Clinical and Forensic Aspects of the Different Subtypes of Argyria
Abstract
:1. Introduction
2. Materials and Methods
3. Chemistry and History of Silver
4. Toxicokinetics of Silver
5. Argyria Subtypes
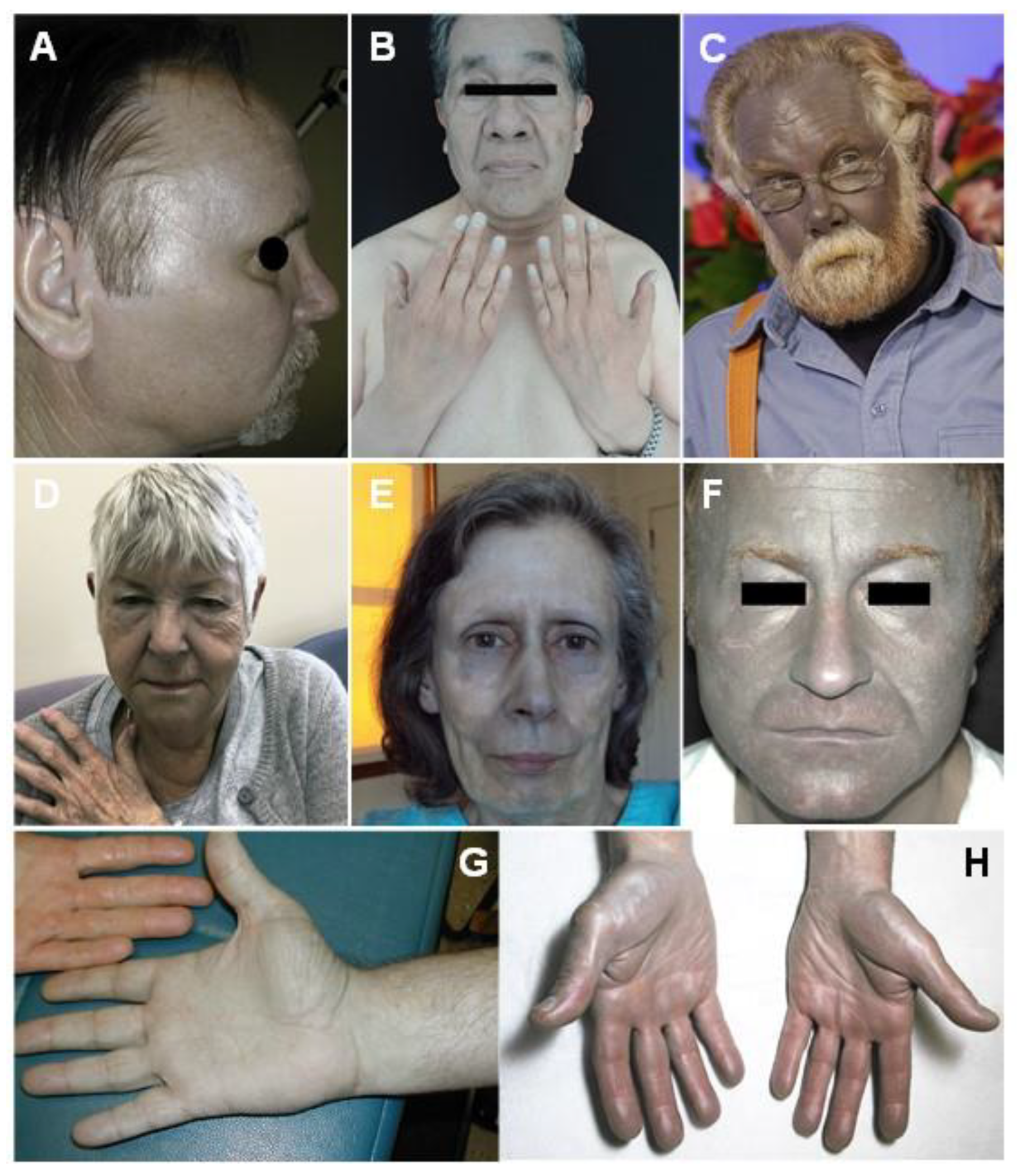
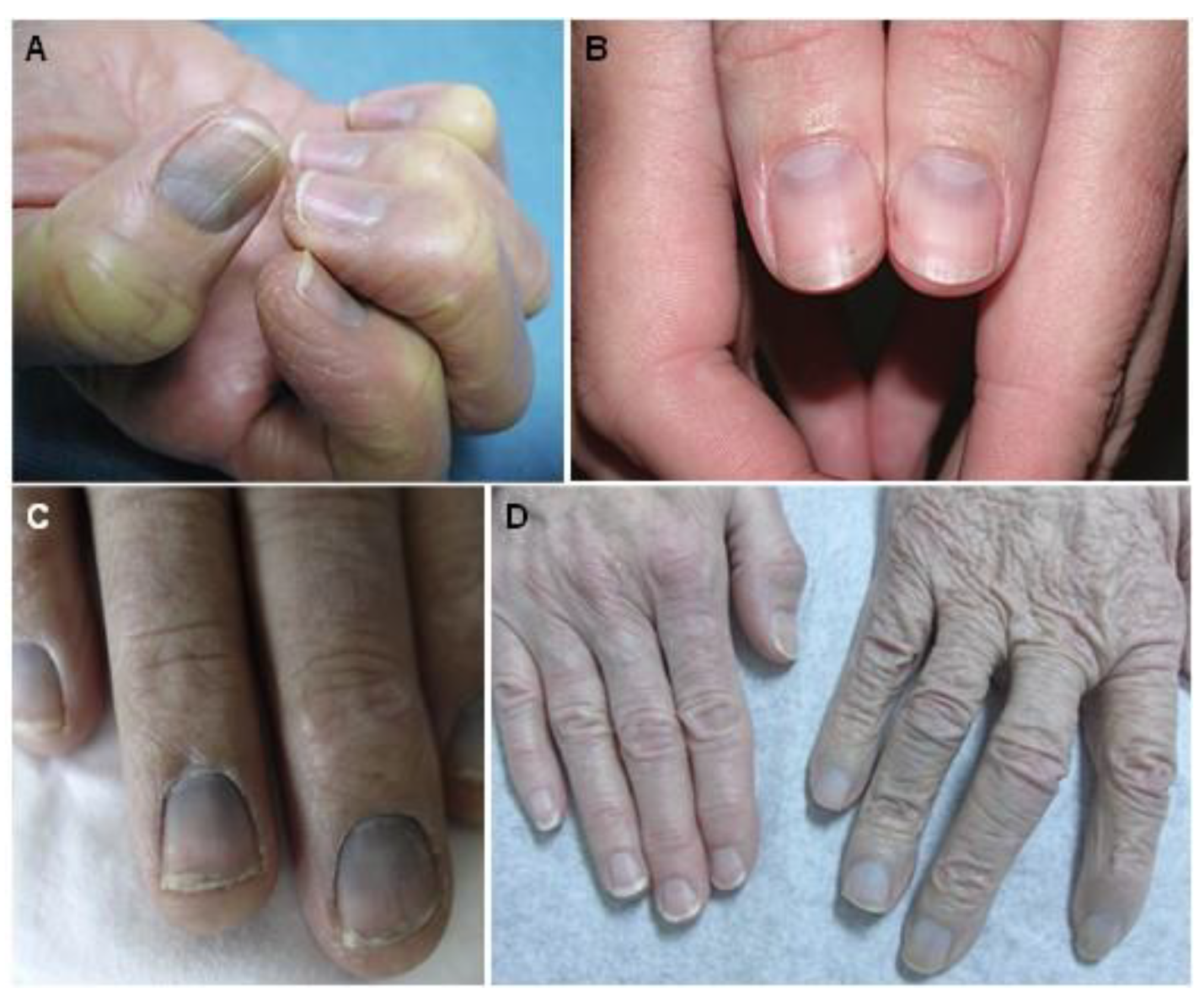
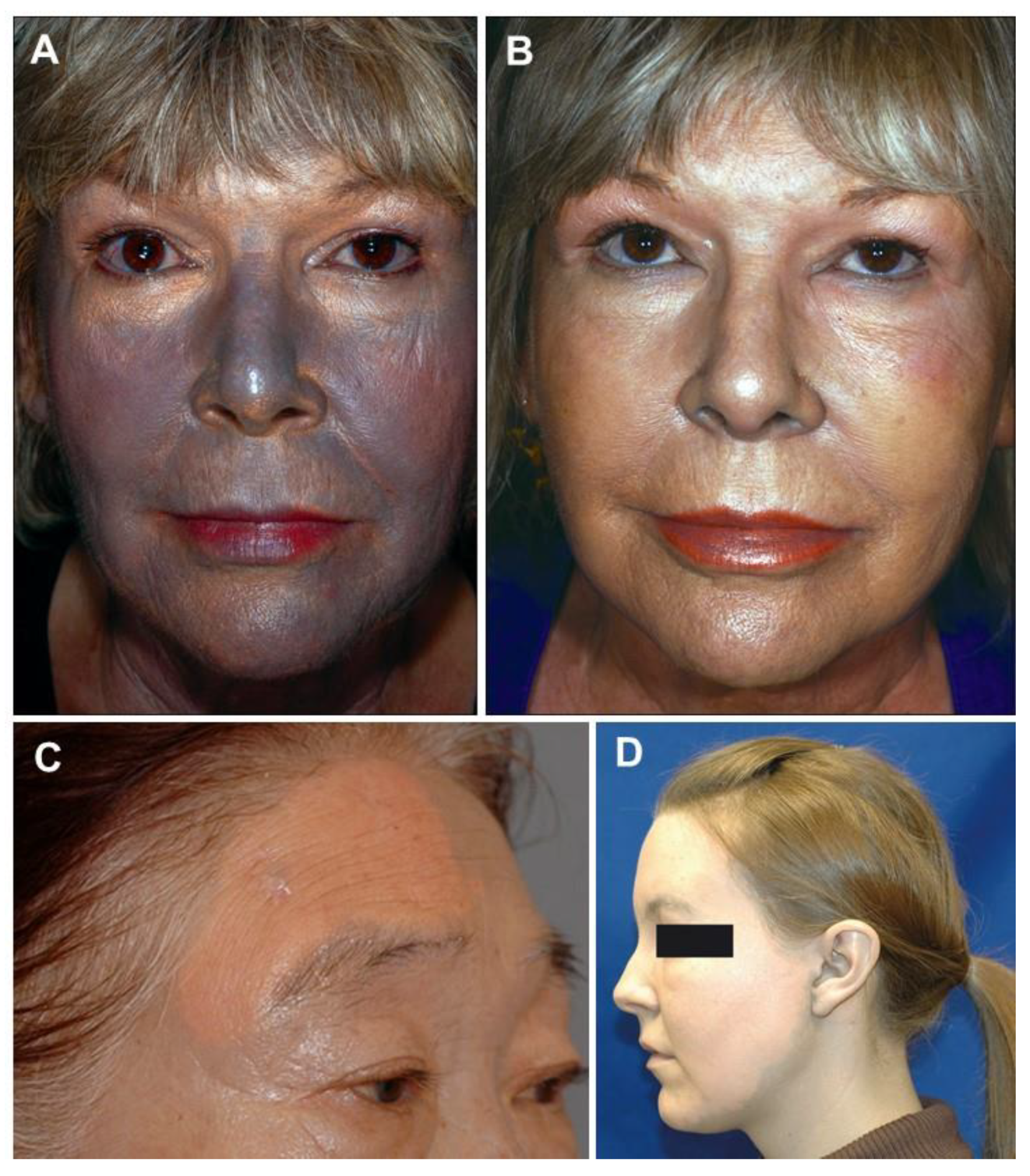
5.1. Generalized Argyria
5.2. Localized Argyria
5.3. Argyrosis
6. Pathophysiology of Argyria
7. Diagnosis of Argyria
7.1. Signs and Symptoms Related to Argyria
7.2. Medical Exams
8. Treatment
8.1. Treatment of Argyria
8.2. Treatment of Amalgam Tattoo
8.3. Treatment of Argyrosis
9. Forensic and Toxicological Aspects
10. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burgert, J.M. Argyria resulting from chronic use of colloidal silver in a patient presenting for colonoscopy. A&A Pract. 2014, 3, 73–75. [Google Scholar] [CrossRef]
- Boix-Vilanova, J.; Del Pozo, L.J.; Martinez, M.; Ramos, D.; Izquierdo, N. Dermoscopy of localised argyria: Apropos of five cases. Australas. J. Dermatol. 2020, 61, e122–e123. [Google Scholar] [CrossRef] [PubMed]
- Bingham, E.; Cohrssen, B. Patty’s Toxicology, 6th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012. [Google Scholar]
- Witkowski, J.A.; Parish, L.C. On being blue: Argyria still exists. Skinmed 2004, 3, 304–305. [Google Scholar] [CrossRef] [PubMed]
- Van de Voorde, K.; Nijsten, T.; Schelfhout, K.; Moorkens, G.; Lambert, J. Long-term use of silver containing nose-drops resulting in systemic argyria. Acta Clin. Belg. 2005, 60, 33–35. [Google Scholar] [CrossRef]
- Kim, Y.; Suh, H.S.; Cha, H.J.; Kim, S.H.; Jeong, K.S.; Kim, D.H. A case of generalized argyria after ingestion of colloidal silver solution. Am. J. Ind. Med. 2009, 52, 246–250. [Google Scholar] [CrossRef]
- Kubba, A.; Kubba, R.; Batrani, M.; Pal, T. Argyria an unrecognized cause of cutaneous pigmentation in Indian patients: A case series and review of the literature. Indian J. Dermatol. Venereol. Leprol. 2013, 79, 805–811. [Google Scholar] [CrossRef]
- Sato, S.; Sueki, H.; Nishijima, A. Two unusual cases of argyria: The application of an improved tissue processing method for X-ray microanalysis of selenium and sulphur in silver-laden granules. Br. J. Dermatol. 1999, 140, 158–163. [Google Scholar] [CrossRef]
- White, J.M.; Powell, A.M.; Brady, K.; Russell-Jones, R. Severe generalized argyria secondary to ingestion of colloidal silver protein. Clin. Exp. Dermatol. 2003, 28, 254–256. [Google Scholar] [CrossRef]
- Lai-Becker, M.W.; Burns, M.M. Silver. In Goldfrank’s Toxicologic Emergencies, 11th ed.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- National Center for Biotechnology Information. PubChem Element Summary for Atomic Number 47, Silver; NCBI: Bethesda, MD, USA, 2004. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 23954, Silver; NCBI: Bethesda, MD, USA, 2004. [Google Scholar]
- Drake, P.L.; Hazelwood, K.J. Exposure-related health effects of silver and silver compounds: A review. Ann. Occup. Hyg. 2005, 49, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Kwon, M.; Ji, J.H.; Kang, C.S.; Ahn, K.H.; Han, J.H.; Yu, I.J. Exposure assessment of workplaces manufacturing nanosized TiO2 and silver. Inhal. Toxicol. 2011, 23, 226–236. [Google Scholar] [CrossRef]
- Hadrup, N.; Lam, H.R. Oral toxicity of silver ions, silver nanoparticles and colloidal silver—A review. Regul. Toxicol. Pharmacol. 2014, 68, 1–7. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Silver as a Drinking-Water Disinfectant; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Klaassen, C.D. Casarett and Doull’s Toxicology: The Basic Science of Poisons, 9th ed.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Wang, Z.; Xia, T.; Liu, S. Mechanisms of nanosilver-induced toxicological effects: More attention should be paid to its sublethal effects. Nanoscale 2015, 7, 7470–7481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, M.C.; Bowen, D.L. Silver products for medical indications: Risk-benefit assessment. J. Toxicol. Clin. Toxicol. 1996, 34, 119–126. [Google Scholar] [CrossRef]
- Wadhera, A.; Fung, M. Systemic argyria associated with ingestion of colloidal silver. Dermatol. Online J. 2005, 11, 12. [Google Scholar]
- Lansdown, A.B. A pharmacological and toxicological profile of silver as an antimicrobial agent in medical devices. Adv. Pharmacol. Sci. 2010, 2010, 910686. [Google Scholar] [CrossRef] [Green Version]
- Mayr, M.; Kim, M.J.; Wanner, D.; Helmut, H.; Schroeder, J.; Mihatsch, M.J. Argyria and decreased kidney function: Are silver compounds toxic to the kidney? Am. J. Kidney Dis. 2009, 53, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Palamar, M.; Midilli, R.; Egrilmez, S.; Akalin, T.; Yagci, A. Black tears (melanodacryorrhea) from argyrosis. Arch. Ophthalmol. 2010, 128, 503–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lansdown, A.B. Silver in health care: Antimicrobial effects and safety in use. Curr. Probl. Dermatol. 2006, 33, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Hanna, C.; Fraunfelder, F.T.; Sanchez, J. Ultrastructural study of argyrosis of the cornea and conjunctiva. Arch. Ophthalmol. 1974, 92, 18–22. [Google Scholar] [CrossRef]
- Bleehen, S.S.; Gould, D.J.; Harrington, C.I.; Durrant, T.E.; Slater, D.N.; Underwood, J.C. Occupational argyria; light and electron microscopic studies and X-ray microanalysis. Br. J. Dermatol. 1981, 104, 19–26. [Google Scholar] [CrossRef]
- Health and Safety Executive. EH40/2005 Workplace Exposure Limits; HSE: London, UK, 2020. [Google Scholar]
- Williams, N. Longitudinal medical surveillance showing lack of progression of argyrosis in a silver refiner. Occup. Med. (Lond.) 1999, 49, 397–399. [Google Scholar] [CrossRef] [Green Version]
- Jonas, L.; Bloch, C.; Zimmermann, R.; Stadie, V.; Gross, G.E.; Schäd, S.G. Detection of silver sulfide deposits in the skin of patients with argyria after long-term use of silver-containing drugs. Ultrastruct. Pathol. 2007, 31, 379–384. [Google Scholar] [CrossRef]
- Lansdown, A.B. Critical observations on the neurotoxicity of silver. Crit. Rev. Toxicol. 2007, 37, 237–250. [Google Scholar] [CrossRef]
- Goebel, H.H.; Muller, J. Ultrastructural observations on silver deposition in the choroid plexus of a patient with argyria. Acta Neuropathol. 1973, 26, 247–251. [Google Scholar] [CrossRef]
- Mirsattari, S.M.; Hammond, R.R.; Sharpe, M.D.; Leung, F.Y.; Young, G.B. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology 2004, 62, 1408–1410. [Google Scholar] [CrossRef] [PubMed]
- Landas, S.; Fischer, J.; Wilkin, L.D.; Mitchell, L.D.; Johnson, A.K.; Turner, J.W.; Theriac, M.; Moore, K.C. Demonstration of regional blood-brain barrier permeability in human brain. Neurosci. Lett. 1985, 57, 251–256. [Google Scholar] [CrossRef]
- Bracey, N.A.; Zipursky, J.S.; Juurlink, D.N. Argyria caused by chronic ingestion of silver. Can. Med. Assoc. J. 2018, 190, E139. [Google Scholar] [CrossRef] [Green Version]
- DiVincenzo, G.D.; Giordano, C.J.; Schriever, L.S. Biologic monitoring of workers exposed to silver. Int. Arch. Occup. Environ. Health 1985, 56, 207–215. [Google Scholar] [CrossRef]
- Sue, Y.M.; Lee, J.Y.; Wang, M.C.; Lin, T.K.; Sung, J.M.; Huang, J.J. Generalized argyria in two chronic hemodialysis patients. Am. J. Kidney Dis. 2001, 37, 1048–1051. [Google Scholar] [CrossRef]
- Dijkstra, M.; Havinga, R.; Vonk, R.J.; Kuipers, F. Bile secretion of cadmium, silver, zinc and copper in the rat. Involvement of various transport systems. Life Sci. 1996, 59, 1237–1246. [Google Scholar] [CrossRef]
- Kwon, H.B.; Lee, J.H.; Lee, S.H.; Lee, A.Y.; Choi, J.S.; Ahn, Y.S. A case of argyria following colloidal silver ingestion. Ann. Dermatol. 2009, 21, 308–310. [Google Scholar] [CrossRef] [Green Version]
