Needling Interventions for Sciatica: Choosing Methods Based on Neuropathic Pain Mechanisms—A Scoping Review
Abstract
:1. Introduction
2. Methods
2.1. Scoping Review
2.2. Search Strategy
2.3. Inclusion/Exclusion Criteria
2.4. Data Mapping
3. Results
3.1. Effects on Pain Intensity
3.2. Efficacy
3.3. Effectiveness
3.4. Common Needling Practices for Sciatica
4. Discussion
4.1. Disinhibition: Considerations for Needling Interventions
4.2. Needle Manipulation, Mast Cells and Mediators
4.3. Adenosine Modulates Neuropathic Pain
4.4. Intersegmental Approach
4.5. Noradrenergic Modulation of NP
4.6. Restorative Effects of Needling for Neuropathic Pain
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lin, C.W.; Verwoerd, A.J.; Maher, C.G.; Verhagen, A.; Pinto, R.; Luijsterburg, P.; Hancock, M.J. How is radiating leg pain defined in randomized controlled trials of conservative treatments in primary care? A systematic review. Eur. J. Pain 2014, 18, 455–464. [Google Scholar] [CrossRef]
- Stafford, M.A.; Peng, P.; Hill, D.A. Sciatica: A review of history, epidemiology, pathogenesis, and the role of epidural steroid injection in management. Br. J. Anaesth. 2007, 99, 461–473. [Google Scholar] [CrossRef] [Green Version]
- Ropper, A.H.; Zafonte, R.D. Sciatica. N. Engl. J. Med. 2015, 372, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Chen, F.-P. Therapeutic approach of acupuncture for sciatica: A brief review. Neuropsychiatry 2017, 7, 149–155. [Google Scholar]
- Konstantinou, K.; Dunn, K.M. Sciatica: Review of epidemiological studies and prevalence estimates. Spine 2008, 33, 2464–2472. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.K.; Kongsted, A.; Kjaer, P.; Koes, B. Diagnosis and treatment of sciatica. BMJ 2019, 367, l6273. [Google Scholar] [CrossRef]
- Coggon, D.; Ntani, G.; Walker-Bone, K.; Palmer, K.T.; Felli, V.E.; Harari, R.; Barrero, L.H.; Felknor, S.A.; Gimeno, D.; Cattrell, A.; et al. Epidemiological Differences Between Localized and Nonlocalized Low Back Pain. Spine 2017, 42, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Porchet, F.; Wietlisbach, V.; Burnand, B.; Daeppen, K.; Villemure, J.G.; Vader, J.P. Relationship between severity of lumbar disc disease and disability scores in sciatica patients. Neurosurgery 2002, 50, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Maier, C.; Attal, N.; Binder, A.; Bouhassira, D.; Cruccu, G.; Finnerup, N.B.; Haanpaa, M.; Hansson, P.; Hullemann, P.; et al. Peripheral neuropathic pain: A mechanism-related organizing principle based on sensory profiles. Pain 2017, 158, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Stynes, S.; Grovle, L.; Haugen, A.J.; Konstantinou, K.; Grotle, M. New insight to the characteristics and clinical course of clusters of patients with imaging confirmed disc-related sciatica. Eur. J. Pain 2020, 24, 171–181. [Google Scholar] [CrossRef]
- Scholz, J.; Finnerup, N.B.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K.D.; et al. The IASP classification of chronic pain for ICD-11: Chronic neuropathic pain. Pain 2019, 160, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrisson, S.A.; Ogollah, R.; Dunn, K.M.; Foster, N.E.; Konstantinou, K. Prevalence, Characteristics, and Clinical Course of Neuropathic Pain in Primary Care Patients Consulting with Low Back-related Leg Pain. Clin. J. Pain 2020, 36, 813–824. [Google Scholar] [CrossRef]
- Zhi, M.-J.; Liu, K.; Zheng, Z.-L.; He, X.; Li, T.; Sun, G.; Zhang, M.; Wang, F.-C.; Gao, X.-Y.; Zhu, B. Application of the chronic constriction injury of the partial sciatic nerve model to assess acupuncture analgesia. J. Pain Res. 2017, 10, 2271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohno, T.; Moore, K.A.; Baba, H.; Woolf, C.J. Peripheral nerve injury alters excitatory synaptic transmission in lamina II of the rat dorsal horn. J. Physiol. 2003, 548, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Lavertu, G.; Cote, S.L.; De Koninck, Y. Enhancing K-Cl co-transport restores normal spinothalamic sensory coding in a neuropathic pain model. Brain 2014, 137, 724–738. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanyan, S.; Stemkowski, P.L.; Stebbing, M.J.; Smith, P.A. Sciatic chronic constriction injury produces cell-type-specific changes in the electrophysiological properties of rat substantia gelatinosa neurons. J. Neurophysiol. 2006, 96, 579–590. [Google Scholar] [CrossRef]
- Goldman, N.; Chen, M.; Fujita, T.; Xu, Q.; Peng, W.; Liu, W.; Jensen, T.K.; Pei, Y.; Wang, F.; Han, X.; et al. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat. Neurosci. 2010, 13, 883–888. [Google Scholar] [CrossRef] [Green Version]
- Shao, X.M.; Shen, Z.; Sun, J.; Fang, F.; Fang, J.F.; Wu, Y.Y.; Fang, J.Q. Strong Manual Acupuncture Stimulation of “Huantiao” (GB 30) Reduces Pain-Induced Anxiety and p-ERK in the Anterior Cingulate Cortex in a Rat Model of Neuropathic Pain. Evid. Based Complementary Altern. Med. 2015, 2015, 235491. [Google Scholar] [CrossRef] [Green Version]
- Vieira, J.S.; Toreti, J.A.; de Carvalho, R.C.; de Araújo, J.E.; Silva, M.L.; Silva, J.R.T. Analgesic Effects Elicited by Neuroactive Mediators Injected into the ST 36 Acupuncture Point on Inflammatory and Neuropathic Pain in Mice. J. Acupunct. Meridian Stud. 2018, 11, 280–289. [Google Scholar] [CrossRef]
- Cha, M.H.; Choi, J.S.; Bai, S.J.; Shim, I.; Lee, H.J.; Choi, S.M.; Lee, B.H. Antiallodynic effects of acupuncture in neuropathic rats. Yonsei Med. J. 2006, 47, 359–366. [Google Scholar] [CrossRef]
- Cidral-Filho, F.J.; da Silva, M.D.; More, A.O.; Cordova, M.M.; Werner, M.F.; Santos, A.R. Manual acupuncture inhibits mechanical hypersensitivity induced by spinal nerve ligation in rats. Neuroscience 2011, 193, 370–376. [Google Scholar] [CrossRef]
