Malocclusion of Molar Teeth Is Associated with Activities of Daily Living Loss and Delirium in Elderly Critically Ill Older Patients
Abstract
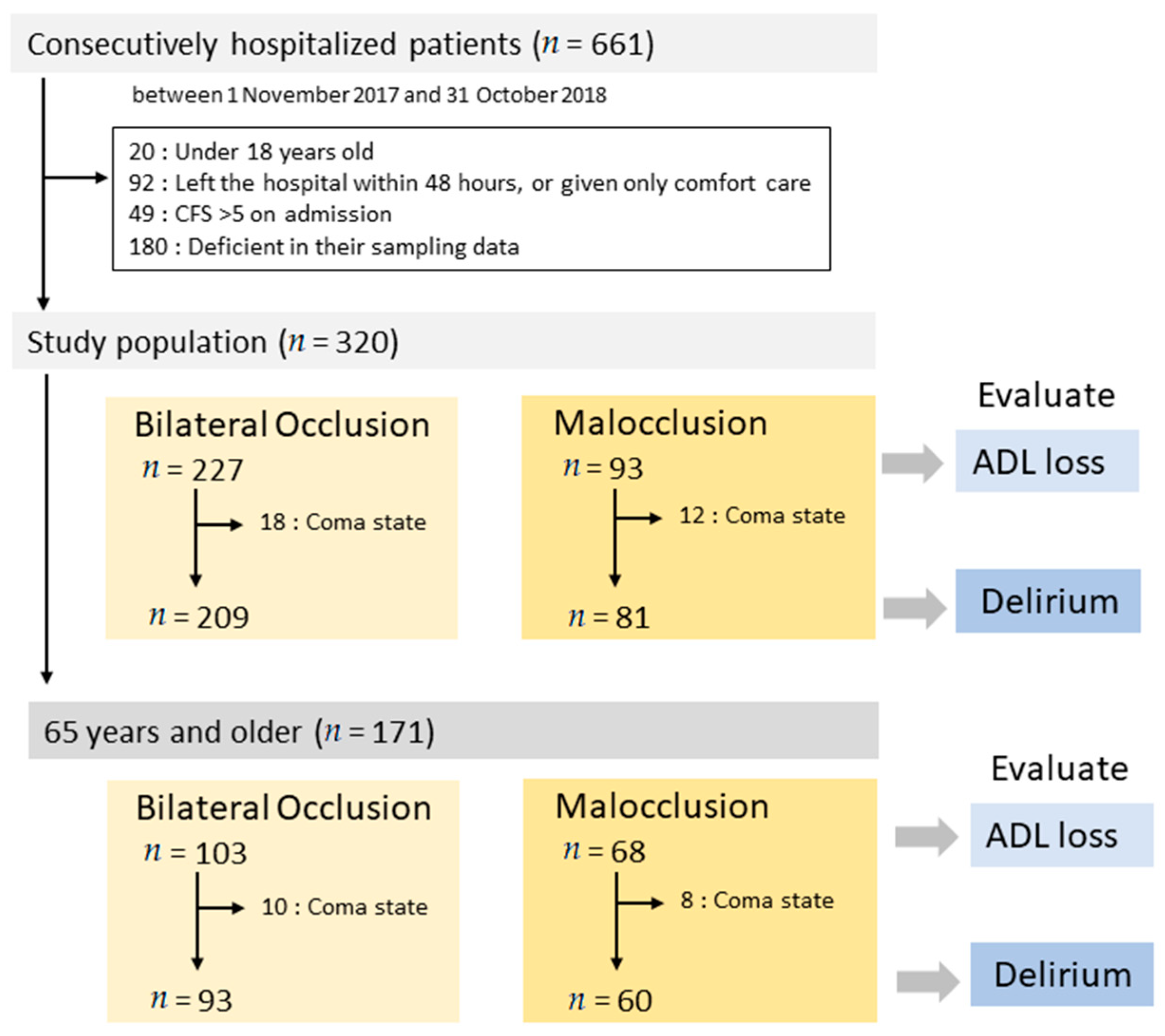
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants and Data Sources
2.3. Definitions
2.3.1. Bilateral Occlusion and Malocclusion
2.3.2. ADL Loss
2.3.3. Neurological Disorders
2.3.4. Delirium
2.4. Exposure and Outcome Measurement
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| 1. Very Fit—People who are robust, active, energetic, and motivated. People in this group exercise regularly and are among the fittest for their age. |
| 2. Well—These people have no active disease symptoms but are less fit compared to those in category 1. They occasionally exercise or engage in activity, e.g., seasonally. |
| 3. Managing well—People whose medical problems are well controlled; however, they are not regularly active beyond routine walking. |
| 4. Vulnerable—While these people are not dependent on others for daily help, their symptoms often limit their activities. A common complaint is feeling “slowed down” and/or being tired during the day. |
| 5. Mildly frail—These people often have more apparent slowing and need help with high order instrumental ADLs (finances, transportation, heavy housework, medications). Mild frailty progressively impairs the ability to shop, walk outside alone, prepare meals, and do housework. |
| 6. Moderately frail—These people need help with all outside activities and with keeping house. They often have problems with stairs, need help with bathing, and might need minimal assistance (cueing, standby) with dressing. |
| 7. Severely frail—These people are completely dependent on others for their personal care impairment. Even so, they appear to be stable and not at high risk of dying (within 6 months). |
| 8. Very Severely frail—These people are completely dependent on others for their personal care as they are approaching the end of life. Typically, they could not even recover from a minor illness. |
| 9. Terminally ill—These people are approaching the end of life. This category applies to people with a life expectancy <6 months who are not otherwise obviously frail. |
Appendix B
| Bilateral | Malocclusion | ||||||
|---|---|---|---|---|---|---|---|
| CFS on Admission | n | CFS at Discharge (Difference of CFS) | Event (%) | n | CFS at Discharge (CFS Different) | Event (%) | p Value |
| 1 | 0.34 | ||||||
| 21 | 1 (0) | 0 | 2 | 1 (0) | 0 | ||
| 2 (1) | 7 (33) | 2 (1) | 0 | ||||
| 3 (2) | 8 (38) | 3 (2) | 1 (50) | ||||
| 4 (3) | 3 (14) | 4 (3) | 0 | ||||
| 5 (4) | 1 (5) | 5 (4) | 1 (50) | ||||
| 6–9 (5–8) | 2 (10) | 6–9 (5–8) | 0 | ||||
| 2 | 0.09 | ||||||
| 83 | 2 (0) | 16 (19) | 19 | 2 (0) | 1 (5) | ||
| 3 (1) | 34 (41) | 3 (1) | 4 (21) | ||||
| 4 (2) | 14 (17) | 4 (2) | 6 (32) | ||||
| 5 (3) | 8 (10) | 5 (3) | 4 (21) | ||||
