Accuracy of a Novel SARS-CoV-2 Antigen-Detecting Rapid Diagnostic Test from Standardized Self-Collected Anterior Nasal Swabs
Abstract
:1. Introduction
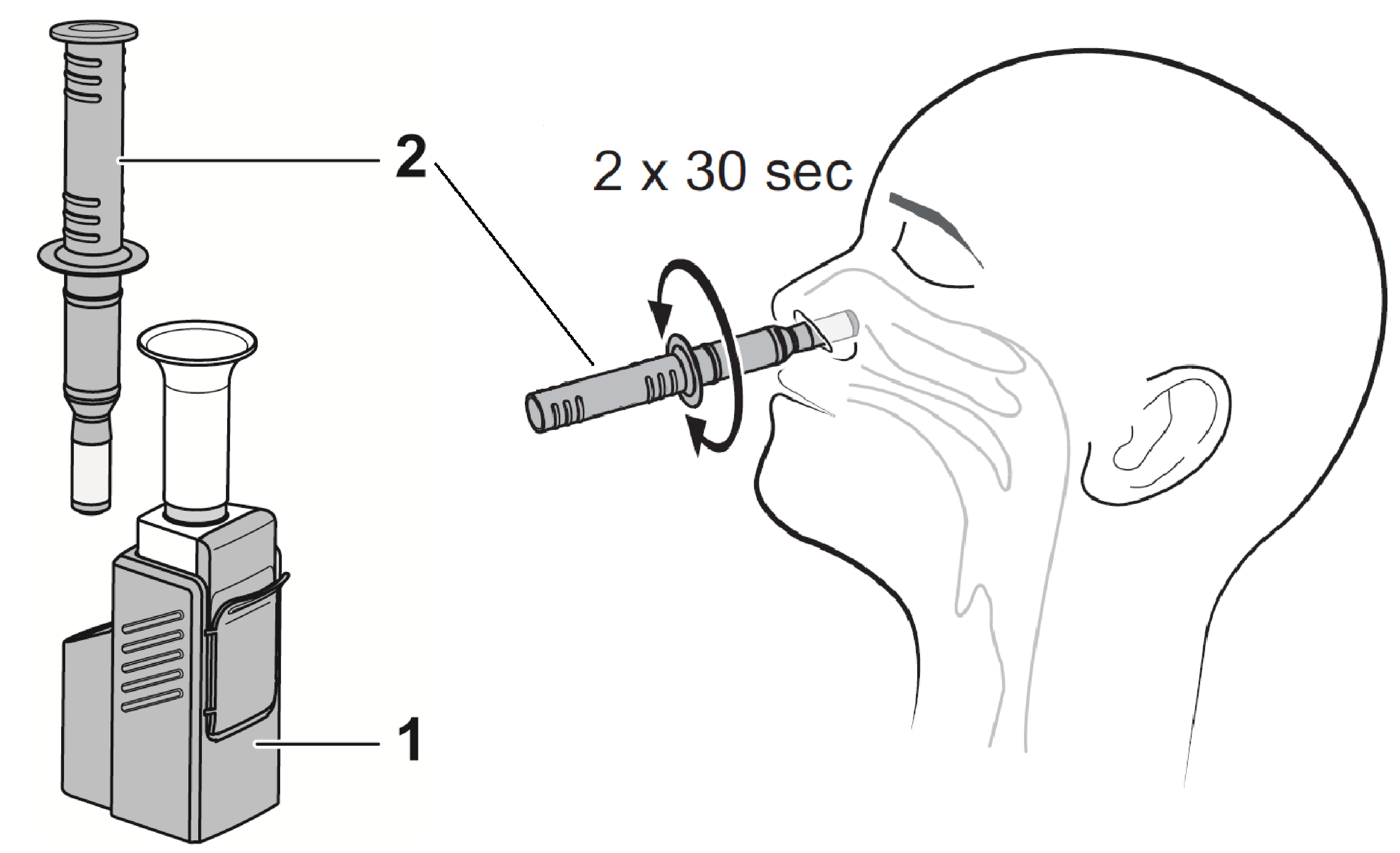
2. Methods
3. Results
3.1. Primary Endpoint
3.2. Secondary Endpoints
3.3. Safety and Usability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foundation of Innovative New Diagnostics (FIND). SARS-COV-2 Diagnostics Pipeline. Available online: http://www.finddx.org/covid-19/pipeline/ (accessed on 13 May 2021).
- SARS-CoV-2 Antigen-Detecting Rapid Diagnostic Tests: An Implementation Guide; World Health Organization: Geneva, Switzerland, 2020.