- Guenova, E.; Schaller, M. Residents’ corner September 2012. CarpeDIEM—Dermatological indications for electron microscopy: Argyria. Eur. J. Dermatol. 2012, 22, 718. [Google Scholar] [CrossRef] [PubMed]
- Gettler, A.O.; Rhoads, C.P.; Weiss, S. A Contribution to the pathology of generalized argyria with a discussion of the fate of silver in the human body. Am. J. Pathol. 1927, 3, 631–652. [Google Scholar]
- Rauber, G.; Duprez, A.; Bibas, H. Argyria with hepatic localization. Apropos of a case. Med. Chir. Dig. 1981, 10, 319–320. [Google Scholar]
- Dietl, H.W.; Anzil, A.P.; Mehraein, P. Brain involvement in generalized argyria. Clin. Neuropathol. 1984, 3, 32–36. [Google Scholar]
- Prescott, R.J.; Wells, S. Systemic argyria. J. Clin. Pathol. 1994, 47, 556–557. [Google Scholar] [CrossRef]
- Brandt, D.; Park, B.; Hoang, M.; Jacobe, H.T. Argyria secondary to ingestion of homemade silver solution. J. Am. Acad. Dermatol. 2005, 53, S105–S107. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.L.; Khosravi, V.; Egbert, B. A case of argyria after colloidal silver ingestion. J. Cutan. Pathol. 2006, 33, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Pezzarossa, E.; Alinovi, A.; Ferrari, C. Generalized argyria. J. Cutan. Pathol. 1983, 10, 361–363. [Google Scholar] [CrossRef]
- Tomi, N.S.; Kränke, B.; Aberer, W. A silver man. Lancet 2004, 363, 532. [Google Scholar] [CrossRef]
- Bianchi, L.; Orlandi, A.; Di Stefani, A.; Ricci, R.; Chimenti, S. “Familial” generalized argyria. Arch. Dermatol. 2006, 142, 789–790. [Google Scholar] [CrossRef]
- Reddy, S.G.; Martin, J.M.t.; Kraus, E.W.; Meffert, J.J. Generalized blue-gray pigmentation—Quiz case. Arch. Dermatol. 2009, 145, 1053–1058. [Google Scholar] [CrossRef]
- Park, S.W.; Shin, H.T.; Lee, K.T.; Lee, D.Y. Medical concern for colloidal silver supplementation: Argyria of the nail and face. Ann. Dermatol. 2013, 25, 111–112. [Google Scholar] [CrossRef] [Green Version]
- Butzmann, C.M.; Technau-Hafsi, K.; Bross, F. “Silver man” argyria of the skin after ingestion of a colloidal silver solution. J. Dtsch. Dermatol. Ges. 2015, 13, 1030–1032. [Google Scholar] [CrossRef]
- Gülseren, D.; Arzberger, E.; Cerroni, L.; Hofmann-Wellenhof, R.; Richtig, E. Reflectance confocal microscopy and dermatopathologic findings of cutaneous argyria after colloidal silver ingestion. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e178–e179. [Google Scholar] [CrossRef] [PubMed]
- Wickless, S.C.; Shwayder, T.A. Medical mystery—The answer. N. Engl. J. Med. 2004, 351, 2349–2350. [Google Scholar] [CrossRef]
- Osińska, J.; Poborc-Godlewska, J.; Kieć-Swierczyńska, M.; Głuszcz, M. 6 cases of argyria among workers engaged in silverplating radio subunits. Med. Pr. 1982, 33, 361–364. [Google Scholar]
- Pariser, R.J. Generalized argyria. Clinicopathologic features and histochemical studies. Arch. Dermatol. 1978, 114, 373–377. [Google Scholar] [CrossRef]
- Plack, W.; Bellizzi, R. Generalized argyria secondary to chewing photographic film. Report of a case. Oral Surg. Oral Med. Oral Pathol. 1980, 49, 504–506. [Google Scholar] [CrossRef]
- Hanada, K.; Hashimoto, I.; Kon, A.; Kida, K.; Mita, R. Silver in sugar particles and systemic argyria. Lancet 1998, 351, 960. [Google Scholar] [CrossRef]
- Molina-Hernandez, A.I.; Diaz-Gonzalez, J.M.; Saeb-Lima, M.; Dominguez-Cherit, J. Argyria after Silver Nitrate Intake: Case Report and Brief Review of Literature. Indian J. Dermatol. 2015, 60, 520. [Google Scholar] [CrossRef]
- Jacobs, R. Argyria: My life story. Clin. Dermatol. 2006, 24, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.W.; Leidy, K.L.; Smith, K.M.; Okeke, U.S. Argyria associated with use of systemic colloidal silver. Fed. Pract. 2011, 28, 39–42. [Google Scholar]
- Plewig, G.; Lincke, H.; Wolff, H.H. Silver-blue nails. Acta Derm. Venereol. 1977, 57, 413–419. [Google Scholar]
- McKenna, J.K.; Hull, C.M.; Zone, J.J. Argyria associated with colloidal silver supplementation. Int. J. Dermatol. 2003, 42, 549. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, S.P.; Goldberg, G.N. Immediate successful treatment of argyria with a single pass of multiple Q-switched laser wavelengths. JAMA Dermatol. 2013, 149, 623–624. [Google Scholar] [CrossRef]
- Lencastre, A.; Lobo, M.; João, A. Argyria—Case report. An. Bras. Dermatol. 2013, 88, 413–416. [Google Scholar] [CrossRef] [Green Version]
- Cinotti, E.; Labeille, B.; Douchet, C.; Cambazard, F.; Perrot, J.L. Dermoscopy, reflectance confocal microscopy, and high-definition optical coherence tomography in the diagnosis of generalized argyria. J. Am. Acad. Dermatol. 2017, 76, S66–S68. [Google Scholar] [CrossRef]
- Rodriguez, V.; Romaguera, R.L.; Heidecker, B. Silver-Containing Wound Cream Leading to Argyria-Always Ask About Alternative Health Products. Am. J. Med. 2017, 130, e145–e146. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.D.; Baker, J.A.; Tosti, A. Chromonychia in an Asymptomatic Vitamin Consumer. Ski. Appendage Disord. 2016, 1, 131–133. [Google Scholar] [CrossRef] [Green Version]
- Kalouche, H.; Watson, A.; Routley, D. Blue lunulae: Argyria and hypercopprecaemia. Australas. J. Dermatol. 2007, 48, 182–184. [Google Scholar] [CrossRef]
- Merchant, F.; Carpenter, T. Blue-gray discoloration of the skin. Am. Fam. Physician 2011, 84, 821–822. [Google Scholar]
- Ellison, D.W.; Chant, A.D.; Harrison, T.A.; Theaker, J.M. Localized argyria: A complication of the repair of inguinal hernia with silver filigree. Br. J. Surg. 1993, 80, 1325. [Google Scholar] [CrossRef] [PubMed]
- Enei, M.L.; Paschoal, F.M.; Valdés, R. Argyria mimicking a blue nevis: Dermoscopy features. An. Bras. Dermatol. 2013, 88, 452–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beutler, B.D.; Lee, R.A.; Cohen, P.R. Localized cutaneous argyria: Report of two patients and literature review. Dermatol. Online J. 2016, 22, 11. [Google Scholar]
- Nagano, T.; Oka, M.; Horikawa, T.; Nishigori, C.; Kotera, M. Single, blue nevus-like localized argyria. J. Dermatol. 2016, 43, 1359–1360. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, D.P.; Buckley, S.; Mishra, V. Localized cutaneous argyria from a nasal piercing successfully treated with a Picosecond 755-nm Q-Switched Alexandrite Laser. Dermatol. Surg. 2017, 43, 1094–1095. [Google Scholar] [CrossRef] [PubMed]
- Isak, V.; Beerli, T.; Cozzio, A.; Flatz, L. A Rare Case of Localized Argyria on the Face. Case Rep. Dermatol. 2019, 11, 23–27. [Google Scholar] [CrossRef]
- Robinson-Bostom, L.; Pomerantz, D.; Wilkel, C.; Mader, R.; Lerner, L.; Dufresne, R.; Flotte, T. Localized argyria with pseudo-ochronosis. J. Am. Acad. Dermatol. 2002, 46, 222–227. [Google Scholar] [CrossRef]
- Buckley, W.R. Localized argyria. Arch. Dermatol. 1963, 88, 531–539. [Google Scholar] [CrossRef]
- Buckley, W.R.; Oster, C.F.; Fassett, D.W. Localized argyria, II. Chemical nature of the silver containing particles. Arch. Dermatol. 1965, 92, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Granstein, R.D.; Sober, A.J. Drug and heavy metal-induced hyperpigmentation. J. Am. Acad. Dermatol. 1981, 5, 1–18. [Google Scholar] [CrossRef]
- Rongioletti, F.; Robert, E.; Buffa, P.; Bertagno, R.; Rebora, A. Blue nevi-like dotted occupational argyria. J. Am. Acad. Dermatol. 1992, 27, 1015–1016. [Google Scholar] [CrossRef]
- Kapur, N.; Landon, G.; Yu, R.C. Localized argyria in an antique restorer. Br. J. Dermatol. 2001, 144, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Sendagorta, E.; Herranz, P.; Casado, B.; Gómez, C.; Ramírez, P.; Feito, M.; García-Cabezas, M.A. Scattered blue maculae in a patient with albinism. Clin. Exp. Dermatol. 2011, 36, 419–420. [Google Scholar] [CrossRef]