| 6–9 (4–7) | 11 (13) | 6–9 (4–6) | 4 (21) | ||||
| 3 | 0.02 | ||||||
| 75 | 3 (0) | 23 (31) | 38 | 3 (0) | 3 (8) | ||
| 4 (1) | 22 (29) | 4 (1) | 11 (29) | ||||
| 5 (2) | 14 (19) | 5 (2) | 8 (21) | ||||
| 6–9 (3–6) | 16 (21) | 6–9 (3–5) | 16 (42) | ||||
| 4 | 0.14 | ||||||
| 32 | 4 (0) | 11 (34) | 25 | 4 (0) | 7 (25) | ||
| 5 (1) | 8 (25) | 5 (1) | 2 (8) | ||||
| 6–9 (2–5) | 13 (41) | 6–9 (2–4) | 16 (64) | ||||
| 5 | 0.20 | ||||||
| 18 | 5 (0) | 7 (39) | 9 | 5 (0) | 1 (11) | ||
| 6–9 (1–4) | 11 (61) | 6–9 (1–3) | 8 (89) | ||||
Appendix C
| p for Interaction | |
|---|---|
| ADL loss | |
| Age*Mal | <0.01 |
| Sex (male)*Mal | 0.07 |
| APACHE*Mal | <0.01 |
| Neuro*Mal | 0.45 |
| Delirium | |
| Age*Mal | <0.01 |
| Sex (male)*Mal | 0.438 |
| APACHE*Mal | <0.01 |
| Neuro*Mal | 0.11 |
References
- Martin, G.S.; Mannino, D.M.; Moss, M. The effect of age on the development and outcome of adult sepsis. Crit. Care Med. 2006, 34, 15–21. [Google Scholar] [CrossRef]
- Heyland, D.; Cook, D.; Bagshaw, S.M.; Garland, A.; Stelfox, H.T.; Mehta, S.; Dodek, P.; Kustogiannis, J.; Burns, K.; Muscedere, J. The very elderly admitted to ICU: A quality finish? Crit. Care Med. 2015, 4313, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Muscedere, J.; Waters, B.; Varambally, A.; Bagshaw, S.M.; Boyd, J.G.; Maslove, D.; Sibley, S.; Rockwood, K. The impact of frailty on intensive care unit outcomes: A systematic review and meta-analysis. Intensiv. Care Med. 2017, 43, 1105–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ely, E.W.; Gautam, S.; Margolin, R.; Francis, J.; May, L.; Speroff, T.; Truman, B.; Dittus, R.; Bernard, G.; Inouye, S.K. The impact of delirium in the intensive care unit on hospital length of stay. Intensiv. Care Med. 2001, 27, 1892–1900. [Google Scholar] [CrossRef]
- Tôrres, L.H.D.N.; Tellez, M.; Hilgert, J.B.; Hugo, F.N.; Sousa, M.D.L.R.D.; Ismail, A.I. Frailty, Frailty Components, and Oral Health: A Systematic Review. J. Am. Geriatr. Soc. 2015, 63, 2555–2562. [Google Scholar] [CrossRef]
- Watanabe, Y.; Hirano, H.; Arai, H.; Morishita, S.; Ohara, Y.; Edahiro, A.; Murakami, M.; Pt, H.S.; Kikutani, T.; Suzuki, T. Relationship between Frailty and Oral Function in Community-Dwelling Elderly Adults. J. Am. Geriatr. Soc. 2017, 65, 66–76. [Google Scholar] [CrossRef]
- Abnet, C.C.; Qiao, Y.-L.; Dawsey, S.M.; Dong, Z.-W.; Taylor, P.R.; Mark, S.D. Tooth loss is associated with increased risk of total death and death from upper gastrointestinal cancer, heart disease, and stroke in a Chinese population-based cohort. Int. J. Epidemiol. 2005, 34, 467–474. [Google Scholar] [CrossRef]
- Baldomero, A.K.; Siddiqui, M.; Lo, C.-Y.; Petersen, A.A.; Pragman, A.E.; Connett, J.; Kunisaki, K.M.; Wendt, C.H. The relationship between oral health and COPD exacerbations. Int. J. Chronic Obs. Pulm. Dis. 2019, ume 14, 881–892. [Google Scholar] [CrossRef] [Green Version]
