Convalescent Plasma Efficacy in Life-Threatening COVID-19 Patients Admitted to the ICU: A Retrospective Cohort Study
Abstract
:1. Background
2. Methods
2.1. Outcome Measures
2.2. Convalescent Plasma Procurement
2.3. Antibody Titer Determination
2.4. Statistical Analysis
3. Results
3.1. Study Population
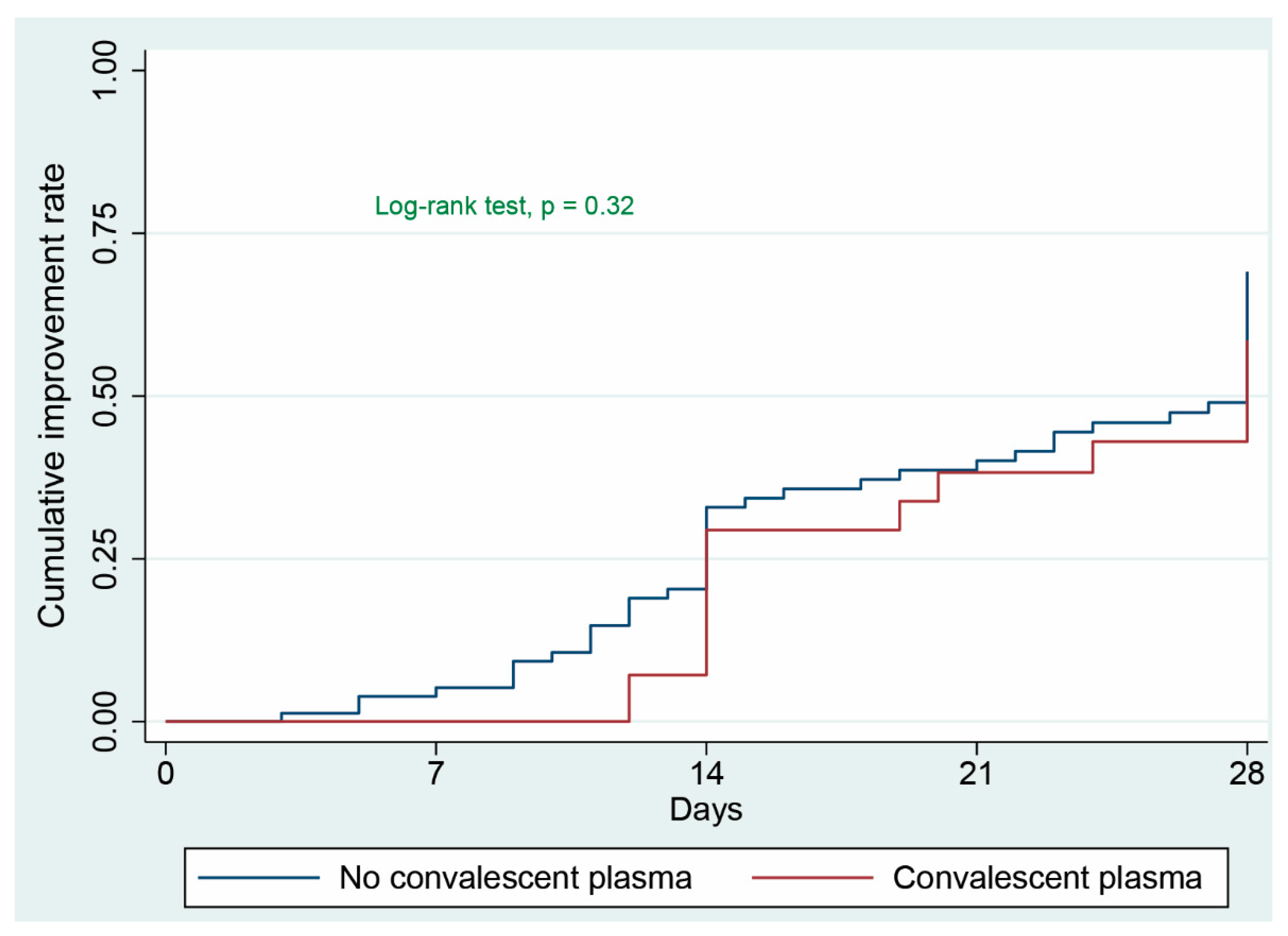
3.2. Primary Clinical Outcome
3.3. Secondary Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- WHO Coronavirus Disease Dashboard. Available online: https://covid19.who.int (accessed on 1 March 2021).