- Liu, C.H.; Kung, Y.Y.; Lin, C.L.; Yang, J.L.; Wu, T.P.; Lin, H.C.; Chang, Y.K.; Chang, C.M.; Chen, F.P. Therapeutic Efficacy and the Impact of the “Dose” Effect of Acupuncture to Treat Sciatica: A Randomized Controlled Pilot Study. J. Pain Res. 2019, 12, 3511–3520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, W.K.; Lau, Y.M.; Zhang, H.Q.; Wong, S.C.; Bian, Z.X. Electroacupuncture versus celecoxib for neuropathic pain in rat SNL model. Neuroscience 2010, 170, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.A.; Williams, N.H.; Sutton, A.J.; Burton, K.; Din, N.U.; Matar, H.E.; Hendry, M.; Phillips, C.J.; Nafees, S.; Fitzsimmons, D.; et al. Comparative clinical effectiveness of management strategies for sciatica: Systematic review and network meta-analyses. Spine J. 2015, 15, 1461–1477. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.; Kwon, O.S.; Bang, S.K.; Kim, D.H.; Baek, M.W.; Ryu, Y.; Bae, J.H.; Fan, Y.; Lee, S.M.; Kim, H.K.; et al. Peripheral Sensory Nerve Tissue but Not Connective Tissue Is Involved in the Action of Acupuncture. Front. Neurosci. 2019, 13, 110. [Google Scholar] [CrossRef] [Green Version]
- Yin, N.; Yang, H.; Yao, W.; Xia, Y.; Ding, G. Mast Cells and Nerve Signal Conduction in Acupuncture. Evid. Based Complementary Alternat. Med. 2018, 2018, 3524279. [Google Scholar] [CrossRef]
- Hsieh, Y.L.; Hong, C.Z.; Liu, S.Y.; Chou, L.W.; Yang, C.C. Acupuncture at distant myofascial trigger spots enhances endogenous opioids in rabbits: A possible mechanism for managing myofascial pain. Acupunct. Med. 2016, 34, 302–309. [Google Scholar] [CrossRef]
- Zhou, K.; Ma, Y.; Brogan, M.S. Dry needling versus acupuncture: The ongoing debate. Acupunct. Med. 2015, 33, 485–490. [Google Scholar] [CrossRef]
- Chou, L.W.; Kao, M.J.; Lin, J.G. Probable mechanisms of needling therapies for myofascial pain control. Evid. Based Complementary Alternat. Med. 2012, 2012, 705327. [Google Scholar] [CrossRef]
- Liu, L.; Skinner, M.A.; McDonough, S.M.; Baxter, G.D. Traditional Chinese Medicine acupuncture and myofascial trigger needling: The same stimulation points? Complementary Ther. Med. 2016, 26, 28–32. [Google Scholar] [CrossRef]
- Baeumler, P.I.; Fleckenstein, J.; Benedikt, F.; Bader, J.; Irnich, D. Acupuncture-induced changes of pressure pain threshold are mediated by segmental inhibition-a randomized controlled trial. Pain 2015, 156, 2245–2255. [Google Scholar] [CrossRef] [PubMed]
- Srbely, J.Z.; Dickey, J.P.; Lee, D.; Lowerison, M. Dry needle stimulation of myofascial trigger points evokes segmental anti-nociceptive effects. J. Rehabil. Med. 2010, 42, 463–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baeumler, P.I.; Fleckenstein, J.; Takayama, S.; Simang, M.; Seki, T.; Irnich, D. Effects of acupuncture on sensory perception: A systematic review and meta-analysis. PLoS ONE 2014, 9, e113731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitner, J.; Westerholz, S.; Heinke, B.; Forsthuber, L.; Wunderbaldinger, G.; Jäger, T.; Gruber-Schoffnegger, D.; Braun, K.; Sandkühler, J. Impaired excitatory drive to spinal GABAergic neurons of neuropathic mice. PLoS ONE 2013, 8, e73370. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Ji, R.R.; Ito, N.; Allchorne, A.J.; Befort, K.; Karchewski, L.A.; Woolf, C.J. Peripheral axonal injury results in reduced mu opioid receptor pre- and post-synaptic action in the spinal cord. Pain 2005, 117, 77–87. [Google Scholar] [CrossRef]
- Keller, A.F.; Beggs, S.; Salter, M.W.; De Koninck, Y. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol. Pain 2007, 3, 27. [Google Scholar] [CrossRef] [Green Version]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Int. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, Y.; Wang, Z.; Wang, C.; Ding, W.; Liu, Z. A Randomized Clinical Trial Comparing the Effectiveness of Electroacupuncture versus Medium-Frequency Electrotherapy for Discogenic Sciatica. Evid. Based Complementary Alternat. Med. 2017, 2017, 9502718. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.K.; Kim, E.; Yoon, K.S.; Jeon, J.H.; Kim, Y.I.; Lee, H.; Kwon, O.; Jung, S.-Y.; Lee, J.-H.; Yang, C. Acupotomy versus Manual Acupuncture for the Treatment of Back and/or Leg Pain in Patients with Lumbar Disc Herniation: A Multicenter, Randomized, Controlled, Assessor-Blinded Clinical Trial. J. Pain Res. 2020, 13, 677. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.-F.; Zheng, X.-Q.; Chen, D.; Lin, J.-L.; Zhou, W.-X.; Wang, H.; Qin, Z.; Wu, A.-M. Can Acupuncture Improve Chronic Spinal Pain? A Systematic Review and Meta-Analysis. Glob. Spine J. 2020, 2192568220962440. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, S.; Zhou, J.; Yao, Q.; Liu, Z. Efficacy and Safety of Acupuncture for Chronic Discogenic Sciatica, a Randomized Controlled Sham Acupuncture Trial. Pain Med. 2019, 20, 2303–2310. [Google Scholar] [CrossRef]
- Lewis, R.; Williams, N.; Matar, H.; Din, N.; Fitzsimmons, D.; Phillips, C.; Jones, M.; Sutton, A.; Burton, K.; Nafees, S.; et al. The clinical effectiveness and cost-effectiveness of management strategies for sciatica: Systematic review and economic model. Health Technol. Assess. 2011, 15, 1–434. [Google Scholar] [CrossRef] [PubMed]
- Luijsterburg, P.A.; Verhagen, A.P.; Ostelo, R.W.; van Os, T.A.; Peul, W.C.; Koes, B.W. Effectiveness of conservative treatments for the lumbosacral radicular syndrome: A systematic review. Eur. Spine J. 2007, 16, 881–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, M.; Wang, X.; Chen, M.; Shen, Y.; Zhang, X.; Yang, J. The Efficacy of Acupuncture for the Treatment of Sciatica: A Systematic Review and Meta-Analysis. Evid. Based Complementary Alternat. Med. 2015, 2015, 192808. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Liu, X.; Wu, J.; Zhai, Y.; Liu, Z. Effectiveness of Acupuncture for Treating Sciatica: A Systematic Review and Meta-Analysis. Evid. Based Complementary Alternat. Med. 2015, 2015, 425108. [Google Scholar] [CrossRef] [Green Version]
- Vickers, A.J.; Vertosick, E.A.; Lewith, G.; MacPherson, H.; Foster, N.E.; Sherman, K.J.; Irnich, D.; Witt, C.M.; Linde, K. Acupuncture for Chronic Pain: Update of an Individual Patient Data Meta-Analysis. J. Pain 2018, 19, 455–474. [Google Scholar] [CrossRef] [Green Version]
