The Relation of Clinic and Ambulatory BP with the Risk of Cardiovascular Events and All-Cause Mortality among Patients on Peritoneal Dialysis
Abstract
1. Introduction
2. Materials and Methods
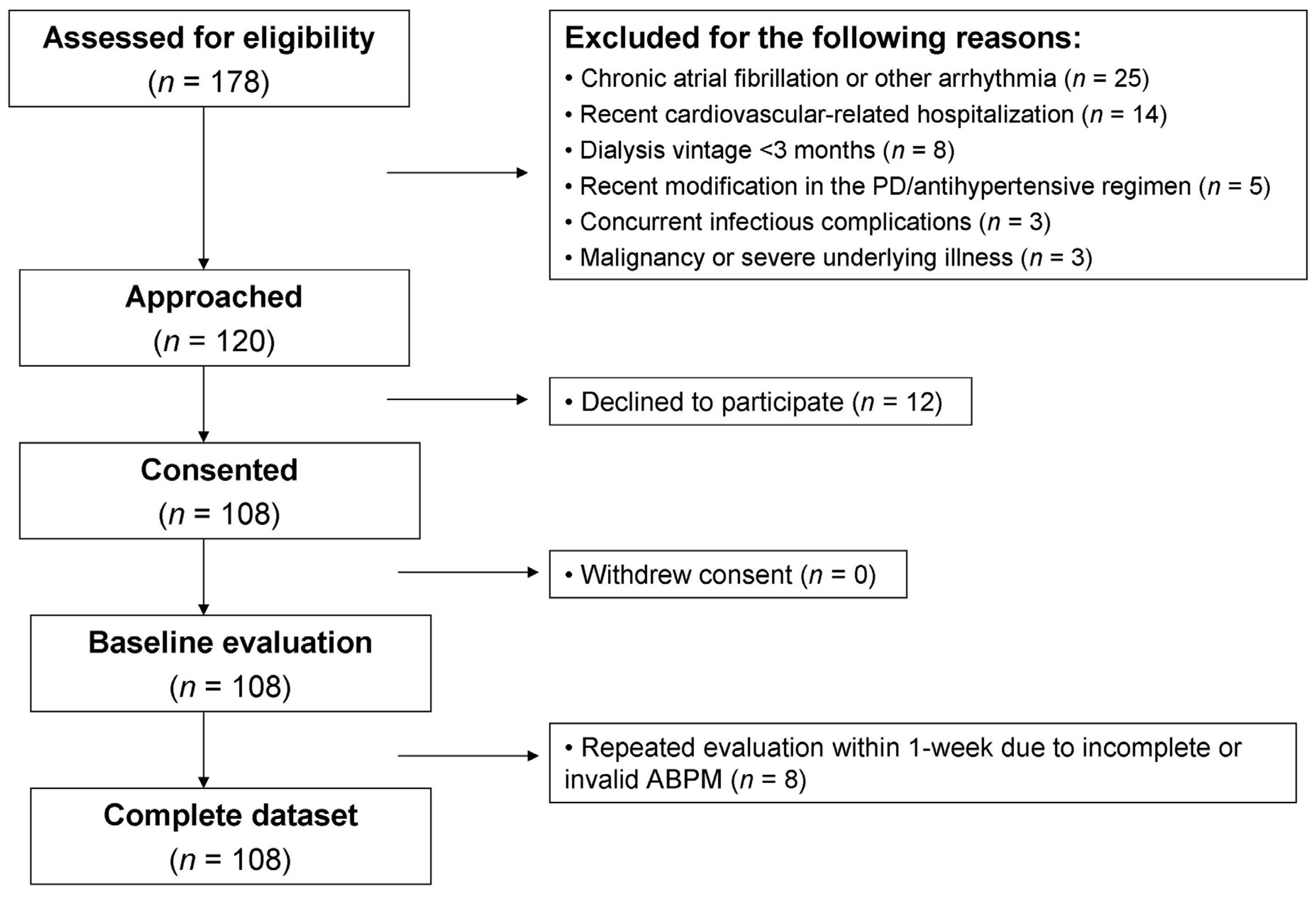
2.1. Study Population
2.2. Predictors
2.2.1. Clinic BP
2.2.2. 24-H Ambulatory BP
2.3. Outcome
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti, R.E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar]
- Udayaraj, U.P.; Steenkamp, R.; Caskey, F.J.; Rogers, C.; Nitsch, D.; Ansell, D.; Tomson, C.R. Blood pressure and mortality risk on peritoneal dialysis. Am. J. Kidney. Dis. 2009, 53, 70–78. [Google Scholar] [CrossRef]
- Xie, X.; Lv, D.; Zheng, H.; Zhang, X.; Han, F.; Chen, J. The associations of blood pressure parameters with all-cause and cardiovascular mortality in peritoneal dialysis patients: A cohort study in China. J. Hypertens. 2020, 38, 2252–2260. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kilpatrick, R.D.; McAllister, C.J.; Greenland, S.; Kopple, J.D. Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: The 58th annual fall conference and scientific sessions. Hypertension 2005, 45, 811–817. [Google Scholar] [CrossRef]
- Zager, P.G.; Nikolic, J.; Brown, R.H.; Campbell, M.A.; Hunt, W.C.; Peterson, D.; Van, S.J.; Levey, A.; Meyer, K.B.; Klag, M.J.; et al. “U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney. Int. 1998, 54, 561–569. [Google Scholar] [CrossRef]
- Georgianos, P.I.; Agarwal, R. Epidemiology, diagnosis and management of hypertension among patients on chronic dialysis. Nat. Rev. Nephrol. 2016, 12, 636–647. [Google Scholar] [CrossRef]
- Vaios, V.; Georgianos, P.I.; Liakopoulos, V.; Agarwal, R. Assessment and Management of Hypertension among Patients on Peritoneal Dialysis. Clin. J. Am. Soc. Nephrol. 2019, 14, 297–305. [Google Scholar] [CrossRef]
- Georgianos, P.I.; Agarwal, R. Blood Pressure and Mortality in Long-Term Hemodialysis-Time to Move Forward. Am. J. Hypertens. 2017, 30, 211–222. [Google Scholar] [CrossRef]
- Agarwal, R. Blood pressure and mortality among hemodialysis patients. Hypertension 2010, 55, 762–768. [Google Scholar] [CrossRef]