- Covid19 Hospitalization Forcasts: Cdc.gov. Available online: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/hospitalizations-forecasts.html (accessed on 1 March 2021).
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E. Remdesivir for the treatment of Covid-19—Prelimiary report: Reply. N Engl. J. Med. 2020, 383, 994. [Google Scholar] [CrossRef] [PubMed]
- The RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- Luke, T.C.; Kilbane, E.M.; Jackson, J.L.; Hoffman, S.L. Meta-analysis. convalescent blood products for Spanish influenza pneumonia: A future H5N1 treatment? Ann. Intern. Med. 2006, 145, 599–609. [Google Scholar] [CrossRef]
- Yeh, K.M.; Chiueh, T.S.; Siu, L.K.; Lin, J.C.; Chan, P.K.; Peng, M.Y.; Wan, H.L.; Chen, J.H.; Hu, B.S.; Perng, C.L.; et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J. Antimicrob. Chemother. 2005, 56, 919–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arabi, Y.M.; Hajeer, A.H.; Luke, T.; Raviprakash, K.; Balkhy, H.; Johani, S.; Al-Dawood, A.; Al-Qahtani, S.; Al-Omari, A.; Al-Hameed, F.; et al. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerg. Infect. Dis. 2016, 22, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.; Senefeld, J.; Mills, J.R.; Johnson, P.W.; Theel, E.S.; Wiggins, C.C.; Bruno, K.A.; Klompas, A.M.; Lesser, E.R.; Kunze, K.L. Effect of Convalescent Plasma on Mortality among Hospitalized Patients with COVID-19: Initial Three-Month Experience. MedRxiv 2020. [Google Scholar] [CrossRef]
- Joyner, M.J.; Carter, R.E.; Senefeld, J.W.; Klassen, S.A.; Mills, J.R.; Johnson, P.W.; Theel, E.S.; Wiggins, C.C.; Bruno, K.A.; Klompas, A.M.; et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N. Engl. J. Med. 2021, 384, 1015–1027. [Google Scholar] [CrossRef]
- Libster, R.; Pérez Marc, G.; Wappner, D.; Coviello, S.; Bianchi, A.; Braem, V.; Esteban, I.; Caballero, M.T.; Wood, C.; Berrueta, M.; et al. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N. Engl. J. Med. 2021, 384, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mukherjee, A.; Kumar, G.; Chatterjee, P.; Bhatnagar, T.; Malhotra, P. Convalescent plasma in the management of moderate covid-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ 2020, 371, m3939. [Google Scholar] [CrossRef]
- Simonovich, V.A.; Burgos Pratx, L.D.; Scibona, P.; Beruto, M.V.; Vallone, M.G.; Vázquez, C.; Savoy, N.; Giunta, D.H.; Pérez, L.G.; Sánchez, M.D.L.; et al. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N. Engl. J. Med. 2021, 384, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, W.; Hu, Y.; Tong, X.; Zheng, S.; Yang, J.; Kong, Y.; Ren, L.; Wei, Q.; Mei, H.; et al. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients with Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Janiaud, P.; Axfors, C.; Schmitt, A.M.; Gloy, V.; Ebrahimi, F.; Hepprich, M.; Smith, E.R.; Haber, N.A.; Khanna, N.; Moher, D.; et al. Association of Convalescent Plasma Treatment with Clinical Outcomes in Patients With COVID-19: A Systematic Review and Meta-analysis. JAMA 2021, 325, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, P.; Righy, C.; Gadelha, M.; Bozza, F.A.; Bozza, P.T.; Gonçalves, B.; Bastos, L.S.; Vale, A.M.; Higa, L.M.; Castilho, L.; et al. Effect of Convalescent Plasma in Critically Ill Patients With COVID-19: An Observational Study. Front. Med. (Lausanne) 2021, 8, 630982. [Google Scholar] [CrossRef] [PubMed]