- Kwon, I.H.; Ahn, H.H.; Ryu, H.J.; Rhyu, I.J. Sudden appearance of black macules on palmar aspect of two university chemistry students. Int. J. Dermatol. 2016, 55, e167–e169. [Google Scholar] [CrossRef]
- Fisher, N.M.; Marsh, E.; Lazova, R. Scar-localized argyria secondary to silver sulfadiazine cream. J. Am. Acad. Dermatol. 2003, 49, 730–732. [Google Scholar] [CrossRef]
- Schwieger-Briel, A.; Kiritsi, D.; Schumann, H.; Meiss, F.; Technau, K.; Bruckner-Tuderman, L. Grey spots in a patient with dystrophic epidermolysis bullosa. Br. J. Dermatol. 2010, 163, 1124–1126. [Google Scholar] [CrossRef]
- Rumayor Piña, A.; Martínez Martínez, M.; Toral Rizo, V.H.; Ajudarte Lopes, M.; Paes de Almeida, O. Cutaneous amalgam tattoo in a dental professional: An unreported occupational argyria. Br. J. Dermatol. 2012, 167, 1184–1185. [Google Scholar] [CrossRef]
- Garcias-Ladaria, J.; Hernandez-Bel, P.; Torregrosa-Calatayud, J.L.; Martínez-Aparicio, A. Localized cutaneous argyria: A report of 2 cases. Actas Dermo. Sifiliogr. 2013, 104, 253–254. [Google Scholar] [CrossRef]
- Al-Niaimi, F. Localized argyria from silver nasal piercing unresponsive to Q-switched laser successfully treated with a 1064 picoseconds laser. J. Cosmet. Dermatol. 2020, 19, 1535–1536. [Google Scholar] [CrossRef]
- Park, M.Y.; Lee, J.S.; Jin, H.J.; You, H.S.; Kim, G.W.; Ko, H.C.; Kim, B.S.; Kim, M.B.; Kim, H.S. Localized argyria: Troublesome side-effect of acupuncture. J. Eur. Acad. Dermatol. Venereol. JEADV 2018, 32, e62–e65. [Google Scholar] [CrossRef]
- Karakasli, A.; Hapa, O.; Akdeniz, O.; Havitcioğlu, H. Dermal argyria: Cutaneous manifestation of a megaprosthesis for distal femoral osteosarcoma. Indian J. Orthop. 2014, 48, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Dummett, C.O. Systemic significance of oral pigmentation and discoloration. Postgrad. Med. 1971, 49, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Buchner, A. Amalgam tattoo (amalgam pigmentation) of the oral mucosa: Clinical manifestations, diagnosis and treatment. Refuat Hapeh Vehashinayim (1993) 2004, 21, 25–28. [Google Scholar]
- Wu, Q.; Yang, Z.; Su, L. Light microscope and energy dispersive X-ray analysis of amalgam pigmentation. Zhonghua Kou Qiang Yi Xue Za Zhi 1995, 30, 140–142. [Google Scholar] [PubMed]
- Bell, C.D.; Cooksey, D.E.; Nickel, W.R. Amalgam tattoo (localized argyria). Arch. Derm. 1952, 66, 523–525. [Google Scholar] [CrossRef]
- Dubach, P.; Caversaccio, M. Images in clinical medicine. Amalgam tattoo. N. Engl. J. Med. 2011, 364, e29. [Google Scholar] [CrossRef] [Green Version]
- Kirchoff, D.A. Localized argyria after a surgical endodontic procedure. Report of a case. Oral Surg. Oral Med. Oral Pathol. 1971, 32, 613–617. [Google Scholar] [CrossRef]
- Kehoe, J.C. Intracanal corrosion of a silver cone producing a localized argyria: Scanning electron microscope and energy dispersive X-ray analyzer analyses. J. Endod. 1984, 10, 199–201. [Google Scholar] [CrossRef]
- Matsumura, T.; Kumakiri, M.; Ohkawara, A.; Himeno, H.; Numata, T.; Adachi, R. Detection of selenium in generalized and localized argyria: Report of four cases with X-ray microanalysis. J. Dermatol. 1992, 19, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Weathers, D.R.; Fine, R.M. Amalgam tattoo of oral mucosa. Arch. Dermatol. 1974, 110, 727–728. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, H.; Katagiri, M. Long-term effects of Ag-containing alloys on mucous tissue present in biopsy samples. Dent. Mater. J. 2004, 23, 340–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrara, G.; Filosa, A.; Mariani, M.P.; Fasanella, L. Occupational Argyria of the Nasal Mucosa. Head Neck Pathol. 2018, 12, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Güemes-Villahoz, N.; Burgos-Blasco, B.; CasoViesca, A.; Benitez-Del-Castillo, J.M. Non invasive methods to diagnose ocular argyrosis. J. Fr. Ophtalmol. 2020, 43, e185–e187. [Google Scholar] [CrossRef] [PubMed]
- Secretan, J.P.; Demontmollin, D. Several cases of industrial argyrosis of the rhino-bronchial mucous membranes. Pract. Otorhinolaryngol. (Basel) 1965, 27, 167–171. [Google Scholar]
- Liakhovitskiĭ, N.S. Argyria of the urethra. Urol. Nefrol. (Mosk.) 1968, 33, 59–61. [Google Scholar]
- Gordon, D.H.; Singla, S.K.; Goode, R.; Pollack, H.M.; Glanz, S. Argyrosis of the urinary tract. Am. J. Roentgenol. 1981, 136, 423–426. [Google Scholar] [CrossRef]
- Matzinger, M.A.; Gray, R.R.; Leekam, R.N.; Grosman, H.; St Louis, E.L. Argyrosis of the urinary tract. J. Clin. Ultrasound 1985, 13, 288–290. [Google Scholar] [CrossRef]
- Kojima, Y.; Uchida, K.; Takiuchi, H.; Wakatsuki, A.; Sakurai, T.; Fujita, Y.; Shirai, D.; Kobayashi, Y. Argyrosis of the urinary tract after silver nitrate instillation: Report of a case. Hinyokika Kiyo 1993, 39, 41–44. [Google Scholar]
- Thomas, K.; Sproston, A.R.; Kingsland, C.R. A case of vaginal argyrosis: All that glistens isn’t gold. BJOG Int. J. Obstet. Gynaecol. 2001, 108, 890–891. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.R.; Milne, J.T.; Porter, W.M. Penile argyria. Br. J. Dermatol. 2006, 155, 1074–1075. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.J.; Cockerell, C.J.; Chisholm, C.; Vandergriff, T.; Jessup, C.; Motaparthi, K.; Elston, D.M. Amalgam Tattoo. In Diagnostic Pathology: Nonneoplastic Dermatopathology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Madi, H.A.; Steel, D.H.W.; Kotagiri, A.K. Multimodal imaging in a patient with ocular argyrosis complicated by diabetic retinopathy. Can. J. Ophthalmol. 2018, 53, e262–e266. [Google Scholar] [CrossRef] [Green Version]
- Jensen, S.F. Argyrosis of conjunctiva in studio photographer. Acta Ophthalmol. (Copenh.) 1962, 40, 544–547. [Google Scholar] [CrossRef]
- Hodson, T.J.; Gillies, W.E. Argyrol, argyrosis and the acquisition of art. Aust. N. Z. J. Ophthalmol. 1985, 13, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Couto, A., Jr.; Molles, S.; Simionato, R.; Kamlot, D. Eye’s and excretory lacrimal apparatus’ argyrosis: Case report. Arq. Bras. Oftalmol. 2009, 72, 836–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiler, H.H.; Lemp, M.A.; Zeavin, B.H.; Suarez, A.F. Argyria of the cornea due to self-administration of eyelash dye. Ann. Ophthalmol. 1982, 14, 822–823. [Google Scholar] [PubMed]
- Karcioglu, Z.A.; Caldwell, D.R. Corneal argyrosis: Histologic, ultrastructural and microanalytic study. Can. J. Ophthalmol. 1985, 20, 257–260. [Google Scholar] [PubMed]
- Pala, G.; Fronterré, A.; Scafa, F.; Scelsi, M.; Ceccuzzi, R.; Gentile, E.; Candura, S.M. Ocular argyrosis in a silver craftsman. J. Occup. Health 2008, 50, 521–524. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Pulgarín, M.; Matilla, M.; Martinez-de-la-Casa, J.M.; Jerez, M.; Benítez-del-Castillo, J.M. Confocal microscopy in ocular argyrosis. Cornea 2010, 29, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Tendler, I.; Pulitzer, M.P.; Roggli, V.; Abramson, D.H.; Marr, B.P. Ocular argyrosis mimicking conjunctival melanoma. Cornea 2017, 36, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Gutman, F.A.; Crosswell, H.H., Jr. Argyrosis of the cornea without clinical conjunctival involvement. Am. J. Ophthalmol. 1968, 65, 183–187. [Google Scholar] [CrossRef]
- Wu, M.; Wang, X.; Shao, T.; Wang, Y. Case Report: In vivo confocal microscopic appearance of corneal argyrosis. Optom. Vis. Sci. 2017, 94, 1066–1069. [Google Scholar] [CrossRef] [PubMed]
- Scroggs, M.W.; Lewis, J.S.; Proia, A.D. Corneal argyrosis associated with silver soldering. Cornea 1992, 11, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Texas Tech University Health Sciences Center. List Eye Atlas Contributors. SOM Ophthalmology Eye Atlas: Conjunctiva. 2021. Available online: https://www.ttuhsc.edu/medicine/ophthalmology/eye-atlas/conjunctiva.aspx (accessed on 12 April 2021).