- Alborzi, P.; Patel, N.; Agarwal, R. Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clin. J. Am. Soc. Nephrol. 2007, 2, 1228–1234. [Google Scholar] [CrossRef]
- Vaios, V.; Georgianos, P.I.; Vareta, G.; Dounousi, E.; Dimitriadis, C.; Eleftheriadis, T.; Papagianni, A.; Zebekakis, P.E.; Liakopoulos, V. Clinic and Home Blood Pressure Monitoring for the Detection of Ambulatory Hypertension Among Patients on Peritoneal Dialysis. Hypertension 2019, 74, 998–1004. [Google Scholar] [CrossRef]
- O’Brien, E.; Mee, F.; Atkins, N.; Thomas, M. Evaluation of three devices for self-measurement of blood pressure according to the revised British Hypertension Society Protocol: The Omron HEM-705CP, Philips HP5332, and Nissei DS-175. Blood Press. Monit. 1996, 1, 55–61. [Google Scholar] [PubMed]
- Franssen, P.M.; Imholz, B.P. Evaluation of the Mobil-O-Graph new generation ABPM device using the ESH criteria. Blood Press. Monit. 2010, 15, 229–231. [Google Scholar] [CrossRef]
- Wei, W.; Tolle, M.; Zidek, W.; van der Giet, M. Validation of the mobil-O-Graph: 24 h-blood pressure measurement device. Blood Press. Monit. 2010, 15, 225–228. [Google Scholar] [CrossRef]
- Sarafidis, P.A.; Lazaridis, A.A.; Imprialos, K.P.; Georgianos, P.I.; Avranas, K.A.; Protogerou, A.D.; Doumas, M.; Athyros, V.G.; Karagiannis, A.I. A comparison study of brachial blood pressure recorded with Spacelabs 90217A and Mobil-O-Graph NG devices under static and ambulatory conditions. J. Hum. Hypertens. 2016, 30, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Parati, G.; Stergiou, G.; O’Brien, E.; Asmar, R.; Beilin, L.; Bilo, G.; Clement, D.; de la Sierra, A.; de Leeuw, P.; Dolan, E.; et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J. Hypertens. 2014, 32, 1359–1366. [Google Scholar] [CrossRef]
- Wang, A.Y.; Brimble, K.S.; Brunier, G.; Holt, S.G.; Jha, V.; Johnson, D.W.; Kang, S.W.; Kooman, J.P.; Lambie, M.; McIntyre, C.; et al. ISPD Cardiovascular and Metabolic Guidelines in Adult Peritoneal Dialysis Patients Part I—Assessment and Management of Various Cardiovascular Risk Factors. Perit. Dial. Int. 2015, 35, 379–387. [Google Scholar] [CrossRef]