- Leite, R.S.; Marlow, N.M.; Fernandes, J.K.; Hermayer, K. Oral Health and Type 2 Diabetes. Am. J. Med. Sci. 2013, 345, 271–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shwe, P.S.; Ward, S.A.; Thein, P.M.; Junckerstorff, R. Frailty, oral health and nutrition in geriatrics inpatients: A cross-sectional study. Gerodontology 2019, 36, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Daly, B.; Thompsell, A.; Sharpling, J.; Rooney, Y.M.; Hillman, L.; Wanyonyi, K.L.; White, S.; Gallagher, J.E. Evidence summary: The relationship between oral health and dementia. Br. Dent. J. 2017, 223, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Farmer, J.; Phillips, R.C.; Singhal, S.; Quiñonez, C. Inequalities in oral health: Understanding the contributions of education and income. Can. J. Public Health 2017, 108, e240–e245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, Y.-K.; Galobardes, B.; Smith, G.D.; McCarron, P.; Jeffreys, M.; Gilthorpe, M.S. Associations between tooth loss and mortality patterns in the Glasgow Alumni Cohort. Heart 2007, 93, 1098–1103. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.W. Complete Edentulism Prior to the Age of 65 Years is Associated with All-Cause Mortality. J. Public Health Dent. 2009, 69, 260–266. [Google Scholar] [CrossRef]
- Ansai, T.; Takata, Y.; Soh, I.; Awano, S.; Yoshida, A.; Sonoki, K.; Hamasaki, T.; Torisu, T.; Sogame, A.; Shimada, N.; et al. Relationship between tooth loss and mortality in 80-year-old Japanese community-dwelling subjects. BMC Public Health 2010, 10, 386. [Google Scholar] [CrossRef] [Green Version]
- Imamura, Y.; Sato, Y.; Kitagawa, N.; Uchida, K.; Osawa, T.; Omori, M.; Okada, Y. Influence of occlusal loading force on occlusal contacts in natural dentition. J. Prosthodont. Res. 2015, 59, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Ikebe, K.; Matsuda, K.-I.; Kagawa, R.; Enoki, K.; Yoshida, M.; Maeda, Y.; Nokubi, T. Association of masticatory performance with age, gender, number of teeth, occlusal force and salivary flow in Japanese older adults: Is ageing a risk factor for masticatory dysfunction? Arch. Oral Biol. 2011, 56, 991–996. [Google Scholar] [CrossRef]
- Ikebe, K.; Matsuda, K.-I.; Murai, S.; Maeda, Y.; Nokubi, T. Validation of the Eichner index in relation to occlusal force and masticatory performance. Int. J. Prosthodont. 2011, 23, 521–524. [Google Scholar]
- Rockwood, K.; Song, X.; Macknight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. Can. Med. Assoc. J. 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, A.; Haas, B.; Ringer, T.J.; McFarlan, A.; Wong, C.L. Canadian Study of Health and Aging Clinical Frailty Scale: Does It Predict Adverse Outcomes among Geriatric Trauma Patients? J. Am. Coll. Surg. 2017, 225, 658–665. [Google Scholar] [CrossRef]