- Leite, C.M.; Botelho, A.S.; Oliveira, J.R.; Cardoso, S.V.; Loyola, A.M.; Gomez, R.S.; Vaz, R.R. Immunolocalization of HLA-DR and metallothionein on amalgam tattoos. Braz. Dent. J. 2004, 15, 99–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amber, K.T.; Winslow, C.Y.; Styperek, A.; Schwartz, P.H.; Shiman, M.I.; Elgart, G. Blue skin. Int. J. Dermatol. 2014, 53, 275–276. [Google Scholar] [CrossRef] [PubMed]
- East, B.W.; Boddy, K.; Williams, E.D.; Macintyre, D.; McLay, A.L. Silver retention, total body silver and tissue silver concentrations in argyria associated with exposure to an anti-smoking remedy containing silver acetate. Clin. Exp. Dermatol. 1980, 5, 305–311. [Google Scholar] [CrossRef]
- Aaseth, J.; Olsen, A.; Halse, J.; Hovig, T. Argyria-tissue deposition of silver as selenide. Scand. J. Clin. Lab. Investig. 1981, 41, 247–251. [Google Scholar] [CrossRef]
- Westhofen, M.; Schäfer, H. Generalized argyrosis in man: Neurotological, ultrastructural and X-ray microanalytical findings. Arch. Otorhinolaryngol. 1986, 243, 260–264. [Google Scholar] [CrossRef]
- Peterson, W.C., Jr. Argyria. Minn. Med. 1968, 51, 533–534. [Google Scholar]
- Shelley, W.B.; Shelley, E.D.; Burmeister, V. Argyria: The intradermal “photograph”, a manifestation of passive photosensitivity. J. Am. Acad. Dermatol. 1987, 16, 211–217. [Google Scholar] [CrossRef]
- Marshall, J.P., 2nd; Schneider, R.P. Systemic argyria secondary to topical silver nitrate. Arch. Dermatol. 1977, 113, 1077–1079. [Google Scholar] [CrossRef] [PubMed]
- Massi, D.; Santucci, M. Human generalized argyria: A submicroscopic and X-ray spectroscopic study. Ultrastruct. Pathol. 1998, 22, 47–53. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, P.; López Aventín, D.; Segura, S.; Gómez-Martín, I.; Lloreta, J.; Ibáñez, J.; Elvira, J.J.; Pujol, R.M. In vivo reflectance confocal microscopy characterization of silver deposits in localized cutaneous argyria. Br. J. Dermatol. 2016, 175, 1052–1055. [Google Scholar] [CrossRef]
- Rich, L.L.; Epinette, W.W.; Nasser, W.K. Argyria presenting as cyanotic heart disease. Am. J. Cardiol. 1972, 30, 290–292. [Google Scholar] [CrossRef]
- Steininger, H.; Langer, E.; Stömmer, P. Generalized argyrosis. Dtsch. Med. Wochenschr. 1990, 115, 657–662. [Google Scholar] [CrossRef]
- Reymond, J.L.; Stoebner, P.; Amblard, P. Cutaneous argyria: An electron microscopic study of four eases with microanalysis X study of one case (author’s transl). Ann. Dermatol. Venereol. 1980, 107, 251–255. [Google Scholar]
- Shall, L.; Stevens, A.; Millard, L.G. An unusual case of acquired localized argyria. Br. J. Dermatol. 1990, 123, 403–407. [Google Scholar] [CrossRef]
- Wootton, C.I.; May, T.; Khan, M.; Walker, S.L. A pigmented lesion on the earlobe. Clin. Exp. Dermatol. 2015, 40, 457–459. [Google Scholar] [CrossRef]
- Arunkajohnsak, S.; Thanomkitti, K.; Kasemsarn, P.; Pattanaprichakul, P.; Jiamton, S.; Eimpunth, S. Successful treatment of acupuncture-induced argyria using Q-switched 1064-nm Nd:YAG laser. JAAD Case Rep. 2020, 6, 984–987. [Google Scholar] [CrossRef]
- Tanita, Y.; Kato, T.; Hanada, K.; Tagami, H. Blue macules of localized argyria caused by implanted acupuncture needles. Electron microscopy and roentgenographic microanalysis of deposited metal. Arch. Dermatol. 1985, 121, 1550–1552. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Lee, S.H. Generalized argyria after habitual use of AgNO3. J. Dermatol. 1994, 21, 50–53. [Google Scholar] [CrossRef]
- Saager, R.B.; Hassan, K.M.; Kondru, C.; Durkin, A.J.; Kelly, K.M. Quantitative near infrared spectroscopic analysis of Q-Switched Nd:YAG treatment of generalized argyria. Lasers Surg. Med. 2013, 45, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Pinto-Almeida, T.; Lobo, I.; Selores, M. Unknown: Bluish-gray macules on the hands of a healthy 34 year old man. Dermatol. Online J. 2013, 19, 18964. [Google Scholar]
- Sakai, N.; Aoki, M.; Miyazawa, S.; Akita, M.; Takezaki, S.; Kawana, S. A case of generalized argyria caused by the use of silver protein as a disinfection medicine. Acta Derm. Venereol. 2007, 87, 186–187. [Google Scholar] [CrossRef] [Green Version]
- Devins, K.M.; Mogavero, H.S., Jr.; Helm, T.N. Localized argyria with pseudo-ochronosis. Cutis 2015, 95, 29–31. [Google Scholar] [PubMed]
- Landas, S.; Bonsib, S.M.; Ellerbroek, R.; Fischer, J. Argyria: Microanalytic-morphologic correlation using paraffin-embedded tissue. Ultrastruct. Pathol. 1986, 10, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Greene, R.M.; Su, W.P. Argyria. Am. Fam. Physician 1987, 36, 151–154. [Google Scholar]
- González-Serva, A. Normal nail anatomy, normal nail histology, and common reaction patterns. In Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy, 4th ed.; Rubin, A.I., Jellinek, N.J., Daniel, C.R., Scher, R.K., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Dudeja, L.; Dudeja, I.; Janakiraman, A.; Babu, M. Ocular argyrosis: A case with silver deposits in cornea and lens. Indian J. Ophthalmol. 2019, 67, 267–268. [Google Scholar] [CrossRef]
- Panigrahi, A.K.; Aruljothi, L.; Prajna, V.N. Argyrosis. Ophthalmology 2017, 124, 1270. [Google Scholar] [CrossRef]
- Geyer, O.; Rothkoff, L.; Lazar, M. Clearing of corneal argyrosis by YAG laser. Br. J. Ophthalmol. 1989, 73, 1009–1010. [Google Scholar] [CrossRef]
- Zografos, L.; Uffer, S.; Chamot, L. Unilateral conjunctival-corneal argyrosis simulating conjunctival melanoma. Arch. Ophthalmol. 2003, 121, 1483–1487. [Google Scholar] [CrossRef] [Green Version]
- Yanoff, M.; Scheie, H.G. Argyrosis of the conjunctiva and lacrimal sac. Arch. Ophthalmol. 1964, 72, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, K.U.; Lee, W.R. Argyrosis of the lacrimal sac. Graefe’s Arch. Clin. Exp. Ophthalmol. 1987, 225, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Calmettes; Deodati; Amalric. Argyrosis of the crystalline lens. Bull. Soc. Ophtalmol. Fr. 1957, 7–8, 534–535. [Google Scholar]
- Stafeeva, K.; Erlanger, M.; Velez-Montoya, R.; Olson, J.L. Ocular argyrosis secondary to long-term ingestion of silver nitrate salts. Clin. Ophthalmol. (Auckl. N. Z.) 2012, 6, 2033–2036. [Google Scholar] [CrossRef] [Green Version]
- Sarnat-Kucharczyk, M.; Pojda-Wilczek, D.; Mrukwa-Kominek, E. Diagnostic methods in ocular argyrosis: Case report. Doc. Ophthalmol. 2016, 133, 129–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimy, E.; Beardsley, R.; Ferrucci, S.; Ilsen, P.; Sarraf, D. Optical coherence tomography findings in ocular argyrosis. Ophthalmic Surg. Lasers Imaging Retin. 2013, 44, E20–E22. [Google Scholar] [CrossRef]
- Kamath, Y.; Sinha, A. Ocular argyrosis in a jeweller. BMJ Case Rep. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Bartley, G.B.; Buller, C.R.; Campbell, R.J.; Bullock, J.D. Pigmented episcleral mass from argyrosis following strabismus surgery. Arch. Ophthalmol. 1991, 109, 775–776. [Google Scholar] [CrossRef]
- Frei, J.; Schröder, B.; Messerli, J.; Probst, A.; Meyer, P. Localized argyrosis 58 years after strabismus operation—An ophthalmological rarity. Klin. Mon. Augenheilkd. 2001, 218, 61–63. [Google Scholar] [CrossRef]
- Moss, A.P.; Sugar, A.; Hargett, N.A.; Atkin, A.; Wolkstein, M.; Rosenman, K.D. The ocular manifestations and functional effects of occupational argyrosis. Arch. Ophthalmol. 1979, 97, 906–908. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Huerta, V.; De Wit-Carter, G.; Hernández-Quintela, E.; Naranjo-Tackman, R. Occupational corneal argyrosis in art silver solderers. Cornea 2003, 22, 604–611. [Google Scholar] [CrossRef]
- McClain, C.M.; Kantrow, S.M.; Abraham, J.L.; Price, J.; Parker, E.R.; Robbins, J.B. Localized cutaneous argyria: Two case reports and clinicopathologic review. Am. J. Dermatopathol. 2013, 35, e115–e118. [Google Scholar] [CrossRef]