- Duplan, B.; Cabanel, G.; Piton, J.L.; Grauer, J.L.; Phelip, X. Acupuncture and sciatica in the acute phase. Double-blind study of 30 cases. Sem. Hop. 1983, 59, 3109–3114. [Google Scholar]
- MacPherson, H.; Maschino, A.C.; Lewith, G.; Foster, N.E.; Witt, C.M.; Vickers, A.J.; Acupuncture Trialists, C. Characteristics of acupuncture treatment associated with outcome: An individual patient meta-analysis of 17,922 patients with chronic pain in randomised controlled trials. PLoS ONE 2013, 8, e77438. [Google Scholar] [CrossRef]
- Jeong, J.K.; Kim, Y.I.; Kim, E.; Kong, H.J.; Yoon, K.S.; Jeon, J.H.; Kang, J.H.; Lee, H.; Kwon, O.; Jung, S.-Y. Effectiveness and safety of acupotomy for treating back and/or leg pain in patients with lumbar disc herniation: A study protocol for a multicenter, randomized, controlled, clinical trial. Medicine 2018, 97, e11951. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, L.Q.; Li, J.L.; Su, X.T.; Yu, F.T.; Shi, G.X.; Yang, J.W.; Liu, C.Z. The Management of Sciatica by Acupuncture: An Expert Consensus Using the Improved Delphi Survey. J. Pain Res. 2021, 14, 13–22. [Google Scholar] [CrossRef]
- Cheng, K.J. Neuroanatomical basis of acupuncture treatment for some common illnesses. Acupunct. Med. 2009, 27, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Ots, T.; Kandirian, A.; Szilagyi, I.; DiGiacomo, S.M.; Sandner-Kiesling, A. The selection of dermatomes for sham (placebo) acupuncture points is relevant for the outcome of acupuncture studies: A systematic review of sham (placebo)-controlled randomized acupuncture trials. Acupunct. Med. 2020, 38, 0964528419889636. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Moon, H.J.; Na, H.S.; Kim, K.J.; Kim, J.H.; Park, J.H.; Lee, S.H.; Rhim, S.S.; Lee, S.G.; Min, B.I. The analgesic effects of automatically controlled rotating acupuncture in rats: Mediation by endogenous opioid system. J. Physiol. Sci. 2006, 56, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Marvizón, J.C.G. Dorsal Horn Neurons Firing at High Frequency, But Not Primary Afferents, Release Opioid Peptides that Produce μ-Opioid Receptor Internalization in the Rat Spinal Cord. J. Neurosci. 2003, 23, 9171–9184. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Wang, J.; Han, C.X.; Torao, I.; Guo, Y. Analysis of interspike interval of dorsal horn neurons evoked by different needle manipulations at ST36. Acupunct. Med. 2014, 32, 43–50. [Google Scholar] [CrossRef]
- Kagitani, F.; Uchida, S.; Hotta, H.; Aikawa, Y. Manual acupuncture needle stimulation of the rat hindlimb activates groups I, II, III and IV single afferent nerve fibers in the dorsal spinal roots. Jpn. J. Physiol. 2005, 55, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Kagitani, F.; Uchida, S.; Hotta, H. Afferent nerve fibers and acupuncture. Auton. Neurosci. 2010, 157, 2–8. [Google Scholar] [CrossRef]
- Lao, L.; Song, B.; Chen, W.; Marvizón, J.C.G. Noxious mechanical stimulation evokes the segmental release of opioid peptides that induce μ-opioid receptor internalization in the presence of peptidase inhibitors. Brain Res. 2008, 1197, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Rong, P.J.; Zhu, B.; Huang, Q.F.; Gao, X.Y.; Ben, H.; Li, Y.H. Acupuncture inhibition on neuronal activity of spinal dorsal horn induced by noxious colorectal distention in rat. World J. Gastroenterol. 2005, 11, 1011–1017. [Google Scholar] [CrossRef]
- Hong, S.; Ding, S.; Wu, F.; Xi, Q.; Li, Q.; Liu, Y.; Zhou, T.; Qing, C.; Guo, Y.; Guo, Y. Strong Manual Acupuncture Manipulation Could Better Inhibit Spike Frequency of the Dorsal Horn Neurons in Rats with Acute Visceral Nociception. Evid. Based Complementary Alternat. Med. 2015, 2015, 675437. [Google Scholar] [CrossRef]
- Chen, W.; McRoberts, J.A.; Marvizon, J.C. mu-Opioid receptor inhibition of substance P release from primary afferents disappears in neuropathic pain but not inflammatory pain. Neuroscience 2014, 267, 67–82. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.Y.; Perez, F.M.; Wang, W.; Guan, X.; Zhao, X.; Fisher, J.L.; Guan, Y.; Sweitzer, S.M.; Raja, S.N.; Tao, Y.X. Dynamic temporal and spatial regulation of mu opioid receptor expression in primary afferent neurons following spinal nerve injury. Eur. J. Pain 2011, 15, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Trafton, J.A.; Abbadie, C.; Marek, K.; Basbaum, A.I. Postsynaptic signaling via the [mu]-opioid receptor: Responses of dorsal horn neurons to exogenous opioids and noxious stimulation. J. Neurosci. 2000, 20, 8578–8584. [Google Scholar] [CrossRef]
- Gradwell, M.A.; Callister, R.J.; Graham, B.A. Reviewing the case for compromised spinal inhibition in neuropathic pain. J. Neural Transm. 2020, 127, 481–503. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Dorsal horn circuitry: Complexity and implications for mechanisms of neuropathic pain. Neurology 2016, 86, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Coull, J.A.; Boudreau, D.; Bachand, K.; Prescott, S.A.; Nault, F.; Sik, A.; De Koninck, P.; De Koninck, Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 2003, 424, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.A.; Sejnowski, T.J.; De Koninck, Y. Reduction of anion reversal potential subverts the inhibitory control of firing rate in spinal lamina I neurons: Towards a biophysical basis for neuropathic pain. Mol. Pain 2006, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, S.; Myers, R.R. Myelinated afferents sprout into lamina II of L3–5 dorsal horn following chronic constriction nerve injury in rats. Brain Res. 1999, 818, 285–290. [Google Scholar] [CrossRef]
- Woolf, C.J.; Shortland, P.; Coggeshall, R.E. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature 1992, 355, 75–78. [Google Scholar] [CrossRef]
- Shortland, P.; Kinman, E.; Molander, C. Sprouting of A-fibre primary afferents into lamina II in two rat models of neuropathic pain. Eur J. Pain 1997, 1, 215–227. [Google Scholar] [CrossRef]