- Afshinnia, F.; Zaky, Z.S.; Metireddy, M.; Segal, J.H. Reverse Epidemiology of Blood Pressure in Peritoneal Dialysis Associated with Dynamic Deterioration of Left Ventricular Function. Perit. Dial. Int. 2016, 36, 154–162. [Google Scholar] [CrossRef]
- Agarwal, R.; Andersen, M.J.; Light, R.P. Location not quantity of blood pressure measurements predicts mortality in hemodialysis patients. Am. J. Nephrol. 2008, 28, 210–217. [Google Scholar] [CrossRef]
- Agarwal, R. Pro: Ambulatory blood pressure should be used in all patients on hemodialysis. Nephrol. Dial. Transplant. 2015, 30, 1432–1437. [Google Scholar] [CrossRef]
- Agarwal, R.; Sinha, A.D.; Light, R.P. Toward a definition of masked hypertension and white-coat hypertension among hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 2003–2008. [Google Scholar] [CrossRef]
- Amar, J.; Vernier, I.; Rossignol, E.; Bongard, V.; Arnaud, C.; Conte, J.J.; Salvador, M.; Chamontin, B. Nocturnal blood pressure and 24-hour pulse pressure are potent indicators of mortality in hemodialysis patients. Kidney Int. 2000, 57, 2485–2491. [Google Scholar] [CrossRef]
- Rahman, M.; Griffin, V.; Heyka, R.; Hoit, B. Diurnal variation of blood pressure; reproducibility and association with left ventricular hypertrophy in hemodialysis patients. Blood Press. Monit. 2005, 10, 25–32. [Google Scholar] [CrossRef]
- Tripepi, G.; Fagugli, R.M.; Dattolo, P.; Parlongo, G.; Mallamaci, F.; Buoncristiani, U.; Zoccali, C. Prognostic value of 24-hour ambulatory blood pressure monitoring and of night/day ratio in nondiabetic, cardiovascular events-free hemodialysis patients. Kidney Int. 2005, 68, 1294–1302. [Google Scholar] [CrossRef]

| Parameter | Overall | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p Value |
|---|---|---|---|---|---|---|
| Range of 24-h ambulatory SBP (mmHg) | - | <114.0 | 114–126 | 126–140.8 | >140.8 | - |
| N | 108 | 27 | 27 | 27 | 27 | - |
| 24-h ambulatory SBP (mmHg) | 126.7 ± 18.4 | 104.6 ± 7.6 | 119.5 ± 3.9 | 132.2 ± 4.3 | 150.6 ± 10.4 | <0.001 |
| 24-h ambulatory DBP (mmHg) | 77.6 ± 12.1 | 66.3 ± 7.8 | 74.1 ± 8.1 | 82.9 ± 8.8 | 87.1 ± 11.3 | <0.001 |
| Age (years) | 62.8 ± 15.8 | 67.0 ± 15.5 | 57.5 ± 17.8 | 61.1 ± 18.2 | 65.7 ± 8.6 | 0.10 |