- Sugden, P.; Azad, S.; Erdmann, M. Argyria caused by an earring. Br. J. Plast. Surg. 2001, 54, 252–253. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Peret, P.; Sans-Sabrafen, J.; Boleda Relats, M. Argyriasis. Report of a case (author’s transl). Med. Clin. (Barc.) 1979, 73, 386–388. [Google Scholar] [PubMed]
- Weber, F.P.; Norman, R.H. Argyria. Proc. R. Soc. Med. 1910, 3, 75–76. [Google Scholar]
- Davis, H. Two Cases of Pigmentation: (1) Argyria; (2) Pigmentation of Unknown Origin. Proc. R. Soc. Med. 1926, 20, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, M.D.; Watson, W.C. Argyria: Report of two cases. Glasg. Med. J. 1954, 35, 25–26. [Google Scholar]
- Kamalova, S.I.; Khamidullin, Z.G. Argyria as a result of the treatment of stomach ulcers with silver nitrate. Kazan Med. J. 1963, 3, 68–69. [Google Scholar]
- Eturska, M.; Obreshkova, E. Argyria in the prolonged use of adsorgan. Vutreshni Boles. 1979, 18, 121–123. [Google Scholar]
- Starzyńska, T.; Mandat, A. A case of argyria after peroral treatment of peptic ulcer with silver albuminate. Wiad. Lek. 1987, 40, 691–693. [Google Scholar] [PubMed]
- Mittag, H.; Knecht, J.; Arnold, R.; Hüttich, C.; Rupec, M. Argyria. A clinical, chemical analytic and micromorphologic study. Hautarzt 1987, 38, 670–677. [Google Scholar] [PubMed]
- Müller, M.; Wiedmann, K.H. Generalized argyria caused by targesin-containing drug used for stomach complaints. Med. Klin. (Munich) 1991, 86, 432–434. [Google Scholar] [PubMed]
- Harrison, G.A. Section of skin from case of pigmentation (argyria). Proc. R. Soc. Med. 1924, 17, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamson, H.G. Case of Trade Argyria. Proc. R. Soc. Med. 1924, 17, 8. [Google Scholar] [CrossRef] [Green Version]
- Bristol Medico Chirurgical Society. Argyria from the use of nasal drops. Bristol Med. Chir. J. (1883) 1952, 69, 143–144. [Google Scholar]
- Smith, S.Z.; Scheen, S.R.; Allen, J.D., Jr.; Arnn, E.T. Argyria. Arch. Dermatol. 1981, 117, 595–596. [Google Scholar] [CrossRef]
- Ondrasik, R.M.; Jordan, P.; Sriharan, A. A clinical mimicker of melanoma with distinctive histopathology: Topical silver nitrate exposure. J. Cutan. Pathol. 2020, 47, 1205–1210. [Google Scholar] [CrossRef]
- Wan, A.T.; Conyers, R.A.; Coombs, C.J.; Masterton, J.P. Determination of silver in blood, urine, and tissues of volunteers and burn patients. Clin. Chem. 1991, 37, 1683–1687. [Google Scholar] [CrossRef]
- Chaby, G.; Viseux, V.; Poulain, J.F.; De Cagny, B.; Denoeux, J.P.; Lok, C. Topical silver sulfadiazine-induced acute renal failure. Ann. Dermatol. Venereol. 2005, 132, 891–893. [Google Scholar] [CrossRef]
- Trop, M.; Novak, M.; Rodl, S.; Hellbom, B.; Kroell, W.; Goessler, W. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J. Trauma 2006, 60, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Payne, C.M.; Bladin, C.; Colchester, A.C.; Bland, J.; Lapworth, R.; Lane, D. Argyria from excessive use of topical silver sulphadiazine. Lancet 1992, 340, 126. [Google Scholar] [CrossRef]
- Maitre, S.; Jaber, K.; Perrot, J.L.; Guy, C.; Cambazard, F. Increased serum and urinary levels of silver during treatment with topical silver sulfadiazine. Ann. Dermatol. Venereol. 2002, 129, 217–219. [Google Scholar]
- Silver, S. Bacterial silver resistance: Molecular biology and uses and misuses of silver compounds. FEMS Microbiol. Rev. 2003, 27, 341–353. [Google Scholar] [CrossRef] [Green Version]
- Sataline, L. Tarnished silver. Lancet 2004, 363, 1166. [Google Scholar] [CrossRef]
- Utikal, J.; Thoelke, A.; Becker, J.C.; Figl, R.; Goerdt, S.; Schadendorf, D.; Ugurel, S. Local cutaneous argyria mimicking melanoma metastases in a patient with disseminated melanoma. J. Am. Acad. Dermatol. 2006, 55, S92–S94. [Google Scholar] [CrossRef]
- Holck, D.E.; Klintworth, G.K.; Dutton, J.J.; Foulks, G.N.; Manning, F.J. Localized conjunctival argyrosis: A late sequela of strabismus surgery. Ophthalmic Surg. Lasers 2000, 31, 495–498. [Google Scholar] [CrossRef]
- Marner, E. Case of severe argyria after eye treatment with argyrol. Ugeskr. Laeger 1953, 115, 1061–1062. [Google Scholar]
- Nielsen, I.O.; Kjaerbo, E. Generalized argyria due to the use of eyedrops containing silver nitrate. Ugeskr. Laeger 1989, 151, 33–34. [Google Scholar]
- Capoen, S.C.; Boullie, M.C.; Mallet, E. Argyria in children. Arch. Fr. Pediatr. 1989, 46, 49–50. [Google Scholar]
- Jurecka, W. Generalized argyrosis. Hautarzt 1986, 37, 628–631. [Google Scholar] [PubMed]
- Konecný, L.; Skerík, P.; Pitha, J. General argyrosis following esophagitis therapy. Ceskoslovenska Otolaryngol. 1966, 15, 101–104. [Google Scholar]
- Marie, J.; Léveque, B.; Watchi, J.M.; Desbois, J.C.; Feingold, J. Argyria in a child following pharyngeal sprays of silver salts repeated for 6 years. Ann. Pediatr. (Paris) 1966, 13, 2657–2659. [Google Scholar]
- Ohbo, Y.; Fukuzako, H.; Takeuchi, K.; Takigawa, M. Argyria and convulsive seizures caused by ingestion of silver in a patient with schizophrenia. Psychiatry Clin. Neurosci. 1996, 50, 89–90. [Google Scholar] [CrossRef]
- Shelton, D.; Goulding, R. Silver poisoning associated with an antismoking lozenge. Br. Med. J. 1979, 1, 267. [Google Scholar] [CrossRef] [Green Version]
- Macintire, D.; McLay, A.L.; East, B.W.; Williams, E.D.; Boddy, K. Silver poisoning associated with an antismoking lozenge. Br. Med. J. 1978, 2, 1749–1750. [Google Scholar] [CrossRef] [Green Version]
- Van Garsse, L.; Versieck, J. General argyria caused by administration of tobacco-withdrawal tablets containing silver acetate. Ned. Tijdschr. Geneeskd. 1995, 139, 2658–2661. [Google Scholar]
- Hau, S.C.; Tuft, S.J. Presumed corneal argyrosis from occlusive soft contact lenses: A case report. Cornea 2009, 28, 703–705. [Google Scholar] [CrossRef]
- Bouts, B.A. Images in clinical medicine. Argyria. N. Engl. J. Med. 1999, 340, 1554. [Google Scholar] [CrossRef]
- Fisher, R.L.; Zukerman, M. Argyrosis following prolonged intranasal medication with argyrol. J. Mich. State Med. Soc. 1948, 47, 1229. [Google Scholar] [PubMed]
- Voldrich, Z.; Holub, M.; Plhoñ, F. Isolated case of general argyrosis after long-term administration of targesine nasal drops. Ceskoslovenska Otolaryngol. 1975, 24, 374–376. [Google Scholar]
- Harman, R.R. Argyria. Br. J. Dermatol. 1977, 97 (Suppl. S15), 60. [Google Scholar] [CrossRef]
- Akimov, V.N. Argyria caused by uncontrolled use of silver protein in vasomotor rhinitis. Vestnik Otorinolaringol. 1980, 6, 77–78. [Google Scholar]
- Raimondo, L.; Garzaro, M.; Molinaro, L.; Bartoli, C.; Provenzano, E.; Pecorari, G. Iatrogenic rhinopharyngeal isolated argyria induced by silver-containing nasal drug. J. Craniofacial Surg. 2014, 25, e149–e151. [Google Scholar] [CrossRef] [PubMed]
- Rusch-Behrend, G.D.; Gutmann, J.L. Management of diffuse tissue argyria subsequent to endodontic therapy: Report of a case. Quintessence Int. 1995, 26, 553–557. [Google Scholar] [PubMed]
- Kissel, S.O.; Hanratty, J.J. Periodontal treatment of an amalgam tattoo. Compend. Contin. Educ. Dent. 2002, 23, 930–932. [Google Scholar]
- D’Haeseleire, P.; Thielens, P.; Bourgois, F.; Vanclooster, R. Are Ag-points, gutta percha and amalgam still applicable for apical sealing? Acta Stomatol. Belg. 1989, 86, 243–250. [Google Scholar]
- Schmalz, G.; Garhammer, P. Biological interactions of dental cast alloys with oral tissues. Dent. Mater. 2002, 18, 396–406. [Google Scholar] [CrossRef]
- Shimamoto, Y.; Shimamoto, H. Systemic argyria secondary to breath freshener “Jintan Silver Pills”. Hiroshima J. Med. Sci. 1987, 36, 245–247. [Google Scholar]