- Taylor, B.K. Spinal inhibitory neurotransmission in neuropathic pain. Curr. Pain Headache Rep. 2009, 13, 208–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Dong, H.; Gao, Y.; Gong, Y.; Ren, Y.; Gu, N.; Zhou, S.; Xia, N.; Sun, Y.-Y.; Ji, R.-R. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J. Clin. Investig. 2013, 123, 4050–4062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Cui, G.W.; Kuai, L.; Xu, J.M.; Zhang, T.T.; Cheng, H.J.; Dong, H.S.; Dong, G.R. Role of Acupoint Area Collagen Fibers in Anti-Inflammation of Acupuncture Lifting and Thrusting Manipulation. Evid. Based Complementary Alternat. Med. 2017, 2017, 2813437. [Google Scholar] [CrossRef]
- Yu, X.; Ding, G.; Huang, H.; Lin, J.; Yao, W.; Zhan, R. Role of collagen fibers in acupuncture analgesia therapy on rats. Connect. Tissue Res. 2009, 50, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Langevin, H.M.; Churchill, D.L.; Cipolla, M.J. Mechanical signaling through connective tissue: A mechanism for the therapeutic effect of acupuncture. FASEB J. 2001, 15, 2275–2282. [Google Scholar] [CrossRef]
- Perreault, T.; Grubb, M.T.; Gendron, B.C.; Perez-Santiago, J.C.; Flannagan, S.O. Mechanisms and Dose Parameters of Manual Needle Stimulation: Clinical Considerations—Part 2. Acupunct. Physiother. 2019, 31, 9–23. [Google Scholar]
- Kwon, S.; Lee, Y.; Park, H.J.; Hahm, D.H. Coarse needle surface potentiates analgesic effect elicited by acupuncture with twirling manipulation in rats with nociceptive pain. BMC Complementary Altern. Med. 2017, 17, 1. [Google Scholar] [CrossRef] [Green Version]
- Bae, S.J.; Lim, J.; Lee, S.; Choi, H.; Jang, J.H.; Kim, Y.K.; Oh, J.Y.; Park, J.H.; Jung, H.S.; Chae, Y.; et al. Augmented Mechanical Forces of the Surface-Modified Nanoporous Acupuncture Needles Elicit Enhanced Analgesic Effects. Front. Neurosci. 2019, 13, 652. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-C.; Chen, M.-Y.; Hsieh, C.-L.; Wu, S.-Y.; Hsu, H.-C.; Lin, Y.-W. TRPV1 is a Responding Channel for Acupuncture Manipulation in Mice Peripheral and Central Nerve System. Cell. Physiol. Biochem. 2018, 49, 1813–1824. [Google Scholar] [CrossRef]
- Huo, R.; Han, S.-P.; Liu, F.-Y.; Shou, X.-J.; Liu, L.-Y.; Song, T.-J.; Zhai, F.-J.; Zhang, R.; Xing, G.-G.; Han, J.-S. Responses of Primary Afferent Fibers to Acupuncture-Like Peripheral Stimulation at Different Frequencies: Characterization by Single-Unit Recording in Rats. Neurosci. Bull. 2020, 36, 907–918. [Google Scholar] [CrossRef]
- Zhang, D.; Ding, G.; Shen, X.; Yao, W.; Zhang, Z.; Zhang, Y.; Lin, J.; Gu, Q. Role of mast cells in acupuncture effect: A pilot study. Explore 2008, 4, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Liu, K.; Xu, D.; Zhang, Y.; He, X.; Liu, H.; Gao, X.; Zhu, B. Mast cell deficiency attenuates acupuncture analgesia for mechanical pain using c-kit gene mutant rats. J. Pain Res. 2018, 11, 483–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Ding, G.; Gu, Q.; Schwarz, W. Single-channel properties of a stretch-sensitive chloride channel in the human mast cell line HMC-1. Eur. Biophys. J. 2010, 39, 757–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Spielmann, A.; Wang, L.; Ding, G.; Huang, F.; Gu, Q.; Schwarz, W. Mast-cell degranulation induced by physical stimuli involves the activation of transient-receptor-potential channel TRPV2. Physiol. Res. 2012, 61, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wang, X.; Xing, B.; Yang, H.; Sa, Z.; Zhang, D.; Yao, W.; Yin, N.; Xia, Y.; Ding, G. Critical roles of TRPV2 channels, histamine H1 and adenosine A1 receptors in the initiation of acupoint signals for acupuncture analgesia. Sci. Rep. 2018, 8, 6523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deleuze, Y.; Thiriet, M.; Sheu, T.W.-H. Modeling and simulation of the interstitial medium deformation induced by the needle manipulation during acupuncture. Commun. Comput. Physics 2015, 18, 850–867. [Google Scholar] [CrossRef] [Green Version]
- Thiriet, M.; Deleuze, Y.; Sheu, T.W. A biological model of acupuncture and its derived mathematical modeling and simulations. Commun. Comput. Physics 2015, 18, 831–849. [Google Scholar] [CrossRef] [Green Version]
- Dimitrov, N.; Atanasova, D.; Tomov, N.; Staykova-Pirovska, Y.; Ivanova, I.; Sivrev, D. Mast cell distribution around the needle tract following acupuncture in zusanli (st36) acupoint in rats. Bulg. J. Vet. Med. 2019, 22, 91–98. [Google Scholar] [CrossRef]
- Yang, H.W.; Liu, X.Y.; Shen, Z.F.; Yao, W.; Gong, X.B.; Huang, H.X.; Ding, G.H. An investigation of the distribution and location of mast cells affected by the stiffness of substrates as a mechanical niche. Int J. Biol. Sci. 2018, 14, 1142–1152. [Google Scholar] [CrossRef]
- Takano, T.; Chen, X.; Luo, F.; Fujita, T.; Ren, Z.; Goldman, N.; Zhao, Y.; Markman, J.D.; Nedergaard, M. Traditional acupuncture triggers a local increase in adenosine in human subjects. J. Pain 2012, 13, 1215–1223. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Sikora, J.; Hu, L.; Shen, X.; Grygorczyk, R.; Schwarz, W. ATP release from mast cells by physical stimulation: A putative early step in activation of acupuncture points. Evid. Based Complementary Alternat. Med. 2013, 2013, 350949. [Google Scholar] [CrossRef]
- Qu, F.; Cui, Y.; Zeng, J.; Zhang, M.; Qiu, S.; Huang, X.; Chen, A. Acupuncture induces adenosine in fibroblasts through energy metabolism and promotes proliferation by activating MAPK signaling pathway via adenosine3 receptor. J. Cell. Physiol. 2020, 235, 2441–2451. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Chen, W.H.; Hsieh, C.L.; Lin, Y.W. Abundant expression and functional participation of TRPV1 at Zusanli acupoint (ST36) in mice: Mechanosensitive TRPV1 as an “acupuncture-responding channel”. BMC Complementary Alternat. Med. 2014, 14, 96. [Google Scholar] [CrossRef] [Green Version]