- Omrani, A.S.; Zaqout, A.; Baiou, A.; Daghfal, J.; Elkum, N.; Alattar, R.A.; Bakdach, D.; Abusriwil, H.; Mostafa, A.M.; Alhariri, B.; et al. Convalescent plasma for the treatment of patients with severe coronavirus disease 2019: A preliminary report. J. Med. Virol. 2021, 93, 1678–1686. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Bonelli, F.; Sarasini, A.; Zierold, C.; Calleri, M.; Bonetti, A.; Vismara, C.; Blocki, F.A.; Pallavicini, L.; Chinali, A.; Campisi, D.; et al. Clinical and Analytical Performance of an Automated Serological Test That Identifies S1/S2-Neutralizing IgG in COVID-19 Patients Semiquantitatively. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef] [Green Version]
- Stuart, E.A.; Lee, B.K.; Leacy, F.P. Prognostic score-based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. J. Clin. Epidemiol. 2013, 66, S84–S90. [Google Scholar] [CrossRef]
- Garrido, M.M.; Kelley, A.S.; Paris, J.; Roza, K.; Meier, D.E.; Morrison, R.S.; Aldridge, M.D. Methods for constructing and assessing propensity scores. Health Serv. Res. 2014, 49, 1701–1720. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, J.B.; June, C.H. Cytokine release syndrome in severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casadevall, A.; Pirofski, L.A. The convalescent sera option for containing COVID-19. J. Clin. Investig. 2020, 130, 1545–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharbharan, A.; Jordans, C.C.; GeurtsvanKessel, C.; den Hollander, J.G.; Karim, F.; Mollema, F.P.; Stalenhoef, J.E.; Dofferhoff, A.; Ludwig, I.; Koster, A.; et al. Convalescent plasma for COVID-19: A randomized clinical trial. Medrxiv 2020. [Google Scholar] [CrossRef]
- Joyner, M.J.; Wright, R.S.; Fairweather, D.; Senefeld, J.W.; Bruno, K.A.; Klassen, S.A.; Carter, R.E.; Klompas, A.M.; Wiggins, C.C.; Shepherd, J.R.; et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J. Clin. Investig. 2020, 130, 4791–4797. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef]
- Misset, B.; Hoste, E.; Donneau, A.F.; Grimaldi, D.; Meyfroidt, G.; Moutschen, M.; Compernolle, V.; Gothot, A.; Desmecht, D.; Garigliany, M.; et al. A multicenter randomized trial to assess the efficacy of CONvalescent plasma therapy in patients with Invasive COVID-19 and acute respiratory failure treated with mechanical ventilation: The CONFIDENT trial protocol. BMC Pulm. Med. 2020, 20, 317. [Google Scholar] [CrossRef]
- Hamilton, F.W.; Lee, T.C.; Arnold, D.T.; Lilford, R.; Hemming, K. Is convalescent plasma futile in COVID-19? A Bayesian reanalysis of the RECOVERY randomised controlled trial. MedRxiv 2021. [Google Scholar] [CrossRef]

| Variables | All Patients (n = 110) | Convalescent Plasma (n = 32) | Non-Convalescent Plasma (n = 78) | p-Value |
|---|---|---|---|---|
| Age, year | 49 (40–58) | 50 (43–60) | 46 (39–57) | 0.22 |
| Male, n (%) | 99 (90) | 29 (90.6) | 70 (89.7) | 1.00 |