- Thompson, R.; Elliott, V.; Mondry, A. Argyria: Permanent skin discoloration following protracted colloid silver ingestion. BMJ Case Rep. 2009, 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, I.S.; Lee, M.Y.; Shin, D.H.; Jung, H.R. Three systemic argyria cases after ingestion of colloidal silver solution. Int. J. Dermatol. 2010, 49, 1175–1177. [Google Scholar] [CrossRef] [PubMed]
- Krejci-Manwaring, J.; West, D.A.; Aires, D.J. What is your diagnosis? Argyria. Cutis 2013, 91, 233–234. [Google Scholar]
- Chhabra, L.; Sareen, P.; Trivedi, N. The silver man: A rare cosmetic complication of alternative medicine. BMJ Case Rep. 2013, 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorayski, P.; Pinkham, M.B.; Muir, J.B.; Pullar, A.P. Severe acute radiation dermatitis in a patient with argyria. Case Rep. Oncol. Med. 2014, 2014, 154349. [Google Scholar] [CrossRef]
- DiGiorgio, C.M.; Wu, D.C.; Goldman, M.P. Successful treatment of argyria using the Picosecond Alexandrite Laser. Dermatol. Surg. 2016, 42, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.; Joo, E.J.; Suh, B.S.; Ham, C.B.; Han, J.M.; Kim, Y.G.; Yeom, J.S.; Choi, J.Y.; Park, J.H. A case of generalized argyria presenting with muscle weakness. Ann. Occup. Environ. Med. 2017, 29, 45. [Google Scholar] [CrossRef] [Green Version]
- Claessens, D.; Zeitz, P.F.; Beckers, H. Bluish-gray discoloration of skin and conjunctiva. Ophthalmologe 2020, 117, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.; Streight, K.L.; Rizk, C.B.; Markus, R. Side-by-side comparison of a Picosecond 755-nm Alexandrite Laser and a Quality-switched 1064-nm Neodymium-doped Yttrium Aluminum Garnet Laser in the treatment of argyria. Cureus 2019, 11, e5206. [Google Scholar] [CrossRef] [Green Version]
- Shao, E.X.; Collins, A.; McCallum, N.; Lim, D. Electron nicroscopy of argyria treated with Picosecond Alexandrite Laser. Dermatol. Surg. 2020, 46, 1246–1249. [Google Scholar] [CrossRef]
- Simon, M.; Buchanan, J.A. Argyria, an unexpected case of skin discoloration from colloidal silver salt ingestion. J. Emerg. Med. 2020, 59, e39–e41. [Google Scholar] [CrossRef]
- Seitz, I.P.; Kowarik, M.C.; Sartor-Pfeiffer, J.; Ziemann, U.; Wilhelm, H.; Bartz-Schmidt, K.U. Occurrence of primary progressive multiple sclerosis in a patient with argyria: Causality or coincidence? Mult. Scler. Relat. Disord. 2020, 46, 102465. [Google Scholar] [CrossRef]
- Han, T.Y.; Chang, H.S.; Lee, H.K.; Son, S.J. Successful treatment of argyria using a low-fluence Q-switched 1064-nm Nd:YAG laser. Int. J. Dermatol. 2011, 50, 751–753. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.L.; Janofsky, J.; Jayaram, G. Argyria as a result of somatic delusions. Am. J. Psychiatry 2008, 165, 649–650. [Google Scholar] [CrossRef] [PubMed]
- Okan, D.; Woo, K.; Sibbald, R.G. So what if you are blue? Oral colloidal silver and argyria are out: Safe dressings are in. Adv. Ski. Wound Care 2007, 20, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Martin, T.G.; Rainey, P.; Robertson, W.O. Believe it or not—Silver still poisons! Vet. Hum. Toxicol. 2002, 44, 291–292. [Google Scholar] [PubMed]
- Tran, H.A.; Song, S. Silver toxicity masquerading as hypocaeruloplasminaemia. Pathology 2007, 39, 456–458. [Google Scholar] [CrossRef]
- Baker, C.D.; Federico, M.J.; Accurso, F.J. Case report: Skin discoloration following administration of colloidal silver in cystic fibrosis. Curr. Opin. Pediatr. 2007, 19, 733–735. [Google Scholar] [CrossRef]
- Hristov, A.C.; High, W.A.; Golitz, L.E. Localized cutaneous argyria. J. Am. Acad. Dermatol. 2011, 65, 660–661. [Google Scholar] [CrossRef]
- Glehr, M.; Leithner, A.; Friesenbichler, J.; Goessler, W.; Avian, A.; Andreou, D.; Maurer-Ertl, W.; Windhager, R.; Tunn, P.U. Argyria following the use of silver-coated megaprostheses: No association between the development of local argyria and elevated silver levels. Bone Jt. J. 2013, 95-B, 988–992. [Google Scholar] [CrossRef]
- Naqvi, A.H.; Shields, J.W.; Abraham, J.L. Nasal argyria (deposition of silver-selenium) in the photographic film industry: Histopathology and microanalysis. Am. J. Otolaryngol. 2007, 28, 430–432. [Google Scholar] [CrossRef]
- Flögel, W.; Widmeier, S.; Hotz, P.; Schärer, L.; Barthelmes, D.; Landau, K.; Thiel, M.A. Corneal and conjunctival findings in systemic silver intoxication. Klin. Mon. Augenheilkd. 2006, 223, 390–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajirian, A.L.; Campbell, R.M.; Robinson-Bostom, L. Localized argyria after exposure to aerosolized solder. Cutis 2006, 78, 305–308. [Google Scholar]
- Cho, E.A.; Lee, W.S.; Kim, K.M.; Kim, S.Y. Occupational generalized argyria after exposure to aerosolized silver. J. Dermatol. 2008, 35, 759–760. [Google Scholar] [CrossRef]
- Gallardo, M.J.; Randleman, J.B.; Price, K.M.; Johnson, D.A.; Acosta, S.; Grossniklaus, H.E.; Stulting, R.D. Ocular argyrosis after long-term self-application of eyelash tint. Am. J. Ophthalmol. 2006, 141, 198–200. [Google Scholar] [CrossRef]
- Sakai, T.; Yamada, N.; Yamamoto, O.; Saito-Shono, T.; Yamate, T.; Ishikawa, K.; Goto, M.; Hatano, Y.; Hongo, N.; Kodera, T.; et al. Argyria due to embedded acupuncture needles and their transcaval migration into the right ventricle without serious complications. Eur. J. Dermatol. 2017, 27, 655–656. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Baba, S.; Uchigasaki, S.; Murase, M. Localized argyria with chrysiasis caused by implanted acupuncture needles. Distribution and chemical forms of silver and gold in cutaneous tissue by electron microscopy and X-ray microanalysis. J. Am. Acad. Dermatol. 1993, 29, 833–837. [Google Scholar] [CrossRef]
- Legat, F.J.; Goessler, W.; Schlagenhaufen, C.; Soyer, H.P. Argyria after short-contact acupuncture. Lancet 1998, 352, 241. [Google Scholar] [CrossRef]
- Takeishi, E.; Hirose, R.; Hamasaki, Y.; Katayama, I. Localized argyria 20-years after embedding of acupuncture needles. Eur. J. Dermatol. 2002, 12, 609–611. [Google Scholar]
- Rackoff, E.M.; Benbenisty, K.M.; Maize, J.C.; Maize, J.C., Jr. Localized cutaneous argyria from an acupuncture needle clinically concerning for metastatic melanoma. Cutis 2007, 80, 423–426. [Google Scholar]
- Alés-Fernández, M.; Ríos-Martín, J.J.; Camacho-Martínez, F.M. Localized argyria secondary to acupuncture mimicking blue nevus. J. Drugs Dermatol. 2010, 9, 1019–1020. [Google Scholar]
- van den Nieuwenhuijsen, I.J.; Calame, J.J.; Bruynzeel, D.P. Localized argyria caused by silver earrings. Dermatologica 1988, 177, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.A.; Fallowfield, M.; Kemmett, D. Localized argyria caused by silver earrings. Br. J. Dermatol. 1996, 135, 484–485. [Google Scholar] [CrossRef]
- Newman, M.; Kolecki, P. Argyria in the ED. Am. J. Emerg. Med. 2001, 19, 525–526. [Google Scholar] [CrossRef] [PubMed]
- Timmins, A.C.; Morgan, G.A. Argyria or cyanosis. Anaesthesia 1988, 43, 755–756. [Google Scholar] [CrossRef]
- Fred, H.L. Case in point. Generalized argyria. Hosp. Pract. 1994, 29, 14. [Google Scholar] [CrossRef] [PubMed]
- Faergemann, J. Medical history. Lancet 2002, 360, 689. [Google Scholar] [CrossRef]
- Baernstein, A.; Smith, K.M.; Elmore, J.G. Singing the blues: Is it really cyanosis? Respir. Care 2008, 53, 1081–1084. [Google Scholar]
- Fernandez-Flores, A.; Nguyen, T.; Cassarino, D.S. Mucocutaneous Hyperpigmentation in a Patient With a History of Both Minocycline and Silver Ingestion. Am. J. Dermatopathol. 2017, 39, 916–919. [Google Scholar] [CrossRef]
- Hays, G.B.; Lyle, C.B., Jr.; Wheeler, C.E., Jr. Slate-Gray Color in Patients Receiving Chlorpromazine. Arch. Dermatol. 1964, 90, 471–476. [Google Scholar] [CrossRef]