- Shen, D.; Shen, X.; Schwarz, W.; Grygorczyk, R.; Wang, L. P2Y13 and P2X7 receptors modulate mechanically induced adenosine triphosphate release from mast cells. Exp. Dermatol. 2020, 29, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Sowa, N.A.; Taylor-Blake, B.; Zylka, M.J. Ecto-5′-nucleotidase (CD73) inhibits nociception by hydrolyzing AMP to adenosine in nociceptive circuits. J. Neurosci. 2010, 30, 2235–2244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowa, N.A.; Voss, M.K.; Zylka, M.J. Recombinant ecto-5′-nucleotidase (CD73) has long lasting antinociceptive effects that are dependent on adenosine A1 receptor activation. Mol. Pain 2010, 6, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulte, G.; Robertson, B.; Fredholm, B.; DeLander, G.; Shortland, P.; Molander, C. Distribution of antinociceptive adenosine A1 receptors in the spinal cord dorsal horn, and relationship to primary afferents and neuronal subpopulations. Neuroscience 2003, 121, 907–916. [Google Scholar] [CrossRef]
- Li, J.; Perl, E.R. Adenosine inhibition of synaptic transmission in the substantia gelatinosa. J. Neurophysiol. 1994, 72, 1611–1621. [Google Scholar] [CrossRef]
- Yang, K.; Fujita, T.; Kumamoto, E. Adenosine inhibits GABAergic and glycinergic transmission in adult rat substantia gelatinosa neurons. J. Neurophysiol. 2004, 92, 2867–2877. [Google Scholar] [CrossRef] [Green Version]
- Sawynok, J. Adenosine and ATP receptors. In Analgesia. Handbook of Experimental Pharmacology; Stein, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 177, pp. 309–328. [Google Scholar] [CrossRef]
- Rhee, J.S.; Wang, Z.M.; Nabekura, J.; Inoue, K.; Akaike, N. ATP facilitates spontaneous glycinergic IPSC frequency at dissociated rat dorsal horn interneuron synapses. J. Physiol. 2000, 524, 471. [Google Scholar] [CrossRef]
- Kan, H.W.; Chang, C.H.; Lin, C.L.; Lee, Y.C.; Hsieh, S.T.; Hsieh, Y.L. Downregulation of adenosine and adenosine A1 receptor contributes to neuropathic pain in resiniferatoxin neuropathy. Pain 2018, 159, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Shehab, S.A.; Hughes, D.I. Simultaneous identification of unmyelinated and myelinated primary somatic afferents by co-injection of isolectin B4 and Cholera toxin subunit B into the sciatic nerve of the rat. J. Neurosci. Methods 2011, 198, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Franklin, J.F.; Lee, H.J. Central expression of synaptophysin and synaptoporin in nociceptive afferent subtypes in the dorsal horn. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lomeli, J.; Quevedo, J.; Linares, P.; Rudomin, P. Local control of information flow in segmental and ascending collaterals of single afferents. Nature 1998, 395, 600–604. [Google Scholar] [CrossRef]
- Koerber, H.R.; Mendell, L.M.; Brown, P.B. Correlation of monosynaptic field potentials evoked by single action potentials in single primary afferent axons and their bouton distributions in the dorsal horn. J. Comp. Neurol. 1990, 294, 133–144. [Google Scholar] [CrossRef]
- Pinto, V.; Szûcs, P.; Derkach, V.A.; Safronov, B.V. Monosynaptic convergence of C-and Aδ-afferent fibres from different segmental dorsal roots on to single substantia gelatinosa neurones in the rat spinal cord. J. Physiol. 2008, 586, 4165–4177. [Google Scholar] [CrossRef]
- Pinto, V.; Szucs, P.; Lima, D.; Safronov, B.V. Multisegmental Aδ-and C-fiber input to neurons in lamina I and the lateral spinal nucleus. J. Neurosci. 2010, 30, 2384–2395. [Google Scholar] [CrossRef]
- Fernandes, E.; Pechincha, C.; Luz, L.; Kokai, E.; Szucs, P.; Safronov, B. Primary afferent-driven presynaptic inhibition of C-fiber inputs to spinal lamina I neurons. Prog. Neurobiol. 2020, 188, 101786. [Google Scholar] [CrossRef] [PubMed]
- Rudomin, P.; Schmidt, R.F. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp. Brain Res. 1999, 129, 1–37. [Google Scholar] [CrossRef]
- Fernandes, E.C.; Luz, L.L.; Mytakhir, O.; Lukoyanov, N.V.; Szucs, P.; Safronov, B.V. Diverse firing properties and Aβ-, Aδ-, and C-afferent inputs of small local circuit neurons in spinal lamina I. Pain 2016, 157, 475–487. [Google Scholar] [CrossRef]
- Jones, T.L.; Sweitzer, S.M.; Peters, M.C.; Wilson, S.P.; Yeomans, D.C. GABAB receptors on central terminals of C-afferents mediate intersegmental Aδ-afferent evoked hypoalgesia. Eur. J. Pain 2005, 9, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Ma, H. Blockade of GABAB receptors facilitates evoked neurotransmitter release at spinal dorsal horn synapse. Neuroscience 2011, 193, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wang, D.; Li, Y.-Q. Distribution and depression of the GABAB receptor in the spinal dorsal horn of adult rat. Brain Res. Bull. 2001, 55, 479–485. [Google Scholar] [CrossRef]
- Quiroz-Gonzalez, S.; Segura-Alegria, B.; Guadarrama-Olmos, J.C.; Jimenez-Estrada, I. Cord dorsum potentials evoked by electroacupuncture applied to the hind limbs of rats. J. Acupunct. Meridian Stud. 2014, 7, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Chavez, D.; Rodriguez, E.; Jimenez, I.; Rudomin, P. Changes in correlation between spontaneous activity of dorsal horn neurones lead to differential recruitment of inhibitory pathways in the cat spinal cord. J. Physiol. 2012, 590, 1563–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.-Y.; Li, S.-R.; Zhao, F.-Y.; Spanswick, D.; Lin, M.-T. Norepinephrine can act via α2-adrenoceptors to reduce the hyper-excitability of spinal dorsal horn neurons following chronic nerve injury. J. Formos. Med. Assoc. 2010, 109, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Hitoto, T.; Tsuruoka, M.; Hiruma, Y.; Matsui, Y. A delta afferent fiber stimulation activates descending noradrenergic system from the locus coeruleus. Neurochem. Res. 1998, 23, 1461–1465. [Google Scholar] [CrossRef]
- Mena-Avila, E.; Milla-Cruz, J.J.; Calvo, J.R.; Hochman, S.; Villalón, C.M.; Arias-Montaño, J.A.; Quevedo, J.N. Activation of α-adrenoceptors depresses synaptic transmission of myelinated afferents and inhibits pathways mediating primary afferent depolarization (PAD) in the in vitro mouse spinal cord. Exp. Brain Res. 2020, 238, 1293–1303. [Google Scholar] [CrossRef]