- Lambert-Niclot, S.; Cuffel, A.; Le Pape, S.; Vauloup-Fellous, C.; Morand-Joubert, L.; Roque-Afonso, A.-M.; Le Goff, J.; Delaugerre, C. Evaluation of a Rapid Diagnostic Assay for Detection of SARS-CoV-2 Antigen in Nasopharyngeal Swabs. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [PubMed]
- Lindner, A.K.; Nikolai, O.; Kausch, F.; Wintel, M.; Hommes, F.; Gertler, M.; Krüger, L.J.; Gaeddert, M.; Tobian, F.; Lainati, F.; et al. Head-to-head comparison of SARS-CoV-2 antigen-detecting rapid test with self-collected nasal swab versus professional-collected nasopharyngeal swab. Eur. Respir. J. 2021, 57, 2003961. [Google Scholar] [CrossRef] [PubMed]
- Nagura-Ikeda, M.; Imai, K.; Tabata, S.; Miyoshi, K.; Murahara, N.; Mizuno, T.; Horiuchi, M.; Kato, K.; Imoto, Y.; Iwata, M.; et al. Clinical Evaluation of Self-Collected Saliva by Quantitative Reverse Transcription-PCR (RT-qPCR), Direct RT-qPCR, Reverse Transcription–Loop-Mediated Isothermal Amplification, and a Rapid Antigen Test To Diagnose COVID-19. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Holmes, E.C.; O’Toole, Á.; Hill, V.; McCrone, J.T.; Ruis, C.; Du Plessis, L.; Pybus, O.G. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat. Microbiol. 2020, 5, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- IBBS. COVID-19: Entlassungskriterien aus der Isolierung; Robert Koch-Institut: Berlin, Germany, 2021; p. 1. [Google Scholar]
- Van Kampen, J.J.A.; Van De Vijver, D.A.M.C.; Fraaij, P.L.A.; Haagmans, B.L.; Lamers, M.M.; Okba, N.; Akker, J.P.C.V.D.; Endeman, H.; Gommers, D.A.M.P.J.; Cornelissen, J.J.; et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19). Nat. Commun. 2021, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nat. Cell Biol. 2020, 581, 465–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Dinnes, J.; Deeks, J.J.; Berhane, S.; Taylor, M.; Adriano, A.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2021, 3, CD013705. [Google Scholar] [CrossRef] [PubMed]


| Inclusion Criteria | Exclusion Criteria | ||
|---|---|---|---|
| General |
|
| |
| Specific | Asymptomatic |
|
|
| Symptomatic |
|
| |
| Ct (E-Gen) | VC-Copies | VoC | Days Symptomatic | Ct (E-Gen) | VC-Copies | VoC | Days Symptomatic |
|---|---|---|---|---|---|---|---|
| 13.7 | >10 Mio | B.1.1.7 | 4 | 20.3 | >10 Mio | none | 7 |
| 14.1 | >10 Mio | B.1.1.7 | 1 | 20.4 | >10 Mio | B.1.1.7 | 5 |
| 14.6 | >10 Mio | B.1.1.7 | 3 | 20.5 | >10 Mio | B.1.1.7 | 2 |
| 14.7 | >10 Mio | none | 2 | 20.7 | >10 Mio | B.1.1.7 | 1 |
| 14.7 | >10 Mio | B.1.1.7 | 1 | 20.8 | >10 Mio | B.1.1.7 | 4 |
| 15.1 | >10 Mio | B.1.1.7 | 1 | 21.1 | >10 Mo | B.1.1.7 | 3 |
| 15.4 | >10 Mio | B.1.1.7 | 3 | 21.2 | >10 Mio | B.1.1.7 | 4 |
| 15.5 | >10 Mio | B.1.1.7 | 3 | 21.4 | >10 Mio | none | 4 |
| 15.7 | >10 Mio | B.1.1.7 | 3 | 21.4 | >10 Mio | B.1.1.7 | 7 |
| 15.8 | >10 Mio | none | 2 | 21.5 | >10 Mio | none | 4 |
| 15.9 | >10 Mio | B.1.1.7 | 1 | 21.5 | >10 Mio | B.1.1.7 | 1 |
| 16.2 | >10 Mio | B.1.1.7 | 2 | 22.4 | >10 Mio | none | 3 |