| Body mass index, kg·m−2 | 26.2 (23.5–30.6) | 25.7 (22.4–27.5) | 27.0 (23.7–31.1) | 0.13 |
| SOFA score | 5 (3–8) | 5 (3–7.5) | 5.5 (3–9) | 0.44 |
| SAPS II score | 31 (24–44) | 30 (23–44) | 31 (24–45) | 0.95 |
| Patients with comorbidities, n (%) | 70 (63.6) | 23 (71.9) | 47 (60.3) | 0.28 |
| Comorbidities distribution, n (%) | ||||
| Diabetes mellitus | 48 (43.6) | 13 (40.6) | 35 (44.9) | 0.68 |
| Hypertension | 45 (40.9) | 14 (43.7) | 31 (39.7) | 0.70 |
| Chronic artery disease | 8 (7.3) | 2 (6.2) | 6 (7.7) | 1.00 |
| Chronic kidney disease | 7 (6.4) | 1 (3.1) | 6 (7.7) | 0.67 |
| Time from symptoms to ICU admission, day | 5 (3.2–7) | 4 (3–6) | 5 (4–8) | 0.04 |
| Vital signs on hospital/ICU admission, day | ||||
| Temperature (max) ≥ 38 °C, n (%) | 48 (43.6) | 16 (50.0) | 32 (41.0) | 0.39 |
| Heart rate (max), beats·min−1 | 105 ± 19 | 107 ± 20 | 104 ± 19 | 0.44 |
| Respiratory rate (max), breaths·min−1 | 32 ± 8 | 36 ± 8 | 31 ± 8 | 0.004 |
| Laboratory data on ICU admission | ||||
| C-reactive protein, mg·L−1 | 139 (63–225) | 121 (49–210) | 141 (64–238) | 0.25 |
| Leucocytes count, ×109·L−1 | 8.9 (6.3–11.9) | 8.4 (5.3–11.3) | 9.3 (7.1–12.0) | 0.22 |
| Lymphocytes count, ×109·L−1 | 0.77 (0.48–1.09) | 0.75 (0.41–1.04) | 0.77 (0.49–1.12) | 0.57 |
| Lymphocytes ≤ 1 × 109·L−1; n (%) | 80 (72.7) | 24 (75.0) | 56 (71.8) | 0.82 |
| Platelet count, ×109·L−1 | 224 (164–298) | 216 (152–298) | 228 (180–299) | 0.51 |
| Procalcitonin, ng·mL−1 | 0.49 (0.20–3.05) | 0.40 (0.16–1.05) | 0.58 (0.21–3.71) | 0.16 |
| INR | 1.2 (1.1–1.2) | 1.1 (1.1–1.2) | 1.2 (1.1–1.3) | 0.044 |
| Activated partial thromboplastin time; s | 34.5 (30.2–35.7) | 34.3 (31.6–38.3) | 35.2 (30.0–39.9) | 0.97 |
| D-dimer, µg·mL−1 (normal reference: < 0.05) | 2.6 (0.9–4.0) | 1.8 (0.7–4.0) | 3.0 (1.2–4.0) | 0.10 |
| D-dimer > 2 µg·mL−1, n (%) | 64 (58.2%) | 15 (46.9) | 49 (62.8) | 0.14 |
| Fibrinogen, g·L−1 | 6.0 (4.8–7.0) | 5.8 (4.7–6.4) | 6.3 (5.0–7.2) | 0.31 |
| Ferritin, µg·L−1 (reference range: 36–480) | 1519 (793–2481) | 1538 (923–2388) | 1484 (749–2501) | 0.93 |
| Interleukin 6, ng·L−1 | 219 (103–899) | 181 (123–823) | 234 (96–904) | 0.85 |
| Alanine aminotransferase, IU·mL−1 | 38 (26–64) | 40 (26–73) | 37 (25–60) | 0.34 |
| Aspartate aminotransferase, IU·mL−1 | 53 (33–91) | 52 (35–84) | 53 (32–91) | 0.89 |
| Total bilirubin, µmol·L−1 | 10.7 (7.5–16.1) | 9.3 (6.9–15.7) | 11.1 (8.1–16.7) | 0.15 |
| Creatinine, µmol·L−1 | 78 (64–144) | 73 (62–89) | 82 (66–167) | 0.12 |
| PaO2/FiO2 ratio, mmHg | 85 (66–144) | 85 (67–121) | 95 (64–158) | 0.41 |
| Lactate levels, mmol·L−1 | 1.4 (1.2–1.7) | 1.5 (1.2–1.8) | 1.4 (1.2–1.7) | 0.35 |
| Treatments, n (%) | ||||
| Invasive mechanical ventilation | 76 (69.1) | 21 (65.3) | 55 (70.5) | 0.61 |
| High flow nasal oxygen therapy | 53 (48.2) | 20 (62.5) | 33 (42.3) | 0.05 |
| Non-invasive ventilation | 44 (40) | 19 (59.4) | 25 (32.0) | 0.008 |
| Vasopressor support | 68 (61.8) | 21 (65.6) | 47 (60.3) | 0.60 |
| Renal replacement therapy | 28 (25.5) | 6 (18.7) | 22 (28.2) | 0.30 |
| Extracorporeal membrane oxygenation | 9 (8.2) | 3 (9.4) | 6 (7.7) | 0.72 |
| Tocilizumab | 94 (85.5) | 27 (84.4) | 67 (85.9) | 1.00 |