- Ely, E.W.; Shintani, A.; Truman, B.; Speroff, T.; Gordon, S.M.; Harrell, J.F.E.; Inouye, S.K.; Bernard, G.R.; Dittus, R.S. Delirium as a Predictor of Mortality in Mechanically Ventilated Patients in the Intensive Care Unit. JAMA 2004, 291, 1753–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghighi, A.; Shafipour, V.; Bagheri-Nesami, M.; Baradari, A.G.; Charati, J.Y. The impact of oral care on oral health status and prevention of ventilator-associated pneumonia in critically ill patients. Aust. Crit. Care 2017, 30, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Capasso, B.; Pezzatini, M.; Cinquepalmi, M.; Antonelli, M.S.; Garaceni, G.; Rampini, A.; Cardella, S.; Castagnola, G.; Maggi, S. Is the social status a new prognostic factor in the Fournier’s gangrene? G. Chir. 2019, 40, 141–144. [Google Scholar] [PubMed]
- Giannico, O.V.; Ambrosino, I.; Patano, F.; Germinario, C.; Quarto, M.; Moretti, A.M. Educational level, marital status and sex as social gender discharge determinants in chronic obstructive pulmonary disease exacerbations: A time-to-event analysis. Monaldi Arch. Chest Dis. 2019, 89. [Google Scholar] [CrossRef]
- Tanaka, T.; Takahashi, K.; Hirano, H.; Kikutani, T.; Watanabe, Y.; Ohara, Y.; Furuya, H.; Tetsuo, T.; Akishita, M.; Iijima, K. Oral Frailty as a Risk Factor for Physical Frailty and Mortality in Community-Dwelling Elderly. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2018, 73, 1661–1667. [Google Scholar] [CrossRef]
- Areso, M.P.; Giralt, M.T.; Sainz, B.; Prieto, M.; García-Vallejo, P.; Gómez, F.M. Occlusal disharmonies modulate central catechola-minergic activity in the rat. J. Dent. Res. 1999, 78, 1204–1213. [Google Scholar] [CrossRef]
- Furuta, M.; Komiya-Nonaka, M.; Akifusa, S.; Shimazaki, Y.; Adachi, M.; Kinoshita, T.; Kikutani, T.; Yamashita, Y. Interrelationship of oral health status, swallowing function, nutritional status, and cognitive ability with activities of daily living in Japanese elderly people receiving home care services due to physical disabilities. Community Dent. Oral Epidemiol. 2012, 41, 173–181. [Google Scholar] [CrossRef]
- van den Boogaard, M.; Pickkers, P.; Slooter, A.J. Development and validation of PRE-DELIRIC (PREdiction of DELIRium in ICu patients) delirium prediction model for intensive care patients: Observational multicentre study. BMJ 2012, 344, e420. [Google Scholar] [CrossRef] [Green Version]
- Choutko-Joaquim, S.; Tacchini-Jacquier, N.; Pralong D’Alessio, G.; Verloo, H. Associations between Frailty and Delirium among Older Patients Admitted to an Emergency Department. Dement. Geriatr. Cogn. Dis. Extra 2019, 9, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Plassman, B.; Crout, J.R.; Liang, J. Cognitive function and oral health among community-dwelling older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Tsakos, G.; Watt, R.G.; Rouxel, P.L.; De Oliveira, C.; Demakakos, P. Tooth Loss Associated with Physical and Cognitive Decline in Older Adults. J. Am. Geriatr. Soc. 2015, 63, 91–99. [Google Scholar] [CrossRef]