| 16.2 | >10 Mio | B.1.1.7 | 3 | 22.4 | >10 Mio | B.1.1.7 | 5 |
| 16.3 | >10 Mio | none | 1 | 22.5 | >10 Mio | none | 3 |
| 16.5 | >10 Mio | none | 4 | 22.5 | >10 Mio | none | 3 |
| 16.5 | >10 Mio | none | 3 | 22.6 | >10 Mio | B.1.1.7 | 3 |
| 16.8 | >10 Mio | none | 6 | 22.7 | 1–10 Mio | none | 3 |
| 17.1 | >10 Mio | B.1.1.7 | 1 | 22.8 | >10 Mio | none | 0 |
| 17.1 | >10 Mio | none | 4 | 24.0 | 1–10 Mio | B.1.1.7 | 1 |
| 17.2 | >10 Mio | B.1.1.7 | 1 | 24.2 | 1–10 Mio | B.1.1.7 | 2 |
| 17.2 | >10 Mio | B.1.1.7 | 1 | 24.4 | 1–10 Mio | none | 0 |
| 17.8 | >10 Mio | B.1.1.7 | 1 | 24.5 | 1–10 Mio | B.1.1.7 | 3 |
| 17.8 | >10 Mio | B.1.1.7 | 5 | 24.8 | 1–10 Mio | B.1.1.7 | 3 |
| 17.9 | >10 Mio | B.1.1.7 | 6 | 25.2 | 1–10 Mio | B.1.1.7 | 7 |
| 18.4 | >10 Mio | B.1.1.7 | 1 | 25.4 | < 1 Mio | none | 5 |
| 18.6 | >10 Mio | B.1.1.7 | 6 | 25.4 | 1–10 Mio | none | 7 |
| 18.9 | >10 Mio | B.1.1.7 | 3 | 25.5 | 1–10 Mio | B.1.1.7 | 5 |
| 19.2 | >10 Mio | B.1.1.7 | 1 | 26.5 | <1 Mio | B.1.1.7 | 3 |
| 19.4 | >10 Mio | B.1.1.7 | 2 | 27.7 | <1 Mio | None26 | 2 |
| 19.5 | >10 Mio | none | 1 | 27.8 | <1 Mio | B.1.1.7 | 3 |
| 19.6 | >10 Mio | none | 3 | 29.7 | <1 Mio | none | 0 |
| 19.9 | >10 Mio | none | 1 | 31.0 | <1 Mio | none | asymptomatic |
| 20.1 | >10 Mio | B.1.1.7 | 2 | 33.0 | <1 Mio | B.1.1.7 | 7 |
| 20.2 | >10 Mio | B.1.1.7 | 5 | 33.3 | <1 Mio | none | 1 |
| 20.3 | >10 Mio | B.1.1.7 | 3 | 33.9 | <1 Mio | none | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osmanodja, B.; Budde, K.; Zickler, D.; Naik, M.G.; Hofmann, J.; Gertler, M.; Hülso, C.; Rössig, H.; Horn, P.; Seybold, J.; et al. Accuracy of a Novel SARS-CoV-2 Antigen-Detecting Rapid Diagnostic Test from Standardized Self-Collected Anterior Nasal Swabs. J. Clin. Med. 2021, 10, 2099. https://doi.org/10.3390/jcm10102099
Osmanodja B, Budde K, Zickler D, Naik MG, Hofmann J, Gertler M, Hülso C, Rössig H, Horn P, Seybold J, et al. Accuracy of a Novel SARS-CoV-2 Antigen-Detecting Rapid Diagnostic Test from Standardized Self-Collected Anterior Nasal Swabs. Journal of Clinical Medicine. 2021; 10(10):2099. https://doi.org/10.3390/jcm10102099
Chicago/Turabian StyleOsmanodja, Bilgin, Klemens Budde, Daniel Zickler, Marcel G. Naik, Jörg Hofmann, Maximilian Gertler, Claudia Hülso, Heike Rössig, Philipp Horn, Joachim Seybold, and et al. 2021. "Accuracy of a Novel SARS-CoV-2 Antigen-Detecting Rapid Diagnostic Test from Standardized Self-Collected Anterior Nasal Swabs" Journal of Clinical Medicine 10, no. 10: 2099. https://doi.org/10.3390/jcm10102099
APA StyleOsmanodja, B., Budde, K., Zickler, D., Naik, M. G., Hofmann, J., Gertler, M., Hülso, C., Rössig, H., Horn, P., Seybold, J., Lunow, S., Bothmann, M., Barrera-Pesek, A., & Mayrdorfer, M. (2021). Accuracy of a Novel SARS-CoV-2 Antigen-Detecting Rapid Diagnostic Test from Standardized Self-Collected Anterior Nasal Swabs. Journal of Clinical Medicine, 10(10), 2099. https://doi.org/10.3390/jcm10102099