- Trimble, J.W.; Mendelson, D.S.; Fetter, B.F.; Ingram, P.; Gallagher, J.J.; Shelburne, J.D. Cutaneous pigmentation secondary to amiodarone therapy. Arch. Dermatol. 1983, 119, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Rhee, D.Y.; Chang, S.E.; Lee, M.W.; Choi, J.H.; Moon, K.C.; Koh, J.K. Treatment of argyria after colloidal silver ingestion using Q-switched 1064-nm Nd:YAG laser. Dermatol. Surg. 2008, 34, 1427–1430. [Google Scholar] [CrossRef]
- Parker, W.A. Argyria and cyanotic heart disease. Am. J. Hosp. Pharm. 1977, 34, 287–289. [Google Scholar] [CrossRef]
- Kejda, J.; Horák, O.; Janecková, R. Argyria imitating cardiopulmonary cyanosis (author’s transl). Ceskoslovenska Dermatol. 1980, 55, 330–333. [Google Scholar]
- Donnard, G.; Sallaberry, M.; Morand, J.J. Argyria and anesthesia. Ann. Fr. Anesth. Reanim. 1996, 15, 226. [Google Scholar] [CrossRef]
- Travis, C. Differential diagnosis cyanosis versus argyria: When your patient remains blue—A 48-year-old trauma patient with persistent cyanosis. J. Emerg. Nurs. 2010, 36, 466–467. [Google Scholar] [CrossRef]
- Jänner, M.; Marschelke, I.; Voigt, H. Localized intramural silver impregnation of the tongue. Differential diagnosis from malignant melanoma. Hautarzt 1980, 31, 510–512. [Google Scholar] [PubMed]
- Landas, S.K.; Mitros, F.A.; Furst, D.E.; LaBrecque, D.R. Lipogranulomas and gold in the liver in rheumatoid arthritis. Am. J. Surg. Pathol. 1992, 16, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Hugar, S.B.; Shulman, J.; Yanta, J.; Nine, J. Ochronosis presenting as methemoglobinemia. J. Forensic Sci. 2019, 64, 913–916. [Google Scholar] [CrossRef]
- Atmatzidis, D.H.; Hoegler, K.; Weiss, A.; Lambert, W.C.; Schwartz, R.A. Unsafe deposits: Overlapping cutaneous manifestations of porphyria cutanea tarda, ochronosis, hemochromatosis, and argyria. Skinmed 2019, 17, 161–170. [Google Scholar]
- Rosenman, K.D.; Moss, A.; Kon, S. Argyria: Clinical implications of exposure to silver nitrate and silver oxide. J. Occup. Med. 1979, 21, 430–435. [Google Scholar]
- Bartlett, R.E. Generalized argyrosis with lens involvement; report of an unusual case. Am. J. Ophthalmol. 1954, 38, 402–403. [Google Scholar] [CrossRef]
- Pifer, J.W.; Friedlander, B.R.; Kintz, R.T.; Stockdale, D.K. Absence of toxic effects in silver reclamation workers. Scand. J. Work. Environ. Health 1989, 15, 210–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, W.H.; Garron, L.K.; Contreras, F.; Hayes, T.L.; Lai, C. Endogenous and exogenous ocular and systemic silver deposition. Trans. Ophthalmol. Soc. U. K. 1980, 100, 171–178. [Google Scholar] [PubMed]
- Zech, P.; Colon, S.; Labeeuw, R.; Blanc-Brunat, N.; Richard, P.; Perol, M. Nephrotic syndrome with silver deposits in the glomerular basement membranes during argyria. Nouv. Press. Med. 1973, 2, 161–164. [Google Scholar]
- Watanabe, Y.; Eguchi, A.; Kamio, M.; Yamaguchi, K.; Ohara, M.; Mochizuki, T. Case of membranous nephropathy associated with argyria. Nihon Jinzo Gakkai Shi 2005, 47, 547–551. [Google Scholar]
- McCague, A.; Joe, V.C. A Case of Argyria and Acute Leukopenia Associated with the Use of an Antimicrobial Soft Silicone Foam Dressing. J. Burn Care Res. 2016, 37, e493–e496. [Google Scholar] [CrossRef]
- Rezk, T.; Penton, J.; Stevenson, A.; Owen-Casey, M.; Little, M.; Cunningham, J.; Salama, A.D. Pauci Immune crescentic glomerulonephritis in a patient with T-cell lymphoma and argyria. BMC Nephrol. 2016, 17, 49. [Google Scholar] [CrossRef] [Green Version]
- Parkes, A. Silver-coated dressing Acticoat. J. Trauma 2006, 61, 239–240. [Google Scholar] [CrossRef]
- Barrie, H.J.; Harding, H.E. Argyro-siderosis of the lungs in silver finishers. Occup. Environ. Med. 1947, 4, 225–229. [Google Scholar] [CrossRef] [Green Version]
- Harding, H.E. Fibrosis in the lungs of a silver finisher. Br. J. Ind. Med. 1948, 5, 70–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepien, K.M.; Morris, R.; Brown, S.; Taylor, A.; Morgan, L. Unintentional silver intoxication following self-medication: An unusual case of corticobasal degeneration. Ann. Clin. Biochem. 2009, 46, 520–522. [Google Scholar] [CrossRef]
- Stepien, K.M.; Taylor, A. Colloidal silver ingestion with copper and caeruloplasmin deficiency. Ann. Clin. Biochem. 2012, 49, 300–301. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.A.; O’Meara, J.M. The feasibility of measuring silver concentrations in vivo with X-ray fluorescence. Phys. Med. Biol. 2004, 49, N259–N266. [Google Scholar] [CrossRef]
- Czitober, H.; Frischauf, H.; Leodolter, I. Quantitative studies in generalized argyron’s using neutron activation analysis. Virchows Arch. A Pathol. Pathol. Anat. 1970, 350, 44–51. [Google Scholar] [CrossRef]
- Johansson, E.A.; Kanerva, L.; Niemi, K.M.; Lakomaa, E.L. Generalized argyria with low ceruloplasmin and copper levels in the serum. A case report with clinical and microscopical findings and a trial of penicillamine treatment. Clin. Exp. Dermatol. 1982, 7, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, T.; Ollswang, A. Failure of 2,3-dimercaptopropanol in treatment of argyria. Arch. Derm. Syphilol. 1948, 57, 743–745. [Google Scholar] [CrossRef] [PubMed]
- Kleckner, M.S., Jr. The use of BAL in generalized argyria. Calif. Med. 1949, 70, 133. [Google Scholar] [PubMed]
- Aaseth, J.; Halse, J.; Falch, J. Chelation of silver in argyria. Acta Pharmacol. Toxicol. (Copenh.) 1986, 59 (Suppl. S7), 471–474. [Google Scholar] [CrossRef] [PubMed]
- Almurayshid, A.; Park, S.; Oh, S.H. Effective laser treatment options for argyria: Review of literatures. J. Cosmet. Dermatol. 2020, 19, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Kim, J.H.; Shin, H.T.; Lee, K.T.; Lee, J.H.; Lee, D.Y.; Lee, J.H.; Yang, J.M. An Effective Modality for Argyria Treatment: Q-Switched 1064-nm Nd:YAG Laser. Ann. Dermatol. 2013, 25, 511–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hovenic, W.; Golda, N. Treatment of argyria using the quality-switched 1064-nm neodymium-doped yttrium aluminum garnet laser: Efficacy and persistence of results at 1-year follow-up. Dermatol. Surg. 2012, 38, 2031–2034. [Google Scholar] [CrossRef] [PubMed]
- Krase, J.M.; Gottesman, S.P.; Goldberg, G.N. Recurrence of Argyria Post Q-Switched Laser Treatment. Dermatol. Surg. 2017, 43, 1308–1311. [Google Scholar] [CrossRef] [PubMed]
- Mock, J.J.; Barbic, M.; Smith, D.R.; Schultz, D.A.; Schultz, S. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J. Chem. Phys. 2002, 116, 6755–6759. [Google Scholar] [CrossRef]
- Jańczuk, Z.; Banach, J. Local argyrosis of oral mucosa or amalgam tattoo. A problem in diagnosis and treatment. Adv. Med. Sci. 2006, 51 (Suppl. S1), 62–65. [Google Scholar] [PubMed]
- Munger, M.A.; Radwanski, P.; Hadlock, G.C.; Stoddard, G.; Shaaban, A.; Falconer, J.; Grainger, D.W.; Deering-Rice, C.E. In vivo human time-exposure study of orally dosed commercial silver nanoparticles. Nanomedicine 2014, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, C.; Rosas-Hernandez, H.; Ramirez-Lee, M.A.; Salazar-García, S.; Ali, S.F. Role of silver nanoparticles (AgNPs) on the cardiovascular system. Arch. Toxicol. 2016, 90, 493–511. [Google Scholar] [CrossRef] [PubMed]
- Abukabda, A.B.; Stapleton, P.A.; Nurkiewicz, T.R. Metal Nanomaterial Toxicity Variations Within the Vascular System. Curr. Environ. Health Rep. 2016, 3, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Teran, C.G.; Sura, S.; Cabandugama, P.; Berson, C. Silver nitrate ingestion: Report of a case with an uneventful course and review of the literature. Clin. Pract. 2011, 1, e43. [Google Scholar] [CrossRef] [PubMed]
- Shoults-Wilson, W.A.; Reinsch, B.C.; Tsyusko, O.V.; Bertsch, P.M.; Lowry, G.V.; Unrine, J.M. Effect of silver nanoparticle surface coating on bioaccumulation and reproductive toxicity in earthworms (Eisenia fetida). Nanotoxicology 2011, 5, 432–444. [Google Scholar] [CrossRef]




| Treatment | Cause | References |