- Lucke, J.A.; De Gelder, J.; Blomaard, L.C.; Fogteloo, A.J.; Alsma, J.; Schuit, S.C.; Brink, A.; De Groot, B.; Blauw, G.J.; Mooijaart, S.P. CAM-ICU may not be the optimal screening tool for early delirium screening in older emergency department patients: A prospective cohort study. Eur. J. Emerg. Med. 2019, 26, 428–432. [Google Scholar] [CrossRef]
- Needham, D.M.; Davidson, J.; Cohen, H.; Hopkins, R.O.; Weinert, C.; Wunsch, H.; Zawistowski, C.; Bemis-Dougherty, A.; Berney, S.C.; Bienvenuet, O.J.; et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit. Care Med. 2012, 40, 502–509. [Google Scholar] [CrossRef]
- Milbrandt, E.B.; Deppen, S.; Harrison, P.L.; Shintani, A.K.; Speroff, T.; Stiles, R.A.; Truman, B.; Bernard, G.R.; Dittus, R.S.; Ely, E.W. Costs associated with delirium in mechanically ventilated patients. Crit. Care Med. 2004, 32, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Girard, T.D.; Jackson, J.C.; Pandharipande, P.P.; Pun, B.T.; Thompson, J.L.; Shintani, A.K.; Gordon, S.M.; Canonico, A.E.; Dittus, R.S.; Bernard, G.R.; et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit. Care Med. 2010, 38, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Gil-Montoya, J.A.; Sanchez-Lara, I.; Carnero-Pardo, C.; Fornieles, F.; Montes, J.; Vilchez, R.; Burgos, J.S.; Gonzalez-Moles, M.A.; Barrios, R.; Bravo, M. Is Periodontitis a Risk Factor for Cognitive Impairment and Dementia? A Case-Control Study. J. Periodontol. 2015, 86, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-S.; Shin, M.-S.; Ahn, Y.-B.; Choi, B.-Y.; Nam, J.-H.; Kim, H.-D. Periodontitis Is Associated with Cognitive Impairment in Elderly Koreans: Results from the Yangpyeong Cohort Study. J. Am. Geriatr. Soc. 2016, 64, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Holmes, C.; Butchart, J. Systemic inflammation and Alzheimer’s disease. Biochem. Soc. Trans. 2011, 39, 898–901. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.; Gatz, M.; Crimmins, E.M. Inflammation as a potential mediator for the association between periodontal disease and Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2008, 4, 865–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, F.E. A critical review of vitamin C for the prevention of age-related cognitive decline and Alzheimer’s disease. J. Alzheimers Dis. 2012, 29, 711–726. [Google Scholar] [CrossRef] [Green Version]
- Larrieu, S.; Letenneur, L.; Helmer, C.; Dartigues, J.F.; Barberger-Gateau, P. Nutritional factors and risk of incident dementia in the PAQUID longitudinal cohort. J. Nutr. Health Aging 2004, 8, 150–154. [Google Scholar]
- Onozuka, M.; Fujita, M.; Watanabe, K.; Hirano, Y.; Niwa, M.; Nishiyama, K.; Saito, S. Mapping brain region activity during chewing: A functional magnetic resonance imaging study. J. Dent. Res. 2002, 81, 743–746. [Google Scholar] [CrossRef]