| Hydroxychloroquine | 45 (40.9) | 10 (31.2) | 35 (44.9) | 0.19 |
| Favipiravir | 28 (25.5) | 8 (25.0) | 20 (25.6) | 1.00 |
| Lopinavir/ritonavir | 30 (27.3) | 7 (21.9) | 23 (29.5) | 0.49 |
| Methylprednisolone | 42 (38.2) | 15 (46.9) | 27 (34.6) | 0.23 |
| WHO 6-point disease severity scale on ICU admission, n (%) | ||||
| Scale 4 | 53 (48.2) | 21 (65.6) | 36 (46.1) | 0.06 |
| Scale 5 | 57 (51.8) | 11 (34.4) | 42 (53.8) |
| Variables | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Convalescent plasma therapy (refer: no) | 0.53 | 0.23–1.22 | 0.14 |
| Age, year | 0.98 | 0.95–1.00 | 0.15 |
| Male, (refer: female) | 1.16 | 0.28–4.74 | 0.83 |
| BMI, kg·m−2 | 0.97 | 0.90–1.04 | 0.38 |
| SOFA score | 1.03 | 0.90–1.18 | 0.68 |
| Lactate, mmol·L−1 | 0.76 | 0.40–1.44 | 0.40 |
| Leucocytes count, ×109·L−1 | 1.00 | 0.93–1.09 | 0.88 |
| PaO2/FiO2 ratio, mmHg | 1.00 | 0.99–1.00 | 0.79 |
| D-dimer, µg·mL−1 | 0.95 | 0.69–1.31 | 0.77 |
| Aspartate aminotransferase, IU·mL−1 | 0.99 | 0.98–0.99 | 0.03 |
| Time from symptoms onset to convalescent plasma infusion, day | 1.00 | 0.92–1.10 | 0.85 |
| Invasive mechanical ventilation, (refer: HFNO/NIV) | 0.59 | 0.23–1.50 | 0.27 |
| Vasopressor support, (refer: no) | 0.43 | 0.20–0.96 | 0.04 |
| Renal replacement therapy, (refer: no) | 0.36 | 0.12–1.08 | 0.07 |
| Methylprednisolone, (refer: no) | 1.05 | 0.58–1.91 | 0.87 |
| Tocilizumab, (refer: no) | 1.02 | 0.34–3.10 | 0.97 |
| Extracorporeal oxygenation membrane, (refer: no) | - | - | - |
| Comorbidities, (refer: no) | 0.82 | 0.45–1.49 | 0.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuzakouk, M.; Saleh, K.; Algora, M.; Nusair, A.; Alameri, J.; Alshehhi, F.; Alkhaja, S.; Badr, M.; Abdallah, K.; De Oliveira, B.; et al. Convalescent Plasma Efficacy in Life-Threatening COVID-19 Patients Admitted to the ICU: A Retrospective Cohort Study. J. Clin. Med. 2021, 10, 2113. https://doi.org/10.3390/jcm10102113
Abuzakouk M, Saleh K, Algora M, Nusair A, Alameri J, Alshehhi F, Alkhaja S, Badr M, Abdallah K, De Oliveira B, et al. Convalescent Plasma Efficacy in Life-Threatening COVID-19 Patients Admitted to the ICU: A Retrospective Cohort Study. Journal of Clinical Medicine. 2021; 10(10):2113. https://doi.org/10.3390/jcm10102113
Chicago/Turabian StyleAbuzakouk, Mohamed, Khaled Saleh, Manuel Algora, Ahmad Nusair, Jawahir Alameri, Fatema Alshehhi, Sara Alkhaja, Mohamed Badr, Khaled Abdallah, Bruno De Oliveira, and et al. 2021. "Convalescent Plasma Efficacy in Life-Threatening COVID-19 Patients Admitted to the ICU: A Retrospective Cohort Study" Journal of Clinical Medicine 10, no. 10: 2113. https://doi.org/10.3390/jcm10102113
APA StyleAbuzakouk, M., Saleh, K., Algora, M., Nusair, A., Alameri, J., Alshehhi, F., Alkhaja, S., Badr, M., Abdallah, K., De Oliveira, B., Nadeem, A., Varghese, Y., Munde, D., Salam, S., Abduljawad, B., Elkambergy, H., Wahla, A., Taha, A., Dibu, J., ... Mallat, J. (2021). Convalescent Plasma Efficacy in Life-Threatening COVID-19 Patients Admitted to the ICU: A Retrospective Cohort Study. Journal of Clinical Medicine, 10(10), 2113. https://doi.org/10.3390/jcm10102113