|---|---|---|
| Gastrointestinal conditions (ingestion of silver-containing colloids/pills) | Iatrogenic, systemic | [135,166,167,168,169,170,171,172,173,174] |
| Leukoplakia patch (topical application of silver nitrate) | Iatrogenic, topical | [175] |
| Epilepsy and other neuropsychiatric conditions (ingestion of silver-containing pills) | Iatrogenic, systemic | [40,131,176,177] |
| Alopecia (ingestion of silver-containing colloids) | Iatrogenic, systemic | [52] |
| Prophylaxis of gonococcal ophthalmia neonatorum (application of silver nitrate collyrium) | Iatrogenic, topical | [46,105,178] |
| Syphilis (topical application of silver arsphenamine) | Iatrogenic, topical | [126,131] |
| Wounds/ulcers/burns (topical application of silver sulfadiazine cream for asepsis, silver nitrate for chemical cautery/hemostasis, and/or use of silver-impregnated suture threads/surgical clips) | Iatrogenic, topical and/or systemic (if bloodstream is reached) | [70,84,85,105,128,141,179,180,181,182,183,184,185,186,187] |
| Strabismus surgery (application of silver nitrate collyrium and/or use of silver-impregnated suture threads/surgical clips) | Iatrogenic, topical | [160,161,188] |
| Trachoma (topical application of silver nitrate for chemical cautery) | Iatrogenic, topical | [121] |
| Conjunctivitis/eye soreness/epiphora (application of silver-containing collyrium) | Iatrogenic, topical | [114,153,154,189,190] |
| Pharyngitis/throat soreness (topical throat application of pulverized silver and/or ingestion of silver-containing tablets) | Iatrogenic, topical (pharyngeal) and/or systemic | [67,144,191,192,193,194] |
| Hematuria (instillation of the urinary tract with silver nitrate preparations) | Iatrogenic, topical | [105,106,107] |
| Smoking cessation (chewing/ingestion of silver-coated sugar particles and/or silver acetate lozenges/pills) | Iatrogenic, systemic | [57,126,195,196,197,198] |
| Varicose veins (injection of silver nitrate as sclerosant) | Iatrogenic, topical, and/or systemic | [130] |
| Intractable diplopia (use of silver nitrate-coated soft lenses) | Iatrogenic, topical | [199] |
| Antiseptic and astringent properties (application of silver-containing vasoconstricting nose drops) | Iatrogenic, topical, and/or systemic (silver is drained posteriorly and ingested) | [5,40,47,55,59,64,126,134,186,200,201,202,203,204,205] |
| Dental restoration (silver-containing filling material for endodontic procedures) | Iatrogenic, topical | [92,93,94,96,97,98,100,185,206,207,208,209] |
| Halitosis (silver-containing breath-freshening pills) | Non-medical, systemic | [8,210] |
| Belief in general health benefits/immune system boosting/alternative medicine (ingestion of silver-containing colloids, application of silver-containing nasal drops) | Non-conventional medicine, systemic | [1,6,9,20,22,34,38,44,45,49,50,51,58,62,66,68,69,125,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228] |
| Skin-breaching trauma with silver-containing material | Accidental, topical | [145,229] |
| Antibiotic properties (use of silver-coated prosthetic implants) | Iatrogenic, topical, and/or systemic (if bloodstream is reached) | [90,230] |
| Photochemical industry | Occupational, topical (skin, eye, and intranasal deposition), and/or systemic (inhalation) | [112,152,231] |
| Occupational silver manipulation, silver soldering/silversmithing in jewelry/art crafting | Occupational, topical (skin and eye) and/or systemic (inhalation) | [54,77,80,119,122,150,163,232,233,234] |
| Eyelash tinting | Non-medical, topical | [115,235] |
| Acupuncture | Non-conventional medicine, topical | [8,89,98,139,140,236,237,238,239,240,241] |
| Silver earrings/piercings | Non-medical, topical | [2,71,72,74,88,137,138,165,242,243] |
| Silver-coated nuts and/or spices (areca and betel nut) | Non-medical, systemic (oral intake) | [7,91] |
| Pathological Condition of Xenobiotic | Description | References |
|---|---|---|
| Hemochromatosis | Generalized skin hyperpigmentation (GA) | [40,56,64,69,126,134,167,178,245,246,247] |
| Lead poisoning | Blue line along the gingival margins at the base of the teeth (LA/amalgam tattoo) | [5,7,40,91] |
| Methemoglobinemia/sulfhemoglobinemia | Generalized skin brownish-blue to gray pigmentation (as in cyanosis) (GA) | [4,40,56,126,134,141,178,245,246,248] |
| Toxic melanodermatitis | Hyperpigmented skin lesions (LA, GA) | [132] |
| Minocycline | Blue staining of teeth or blue-gray skin lesions (GA) | [7,64,132,249] |
| Chlorpromazine/Phenothiazines | Slate gray-bluish skin discoloration in sun-exposed areas (GA) | [64,132,178,250] |
| Amiodarone | Slate gray-bluish skin discoloration in sun-exposed areas (GA) | [7,64,125,251] |
| Antimalarial agents | Blue-gray discolorations of the mouth and skin (LA, GA) | [55,79,132,246] |
| Clofazimine | Grayish skin plaques or generalized gray skin pigmentation (LA, GA) | [7,132] |
| Cyanosis/cyanotic heart disease | Generalized skin brownish-blue to gray pigmentation (GA) | [1,8,20,36,40,43,45,53,56,66,69,131,169,178,200,245,246,248,252,253,254,255,256] |
| Nevus | Flat or raised pigmented skin lesion (LA) | [2,71,73,76,82,133,137,164,229,241] |
| End-stage renal disease/uremia | Heterogenous skin lesions (LA, GA) | [36] |
| Melanoma of the skin/conjunctiva/oral mucosa | Pigmented lesion, usually asymmetric, with heterogenous color and time-evolving (LA/amalgam tattoo) | [23,82,92,95,96,100,101,119,133,159,178,188,240,246,257] |
| Chrysiasis | Slate-gray to blue skin pigmentation, especially in sun-exposed areas, nail pigmentation (LA, GA, azure lunula) | [36,64,79,113,178,237,246,258] |
| Iron salts | Brown to red skin lesions (LA) | [79] |
| Ochronosis | Bluish-black skin lesions (LA, GA) | [64,76,113,259,260] |
| Wilson’s disease | Generalized skin hyperpigmentation, Kayser-Fleischer rings (GA, OA) | [55,113] |
| Tobacco (chewing) | Brownish-black staining of the oral mucosa (LA/amalgam tattoo) | [91] |
| Chlorophyll (mouthwash) | Blackening of the tongue (LA/amalgam tattoo) | |
| Sodium perborate (mouthwash) | Blackening of the tongue (LA/amalgam tattoo) | |
| Ariboflavinosis | Diffuse bluish-purple discoloration of the buccal mucosa (GA) | |
| Peutz-Jeghers syndrome | Dark blue-brown hyperpigmented gingival macules (LA/amalgam tattoo) | |
| Addison’s disease | Generalized bronze-like skin pigmentation, diffuse pigmentation of gingiva, tongue, and buccal mucosa (GA) | [53,58,69,91,134,178,214,245] |
| Bismuthosis | Blue-black, sharply limited pigmentation of marginal gingivae, nail pigmentation (LA/amalgam tattoo, GA, azure lunula) | [36,79,91,134,178,246] |
| Mercurialism | Diffuse blue-gray to black gingival pigmentation, nail pigmentation (LA/amalgam tattoo, azure lunula) | [36,79,91,140,178] |
| Arseniasis | Generalized skin hyperpigmentation with hyperkeratosis (GA) | [5,7,79,91] |
| Accidental tattoo | Dark-blue/black macules/patterns (LA) | [133] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, L.; Dinis-Oliveira, R.J. Clinical and Forensic Aspects of the Different Subtypes of Argyria. J. Clin. Med. 2021, 10, 2086. https://doi.org/10.3390/jcm10102086
Mota L, Dinis-Oliveira RJ. Clinical and Forensic Aspects of the Different Subtypes of Argyria. Journal of Clinical Medicine. 2021; 10(10):2086. https://doi.org/10.3390/jcm10102086
Chicago/Turabian StyleMota, Luís, and Ricardo Jorge Dinis-Oliveira. 2021. "Clinical and Forensic Aspects of the Different Subtypes of Argyria" Journal of Clinical Medicine 10, no. 10: 2086. https://doi.org/10.3390/jcm10102086
APA StyleMota, L., & Dinis-Oliveira, R. J. (2021). Clinical and Forensic Aspects of the Different Subtypes of Argyria. Journal of Clinical Medicine, 10(10), 2086. https://doi.org/10.3390/jcm10102086