- Momose, T.; Nishikawa, J.; Watanabe, T.; Sasaki, Y.; Senda, M.; Kubota, K.; Sato, Y.; Funakoshi, M.; Minakuchi, S. Effect of mastication on regional cerebral blood flow in humans examined by pos-itron-emission tomography with 15O-labelled water and magnetic resonance imaging. Arch. Oral. Biol. 1997, 42, 57–61. [Google Scholar] [CrossRef]

| Variables | Total | Bilateral Occlusion | Malocclusion | p Value |
|---|---|---|---|---|
| N | 320 | 227 | 93 | |
| Age (years) | 69 (57–79) | 62 (48–74) | 69 (64–77) | <0.01 * |
| Sex (male) (%) | 179 (56) | 128 (57) | 51 (54) | 0.62 # |
| CFS on admission (%) | <0.01 ## | |||
| Scale 1 | 21 (7) | 19 (8) | 2 (2) | |
| Scale 2 | 101 (32) | 83 (37) | 19 (20) | |
| Scale 3 | 113 (35) | 75 (33) | 38 (41) | |
| Scale 4 | 57 (18) | 32 (14) | 25 (27) | |
| Scale 5 | 27 (8) | 18 (8) | 9 (10) | |
| APACHE II score | 15 (11–21) | 13 (10–18) | 17 (13–24) | <0.01 * |
| Neurological disorders (%) | 128 (40) | 87 (39) | 41 (43) | 0.46 # |
| Death in Hospital (%) | 16 (5) | 9 (4) | 7 (7) | 0.35 # |
| ADL loss (%) | 93 (29) | 50 (22) | 44 (46) | <0.01 # |
| VFDs (mean ± SD, median) | 21.2 ± 9.6, 27 | 22.6 ± 8.4, 27 | 18.1 ± 11.2, 24 | 0.50 * |
| N | 290 | 209 | 81 | |
| Delirium (%) | 68 (23) | 39 (19) | 29 (36) | <0.01 # |
| Variables | Total | Bilateral Occlusion | Malocclusion | p Value |
|---|---|---|---|---|
| N | 171 | 103 | 68 | |
| Age (years) | 75 (69–82) | 76 (69–82) | 74.5 (69–79) | 0.20 * |
| Sex (male) (%) | 83 (49) | 50 (49) | 33 (49) | 1.00 # |
| CFS on admission (%) | <0.01 ## | |||
| Scale 1 | 0 | 0 | 0 | |
| Scale 2 | 29 (17) | 22 (21) | 7 (10) | |
| Scale 3 | 73 (43) | 41 (40) | 32 (47) | |
| Scale 4 | 45 (26) | 24 (23) | 21 (31) | |
| Scale 5 | 24 (14) | 16 (16) | 8 (12) | |
| APACHE II score | 16 (13–22) | 15 (12–19) | 19 (13–24) | <0.01 * |
| Neurological disorders (%) | 74 (43) | 49 (48) | 25 (37) | 0.16 # |
| Death in Hospital (%) | 10 (6) | 4 (4) | 4 (6) | 0.42 # |
| ADL loss (%) | 58 (34) | 26 (25) | 32 (47) | <0.01 # |
| N | 153 | 93 | 60 | |
| Delirium (%) | 49 (32) | 29 (31) | 20 (33) | 0.30 # |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variables | Crude OR | 95% CI | p Value | Adjusted OR | 95% CI | p Value |
| Age | 1.03 | 1.00–1.04 | <0.01 | 1.00 | 0.98–1.02 | 0.94 |
| Sex (male) | 1.03 | 0.63–1.67 | 0.89 | 1.30 | 0.74–2.31 | 0.35 |
| CFS on admission | 2.19 | 1.48–5.77 | <0.01 | 1.94 | 1.40–2.71 | <0.01 |
| APACHE II score | 1.12 | 1.04–2.71 | <0.01 | 1.09 | 1.05–1.14 | <0.01 |
| Neurological disorders | 2.03 | 1.24–3.30 | <0.01 | 2.55 | 1.44–4.57 | <0.01 |
| Malocclusion | 3.02 | 1.81–5.03 | <0.01 | 2.03 | 1.13–3.64 | 0.02 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variables | Crude OR | 95% CI | p Value | Adjusted OR | 95% CI | p Value |
| Age | 1.09 | 1.04–1.14 | <0.01 | 1.08 | 1.02–1.15 | <0.01 |
| Sex (male) | 0.89 | 0.47–1.67 | 0.71 | 1.11 | 0.51–2.40 | 0.80 |
