognosis of patients with NSTEMI. | There was a significant correlation between an increase in SUA levels and an increase in mortality (p < 0.001). | SUA is an independent risk factor of long-term mortality in patients with NSTEMI, and is associated with higher in-hospital death rates. |
| Centola, M. et al. [43], 2020 | Retrospective | 1088 consecutive patients with ACS. | Association between admission SUA levels and in-hospital outcomes in patients with ACS and to investigate the prognostic value of SUA added to GRACE score. | SUA (OR 1.72 95% CI 1.33–2.22, p < 0.0001) and GRACE score (OR 1.04 95% CI 1.02–1.06, p < 0.0001) were significantly associated with an increased risk of in-hospital death. | High admission levels of SUA are independently associated with in-hospital adverse outcomes and mortality in a population of ACS patients. The inclusion of SUA to GRACE risk score predicts more accurately in-hospital mortality. |
| Guo, W. et al. [44], 2019 | Prospective | 1005 AMI patients who underwent PCI. | Prognostic role of hyperuricemia in patients with AMI who underwent PCI. | Mortality for patients with hyperuricemia was higher than that of patients with normal SUA (HR: 1.97; 95% CI: 1.11–3.49; p = 0.019). | Preprocedural hyperuricemia is a significant and independent predictor of long-term mortality for patients with AMI who underwent PCI. |
| Casiglia, E. et al. [45], 2020 | Multicentre, observational cohort study | 23,467 individuals. | Prognostic cut-off values of SUA in predicting fatal MI. | There was an independent association between SUA and fatal MI in the whole database (HR: 1.381, 95% CI: 1.096–1.758, p = 0.006) and in women (HR: 1.514, CI: 1.105–2.075, p < 0.01), but not in men. | SUA is an independent risk factor for fatal MI after adjusting for potential confounding variables, and a prognostic cut-off value associated to fatal MI can be identified at least in women. |
| Xu, J.J. et al. [33], 2020 | Prospective cohort study | 1920 AMI patients. | Related factors of premature AMI (man ≤ 50 years old, woman ≤ 60 years old). | SUA level (OR = 1.02, 95% CI 1.01–1.04, p < 0.01), was an independent related factor of premature AMI. | Metabolic abnormalities, including high SUA, are risk factors of premature AMI. |
| Saito, Y. et al. [46], 2015 | Prospective | 81 patients with ACS who underwent intravascular ultrasound-guided PCI. | The relation between SUA level and plaque composition of nonculprit lesions in patients with ACS. | Greater lipid (59.1 ± 9.1% vs. 49.7 ± 10.9% vs. 51.1 ± 9.3%, p = 0.001) and less fibrous components (36.8 ± 7.8% vs. 44.3 ± 7.8% vs. 43.2 ± 6.7%, p < 0.001) were present in the high than in the low and intermediate SUA levels tertile groups. | Elevated SUA level is associated with greater lipid content of coronary plaque in patients with ACS than in patients with normal levels. |
| Akpek, M. et al. [47], 2011 | Prospective | 289 STEMI patients treated with primary PCI. | The association of uric acid levels with coronary blood flow in STEMI. | A uric acid level ≥5.4 mg/dL had a 77% sensitivity and 70% specificity in predicting no-reflow. Uric acid levels (OR 2.75, <95% CI 1.93–3.94; p < 0.0001) was an independent predictor of in-hospital MACE. | Plasma uric acid level on admission is a strong and independent predictor of poor coronary blood flow following primary PCI and in hospital MACE among patients with STEMI. |
| Verdoia, M. et al. [48], 2014 | Prospective | 1272 consecutive patients undergoing PCI. | The association between SUA levels and periprocedural MI in patients undergoing PCI. | SUA did not affect the risk of periprocedural MI (p = 0.29; adjusted OR = 1.11 [0.93–1.32], p = 0.26) or periprocedural myonecrosis (p = 0.97; adjusted OR = 0.99 [0.86–1.14], p = 0.89). | SUA is not associated with an increase in the risk of periprocedural MI in patients undergoing percutaneous coronary revascularization. |
| Tian, X. et al. [49], 2020 | Prospective | 71,449 Chinese participants. | The association between both baseline SUA and changes in SUA and the risk of MI. | In 837 MI cases identified during follow-up, MI risk was only associated with stable high SUA (HR: 1.42 95% CI: 1.02–1.92, p = 0.03), compared with those with stable low SUA. There was no association between hyperuricemia at baseline and MI (HR 1.14, 95% CI: 0.91–1.42, p = 0.19). | Only stable high SUA is associated with increased higher risk of MI. Changes in SUA levels in any other direction or high SUA levels at baseline were not associated with risk of MI. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Țăpoi, L.; Șalaru, D.L.; Sascău, R.; Stătescu, C. Uric Acid—An Emergent Risk Marker for Thrombosis? J. Clin. Med. 2021, 10, 2062. https://doi.org/10.3390/jcm10102062
Țăpoi L, Șalaru DL, Sascău R, Stătescu C. Uric Acid—An Emergent Risk Marker for Thrombosis? Journal of Clinical Medicine. 2021; 10(10):2062. https://doi.org/10.3390/jcm10102062
Chicago/Turabian StyleȚăpoi, Laura, Delia Lidia Șalaru, Radu Sascău, and Cristian Stătescu. 2021. "Uric Acid—An Emergent Risk Marker for Thrombosis?" Journal of Clinical Medicine 10, no. 10: 2062. https://doi.org/10.3390/jcm10102062
APA StyleȚăpoi, L., Șalaru, D. L., Sascău, R., & Stătescu, C. (2021). Uric Acid—An Emergent Risk Marker for Thrombosis? Journal of Clinical Medicine, 10(10), 2062. https://doi.org/10.3390/jcm10102062