- Kim, W.; Kim, S.K.; Min, B.I. Mechanisms of electroacupuncture-induced analgesia on neuropathic pain in animal model. Evid. Based Complementary Alternat Med. 2013, 2013, 436913. [Google Scholar] [CrossRef] [Green Version]
- Gassner, M.; Ruscheweyh, R.; Sandkuhler, J. Direct excitation of spinal GABAergic interneurons by noradrenaline. Pain 2009, 145, 204–210. [Google Scholar] [CrossRef]
- Sonohata, M.; Doi, A.; Yasaka, T.; Uta, D.; Mawatari, M.; Yoshimura, M. Noradrenaline modulates mechanically evoked responses in the rat spinal dorsal horn: An in vivo patch-clamp study. J. Pain Res. 2019, 12, 1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.Y.; Zhang, Q.S.; Yang, J.; Sun, F.J.; Wang, D.X.; Wang, C.H.; He, W.Y. The role of arginine vasopressin in electroacupuncture treatment of primary sciatica in human. Neuropeptides 2015, 52, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.-H.; Wang, Y.-L.; Yuan, Y.; Liu, X.-S.; Liu, X.-F.; Wang, D.-X.; Lin, B.-C.; Yang, J. The roles of vasopressin in acupuncture analgesia. J. Pharm. Med. 2015, 3, 11–16. [Google Scholar]
- Zhou, X.J.; Yang, J.; Yan, F.L.; Wang, D.X.; Li, X.Y.; Fan, X.Q.; Hao, F.; Yan, X.Q.; Li, X.P.; Li, H.; et al. Norepinephrine plays an important role in antinociceptive modulation of hypothalamic paraventricular nucleus in the rat. Int. J. Neurosci. 2010, 120, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Carbone, E. Noradrenergic inhibition of presynaptic TRPV1 channels: A new pathway of pain control. J. Physiol. 2017, 595, 2413. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.Y.; Gao, Y.H.; Qiao, L.N.; Zhang, J.L.; Duan-Mu, C.L.; Yan, Y.X.; Chen, S.P.; Liu, J.L. Repeated electroacupuncture treatment attenuated hyperalgesia through suppression of spinal glial activation in chronic neuropathic pain rats. BMC Complementary Alternat. Med. 2018, 18, 74. [Google Scholar] [CrossRef]
- Liang, Y.; Qiu, Y.; Du, J.; Liu, J.; Fang, J.; Zhu, J.; Fang, J. Inhibition of spinal microglia and astrocytes contributes to the anti-allodynic effect of electroacupuncture in neuropathic pain induced by spinal nerve ligation. Acupunct. Med. 2016, 34, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Coull, J.A.; Beggs, S.; Boudreau, D.; Boivin, D.; Tsuda, M.; Inoue, K.; Gravel, C.; Salter, M.W.; De Koninck, Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 2005, 438, 1017–1021. [Google Scholar] [CrossRef]
- Lee-Hotta, S.; Uchiyama, Y.; Kametaka, S. Role of the BDNF-TrkB pathway in KCC2 regulation and rehabilitation following neuronal injury: A mini review. Neurochem. Int. 2019, 128, 32–38. [Google Scholar] [CrossRef]
- Lee, K.Y.; Ratte, S.; Prescott, S.A. Excitatory neurons are more disinhibited than inhibitory neurons by chloride dysregulation in the spinal dorsal horn. Elife 2019, 8, e49753. [Google Scholar] [CrossRef]
- Rivera, C.; Li, H.; Thomas-Crusells, J.; Lahtinen, H.; Viitanen, T.; Nanobashvili, A.; Kokaia, Z.; Airaksinen, M.S.; Voipio, J.; Kaila, K.; et al. BDNF-induced TrkB activation down-regulates the K+-Cl- cotransporter KCC2 and impairs neuronal Cl- extrusion. J. Cell Biol. 2002, 159, 747–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, W.Z.; Li, S.S.; Jiang, X.; Qian, X.R.; Yang, G.H.; Gu, P.P.; Lu, B.; Jiang, S.H. Effect of electro-acupuncture on the BDNF-TrkB pathway in the spinal cord of CCI rats. Int. J. Mol. Med. 2018, 41, 3307–3315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, M.; Sun, Y.L.; Xia, Y.Y.; Huang, Z.H.; Huang, C.; Xing, G.G. Electroacupuncture Modulates Spinal BDNF/TrkappaB Signaling Pathway and Ameliorates the Sensitization of Dorsal Horn WDR Neurons in Spared Nerve Injury Rats. Int. J. Mol. Sci. 2020, 21, 6524. [Google Scholar] [CrossRef]
- Li, S.S.; Tu, W.Z.; Jia, C.Q.; Jiang, X.; Qian, X.R.; Yang, G.H.; Hu, Q.M.; Chen, W.C.; Lu, B.; Jiang, S.H. KCC2-GABAA pathway correlates with the analgesic effect of electro-acupuncture in CCI rats. Mol. Med. Rep. 2018, 17, 6961–6968. [Google Scholar] [CrossRef] [PubMed]
- Perreault, T.; Flannagan, S.O.; Grubb, M.T.; Grubb, R. Mechanisms and dose parameters of electric needle stimulation: Clinical considerations—Part 1. Acupunct. Physiother. 2018, 30, 17–26. [Google Scholar]
- Langevin, H.M.; Schnyer, R.; MacPherson, H.; Davis, R.; Harris, R.E.; Napadow, V.; Wayne, P.M.; Milley, R.J.; Lao, L.; Stener-Victorin, E.; et al. Manual and electrical needle stimulation in acupuncture research: Pitfalls and challenges of heterogeneity. J. Alternat. Complementary Med. 2015, 21, 113–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.C.; Lee, J.Y.; Moon, Y.J.; Kim, S.W.; Oh, T.H.; Yune, T.Y. Acupuncture-mediated inhibition of inflammation facilitates significant functional recovery after spinal cord injury. Neurobiol. Dis. 2010, 39, 272–282. [Google Scholar] [CrossRef]
- Choi, D.C.; Lee, J.Y.; Lim, E.J.; Baik, H.H.; Oh, T.H.; Yune, T.Y. Inhibition of ROS-induced p38MAPK and ERK activation in microglia by acupuncture relieves neuropathic pain after spinal cord injury in rats. Exp. Neurol. 2012, 236, 268–282. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, D.C.; Oh, T.H.; Yune, T.Y. Analgesic effect of acupuncture is mediated via inhibition of JNK activation in astrocytes after spinal cord injury. PLoS ONE 2013, 8, e73948. [Google Scholar] [CrossRef] [Green Version]


| PubMed/MEDLINE Search Formula |
|---|
| (“dry needling” OR acupuncture) AND (sciatica OR “neuropathic pain” OR radiculopathy) |
| CINAHL |
| (“dry needling” OR “dry needling” OR acupuncture OR acupuncture) AND (sciatica OR “neuropathic pain” OR “neuropathic pain” OR radiculopathy) |
| Cochrane Database of Systematic Reviews |
| # 1 acupuncture OR “dry needling” OR (mh acupuncture) |
| # 2 sciatica OR “neuropathic pain” OR radiculopathy (MeSh) |
| # 3 # 1 AND # 2 |
| Scoping Review Studies |
|---|
| Inclusion criteria |
| Language: English |