| CFS on admission | 2.37 | 1.60–3.50 | <0.01 | 2.20 | 1.38–3.49 | <0.01 |
| APACHE II score | 1.09 | 1.03–1.14 | <0.01 | 1.06 | 1.00–1.12 | 0.04 |
| Neurological disorders | 1.87 | 0.99–3.55 | 0.06 | 2.86 | 1.32–6.23 | <0.01 |
| Malocclusion | 2.63 | 1.37–5.05 | <0.01 | 3.25 | 1.44–7.32 | <0.01 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variables | Crude OR | 95% CI | p Value | Adjusted OR | 95% CI | p Value |
| Age | 1.03 | 1.01–1.05 | <0.01 | 1.01 | 0.99–1.03 | 0.37 |
| Sex (male) | 0.89 | 0.56–1.43 | 0.64 | 1.04 | 0.61–1.76 | 0.88 |
| CFS on admission | 1.78 | 1.39–2.26 | <0.01 | 1.40 | 1.05–1.95 | 0.02 |
| APACHE II score | 1.11 | 1.07–1.15 | <0.01 | 1.09 | 1.05–1.13 | <0.01 |
| Neurological disorders | 1.82 | 1.13–2.94 | <0.01 | 1.97 | 1.16–3.36 | 0.01 |
| Malocclusion | 2.14 | 1.29–3.53 | <0.01 | 1.33 | 0.76–2.34 | 0.32 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variables | Crude OR | 95% CI | p Value | Adjusted OR | 95% CI | p Value |
| Age | 1.10 | 1.05–1.16 | <0.01 | 1.13 | 1.06–1.20 | <0.01 |
| Sex (male) | 0.89 | 0.48–1.67 | 0.72 | 1.18 | 0.55–2.54 | 0.68 |
| CFS on admission | 1.32 | 0.94–1.87 | 0.11 | 1.01 | 0.65–1.58 | 0.97 |
| APACHE II score | 1.06 | 1.01–1.10 | 0.01 | 1.07 | 1.01–1.13 | 0.02 |
| Neurological disorders | 4.03 | 2.07–7.84 | <0.01 | 5.82 | 2.63–12.90 | <0.01 |
| Malocclusion | 1.92 | 1.01–3.64 | 0.05 | 2.61 | 1.14–5.95 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujinami, Y.; Hifumi, T.; Ono, Y.; Saito, M.; Okazaki, T.; Shinohara, N.; Akiyama, K.; Kunikata, M.; Inoue, S.; Kotani, J.; et al. Malocclusion of Molar Teeth Is Associated with Activities of Daily Living Loss and Delirium in Elderly Critically Ill Older Patients. J. Clin. Med. 2021, 10, 2157. https://doi.org/10.3390/jcm10102157
Fujinami Y, Hifumi T, Ono Y, Saito M, Okazaki T, Shinohara N, Akiyama K, Kunikata M, Inoue S, Kotani J, et al. Malocclusion of Molar Teeth Is Associated with Activities of Daily Living Loss and Delirium in Elderly Critically Ill Older Patients. Journal of Clinical Medicine. 2021; 10(10):2157. https://doi.org/10.3390/jcm10102157
Chicago/Turabian StyleFujinami, Yoshihisa, Toru Hifumi, Yuko Ono, Masafumi Saito, Tomoya Okazaki, Natsuyo Shinohara, Kyoko Akiyama, Misa Kunikata, Shigeaki Inoue, Joji Kotani, and et al. 2021. "Malocclusion of Molar Teeth Is Associated with Activities of Daily Living Loss and Delirium in Elderly Critically Ill Older Patients" Journal of Clinical Medicine 10, no. 10: 2157. https://doi.org/10.3390/jcm10102157
APA StyleFujinami, Y., Hifumi, T., Ono, Y., Saito, M., Okazaki, T., Shinohara, N., Akiyama, K., Kunikata, M., Inoue, S., Kotani, J., & Kuroda, Y. (2021). Malocclusion of Molar Teeth Is Associated with Activities of Daily Living Loss and Delirium in Elderly Critically Ill Older Patients. Journal of Clinical Medicine, 10(10), 2157. https://doi.org/10.3390/jcm10102157