| Article type: Systematic reviews, meta-analyses, randomized controlled trials, pilot studies |
| Subject: Dry needling or acupuncture for sciatica or neuropathic pain related to radiculopathy of lumbar region due to sciatic nerve compression |
| Exclusion criteria |
| Language: Non-English language |
| Article type: Case series, case study, cohort study, study protocol, narrative review, articles not in English |
| Subject: Mechanisms studies or experimental animal studies, studies on low back pain or spinal pain without specifying patients with nerve root compression and symptoms consistent with sciatica. Studies on patients with lumbar spinal stenosis. Studies on patients with neuropathic pain not of spinal or nerve root origin to include; chemotherapy-induced peripheral neuropathy, spinal cord injury, multiple sclerosis, cancer related neuropathic pain, post herpetic neuralgia, piriformis syndrome, diabetic neuropathy. Studies using warming acupuncture or injectates with the needling procedures |
| Mechanisms Studies |
| Inclusion Criteria |
| Language: English |
| Article type: Systematic reviews, meta-analyses, randomized controlled trials or pilot studies, narrative reviews, experimental in vivo animal studies, clinical studies, laboratory studies, modeling and simulation studies. |
| Subject: Physiological mechanisms studies (human and animal subjects) on the use of dry needling, manual acupuncture or electroacupuncture for neuropathic pain related to sciatic nerve injury/compression models. Mechanistic studies on animal models under normal, inflammatory and/or neuropathic pain conditions |
| Exclusion criteria |
| Language: Non-English language |
| Article type: Studies not relevant to the neurophysiological or mechanical effects of needling interventions. Studies not relevant to pathophysiological mechanisms of sciatica, nerve injury or neuropathic pain. |
| Study | Study Design | Number of Patients | Pain Outcome | Follow Up | Rationale | Inclusion Criteria |
|---|---|---|---|---|---|---|
| Ji et al., 2015 [44] | Systematic Review and Meta-Analysis. | 12 Studies (randomized or quasi-randomized clinical trials) involving 1842 participants | VAS (n = 3) | Not reported | All 12 studies used TCM rationale for point selection | Studies chose participants with either subjective signs of sciatica or positive clinical examination tests or both. Conformity was limited on inclusion criteria among studies. |
| Huang et al., 2020 [40] | Systematic Review and Meta-Analysis. | 24 RCTs included in systematic review, 22 RCTs in Meta-analysis. Only 3 RCTs on sciatica involving 196 patients | VAS (n = 2), NRS (n = 1) | Kim et al., 2016: weeks 6 and 12, see below for Huang et al. [41] and Zhang et al. [38] | Kim et al., 2016: point selection was at the discretion of Korean Medical Doctors and was individualize. See below for Huang et al., 2019 and Zhang et al., 2017 | Kim et al., 2016: required clinical and radiological confirmation along with symptoms of radiating pain in the leg. See below for Huang et al. [41] and Zhang et al. [38] |
| Huang et al., 2019 [41] | RCT | 44 patients | VAS | Weeks 1, 2, 3, 4, 16, and 28. Primary outcome was VAS at 4 weeks. | Selection of points was based on expert consensus and protocol of a previous trial. | Patients with chronic sciatica caused by lumbar disc herniation. Diagnosis was based on MRI, CT and examination of symptoms by experienced physicians. |
| Lewis et al., 2015 [24] | Systematic Review and Network Meta-Analysis. 122 studies included | Only a single RCT on acupuncture was included, Duplan, 1983 (French) involving 30 patients | No data reported. | No data reported | Not reported | Patients with clinical diagnosis of sciatica based on nerve root pain and referred pain |
| Liu et al., 2019 [22] | Randomized Controlled Pilot Study | 30 patients | VAS | 4 weeks | Acupoint selection was based on acupuncturist experience and TCM theory. However, sciatic dermatomes were considered in point selection | Patients selected based on radicular pain in L4, L5, S1 dermatomes, findings of radicular pain, motor, sensory or reflex deficits on neurological exam, positive SLR, leg pain upon sneezing, coughing or straining and positive MRI showing unilateral disc herniation with impingement on L4, L5 or S1 nerve root. |
| Luijsterburg et al., 2007 [43] | Systematic Review. 30 publications included | Only a single RCT on acupuncture was included, Duplan, 1983 (French) involving 30 patients | No data reported. | No data reported | Not reported | Patients with clinical diagnosis of sciatica based on nerve root pain and referred pain |
| Qin et al., 2015 [45] | Systematic Review and Meta-Analysis | 11 RCTs included with 932 participants. 9 were in Chinese, 2 were in English | VAS (n = 3) | Reported only in 1 study as 6 months | All studies adopted a treatment theory based on TCM theory and clinical experience. | Patients with sciatica of the nerve roots along with lumbar disc herniation (n = 8 studies). Patients diagnosed with sciatica of the nerve trunk without lumbar disc herniation (n = 3 studies) |
| Zhang et al., 2017 [38] | RCT | 100 patients | NRS | Weeks 1, 2, 3, 4, 16, and 28. Primary outcome was meanchange in NRS at week 4 | Protocol based on specialist consensus and results of a previous pilot trial | Included participants with sciatica symptoms that correlated with MRI or CT findings of lumbar disc herniation |
| Jeong et al., 2020 [39] | RCT | 146 patients | VAS | Weeks 2, 4 and 6. Primary outcome was mean change in VAS at week 4 | Acupuncture rationale not specified | Included patients diagnosed with LDH based on clinical examination with positive MRI or CT and symptoms of low back pain, radiating pain, and paresthesia or weakness in the lower extremities |
| Lewis et al., 2011 [42] | Systematic Review. Cost-effectiveness of treatments for sciatica. 270 studies | Only a single RCT on acupuncture was included, Duplan, 1983 (French) involving 30 patients | No data provided | No data reported | Not reported | Patients with clinical diagnosis of sciatica based on nerve root pain and referred pain |
| Study | Interventions | Needle Placement | Needle Manipulation | Retention Time | Frequency/ Duration |
|---|---|---|---|---|---|
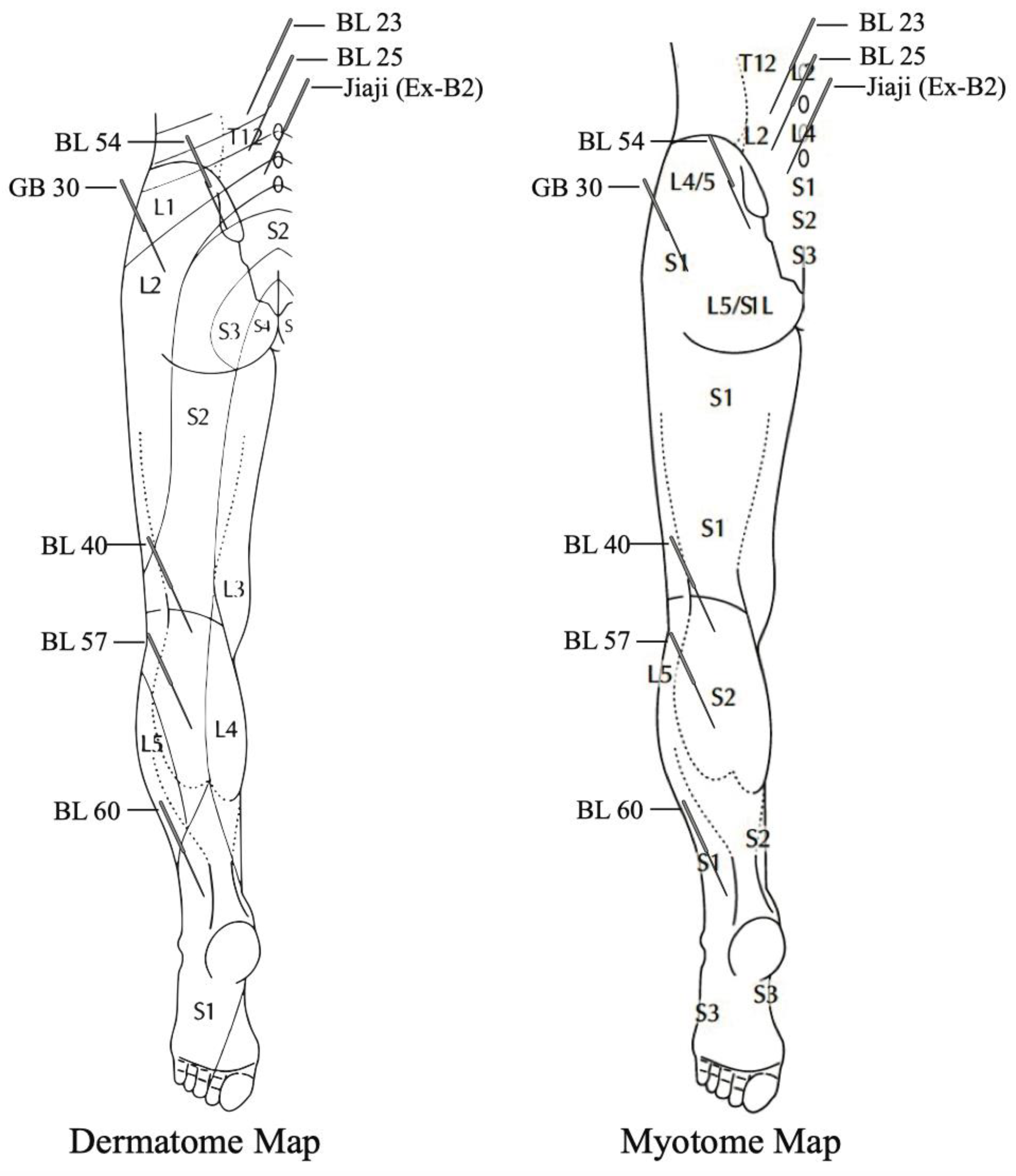
| Ji et al., 2015 [44] | MA or EA vs. Conventional Western Medicine (oral drugs, external drugs or injections) | Common points: GB 30 (n = 12 studies), BL 54 (n = 7 studies), BL 40 (n = 8 studies), GB 34 (n = 5 studies), BL 25 (n = 6 studies), BL 23 (n = 5 studies), BL 60 (n = 8 studies), BL 57 (n = 6 studies), GB 39 (n = 6 studies) | Manual stimulation (n = 8 studies) electric stimulation (n = 4 studies) 10 out of 12 studies elicited de qi or other sensation | Ranged from 5 to 30 min for either MA or EA | Number of sessions ranged from 6 to 40. Frequency ranged from once per day ×6–15 days to 2 times per week for 3 weeks to 3 times per week for 2 weeks |
| Huang et al., 2020 [40] | MA vs. Sham Acupuncture, EA vs. Medium Frequency Electrotherapy (MFE), MA + EA vs. usual care alone (Physical Therapy) | Huang et al., 2019 [41]: (B) BL 23, BL 25, BL 40, BL 57). Zhang et al. [38]: BL 25 on affected side, Jiaji (Ex-B2) bilaterally at spinal level of lumbar disc herniation. Kim et al., 2016: BL23, BL24, BL25 or BL26 or Jiaji points at L2–L5 spinal levels. Other used points were BL57, BL60, GB39, GB34 and tender points | Kim et al., 2016 Manual stimulation 15–50 mm depth, lift-thrust and needle rotation to elicit de qi. Electrical stimulation applied with alternating 2–100 Hz frequency | Kim et al., 2016 retention time 20 min with EA 2–100 Hz alternating. See below for Huang et al. [41] and Zhang et al. [38] | Kim et al., 2016 = 12–16 sessions over a 6-week period. See below for Huang et al. [41] and Zhang et al. [38] |
| Huang et al., 2019 [41] | MA (n = 23) vs. Sham Acupuncture (n = 21) | Acupuncture to (B) BL 23, BL 25, BL 40, BL 57. Sham group used blunt needles on same points without insertion | Manual stimulation, depth of needling 40–70 mm into BL 25, 30 mm into BL 40 and BL 57 Needle twirling, lifting and thrusting were used to elicit de qi | 30 min | 3 ×/week for 4 weeks 12 sessions |
| Lewis et al., 2015 [24] | EA vs. sham acupuncture | No data reported | EA | Not reported | 5 session of EA |
| Liu et al., 2019 [22] | High dose MA vs. Low dose MA | High Dose = 18 points BL 23, BL 25, BL 27, GB 30, BL 37, BL 54, BL 36, GB 31, BL 40, ST 36, GB 34, SP 9, BL 58, SP 6, GB 39, BL 60, KI 3, BL 62. Low Dose = 6 points BL 23, GB 30, BL 40 GB 34, BL 60, GB 39 | Manual stimulation = needle rotation at 5–30 mm depth and elicited de qi | 20–30 min | 2 ×/week for 4 weeks 8 sessions |
| Luijsterburg et al., 2007 [43] | 30 patients with sciatica (15 in acupuncture group and 15 placebo acupuncture) | No data reported. | EA | Not reported | 5 session of EA |
| Qin et al., 2015 [45] | MA (n = 2 studies), EA (n = 6) studies, Warming Acupuncture (n = 2 studies). Comparison interventions included; conventional medication (n = 8 studies), acupuncture with meds compared to meds alone (n = 2 studies), 1 trial compared acupuncture with sham acupuncture | Number of points used ranged from 1 to 10 across studies. Most commonly used points were GB 30 (n= 9 studies) BL 40 (n = 8 studies), BL 67 (n = 4 studies). Other common points were BL 54 (n = 4 studies), Jiaji (EX-B2) (n = 6 studies), BL 57 (n = 3 studies), BL 23 + BL 25 (n = 2 studies) | MA (n = 2 studies). EA (n = 6 studies). All 11 studies reported de qi needle sensation of soreness and numbness | Retention time varied from 20–45 min | 1 to 4 weeks. Frequency ranged from 1 to 3 sessions per day for 7–10 days (n = 9 studies) or 2 to 4 sessions 3 times per week (n = 2 studies) |
| Zhang et al., 2017 [38] | EA (n = 50) vs. MFE (n = 50) | BL 25 on affected side, Jiaji (Ex-B2) bilaterally at spinal level of lumbar disc herniation. MFE = surface electrodes applied over same points as acupuncture group | Manual stimulation (BL 25 up to 3 inch depth and Jiaji (Ex-B2) up to 1.5 inch depth, + electrical stimulation = 50 Hz | 20 min | 5 times per week for 2 weeks then 3 sessions per week for 2 weeks. |
| Jeong et al., 2020 [39] | MA (n = 73) vs. Acupotomy (n = 73) | MA = GV 3 and (B) BL 23, BL 24, BL 25, BL 26, GB 30, BL 40, BL 60 Acupotomy = 2–6 points at lumbar levels of disc herniation | MA = Manual needle rotation 3–5 times after insertion 20 mm for BL 40 and BL 60, 30 mm depth for all others. Acupotomy = 50–70 mm depth to 2–6 points | MA = 15 min Acupotomy = immediate removal after manipulation | MA = 4 sessions over a 2-week period |
| Lewis et al., 2011 [42] | 30 patients with sciatica (15 in acupuncture group and 15 placebo acupuncture). | No data provided | EA | No data provided | 5 session of acupuncture |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perreault, T.; Fernández-de-las-Peñas, C.; Cummings, M.; Gendron, B.C. Needling Interventions for Sciatica: Choosing Methods Based on Neuropathic Pain Mechanisms—A Scoping Review. J. Clin. Med. 2021, 10, 2189. https://doi.org/10.3390/jcm10102189
Perreault T, Fernández-de-las-Peñas C, Cummings M, Gendron BC. Needling Interventions for Sciatica: Choosing Methods Based on Neuropathic Pain Mechanisms—A Scoping Review. Journal of Clinical Medicine. 2021; 10(10):2189. https://doi.org/10.3390/jcm10102189
Chicago/Turabian StylePerreault, Thomas, César Fernández-de-las-Peñas, Mike Cummings, and Barry C. Gendron. 2021. "Needling Interventions for Sciatica: Choosing Methods Based on Neuropathic Pain Mechanisms—A Scoping Review" Journal of Clinical Medicine 10, no. 10: 2189. https://doi.org/10.3390/jcm10102189
APA StylePerreault, T., Fernández-de-las-Peñas, C., Cummings, M., & Gendron, B. C. (2021). Needling Interventions for Sciatica: Choosing Methods Based on Neuropathic Pain Mechanisms—A Scoping Review. Journal of Clinical Medicine, 10(10), 2189. https://doi.org/10.3390/jcm10102189