| Male sex (n, %) | 70, (64.8%) | 19, (70.4%) | 17, (63.0%) | 15, (55.6) | 19, (70.4%) | 0.62 |
| Time on PD (months) | 25.9 ± 28.5 | 19.3 ± 15.9 | 28.3 ± 37.1 | 29.6 ± 35.7 | 26.2 ± 19.1 | 0.56 |
| Mode of PD (n, %) | 0.16 | |||||
| Continuous ambulatory PD (n, %) | 52, (48.1%) | 18, (66.7%) | 12, (44.4%) | 11, (40.7%) | 11, (40.7%) | |
| Automated PD (n, %) | 56, (51.9%) | 9, (33.3%) | 15, (55.6%) | 16, (59.3%) | 16, (59.3%) | |
| BMI (kg/m2) | 26.4 ± 4.5 | 26.2 ± 3.6 | 25.9 ± 4.2 | 26.0 ± 4.9 | 27.6 ± 5.0 | 0.47 |
| Presence of diabetes (n, %) | 39, (36.1%) | 11, (40.7%) | 7, (25.9%) | 8, (29.6%) | 13, (48.1%) | 0.30 |
| Pre-existing cardiovascular disease (n, %) | 48, (44.4%) | 18, (66.7%) | 12, (44.4%) | 6, (22.2%) | 12, (44.4%) | <0.05 |
| Hemoglobin (g/dL) | 11.5 ± 1.5 | 11.6 ± 1.8 | 11.9 ± 1.1 | 11.7 ± 1.6 | 10.9 ± 1.2 | 0.08 |
| Serum albumin (g/dL) | 3.7 ± 0.4 | 3.6 ± 0.5 | 3.8 ± 0.3 | 3.8 ± 0.4 | 3.6 ± 0.4 | 0.07 |
| Antihypertensive drug use (n, %) | 98, (90.7%) | 24 (88.9%) | 24 (88.9%) | 25, (92.6%) | 25, (92.6%) | 0.55 |
| ACEIs or ARBs (n, %) | 43, (39.8%) | 3, (11.1%) | 12, (44.4%) | 12, (44.4%) | 16, (59.3%) | 0.001 |
| CCBs (n, %) | 61, (56.5%) | 6, (22.2%) | 16, (59.3%) | 18, (66.7%) | 21, (77.8%) | <0.001 |
| β-blockers (n, %) | 90, (83.3%) | 22, (81.5%) | 23, (85.2%) | 21, (77.8%) | 24, (88.9%) | 0.72 |
| Clinic SBP (mmHg) | 132.9 ± 19.4 | 112.3 ± 11.4 | 130.6 ± 12.7 | 135.6 ± 13.5 | 153.0 ± 14.3 | <0.001 |
| Clinic DBP (mmHg) | 78.0 ± 12.9 | 69.7 ± 9.9 | 74.8 ± 12.8 | 83.6 ± 11.6 | 84.0 ± 11.8 | <0.001 |
| SBP | Unadjusted Analysis | Adjusted Analysis * | |||||
|---|---|---|---|---|---|---|---|
| Clinic | Range (mmHg) | HR | 95% CI | p Value | HR | 95% CI | p Value |
| Quartile 1 | <119.2 | 1 | 1 | ||||
| Quartile 2 | 119.2–132.0 | 0.201 | 0.057–0.711 | <0.05 | 0.255 | 0.069–0.940 | <0.05 |
| Quartile 3 | 132.0–145.7 | 1.028 | 0.486–2.176 | 0.94 | 1.472 | 0.651–3.331 | 0.35 |
| Quartile 4 | >145.7 | 1.750 | 0.851–3.598 | 0.13 | 1.648 | 0.766–3.547 | 0.20 |
| Model fit (χ2): 16.5 p = 0.001 | Model fit (χ2): 42.0 p < 0.001 | ||||||
| 24-h Ambulatory | |||||||
| Quartile 1 | <114.0 | 1 | 1 | ||||
| Quartile 2 | 114.0–126.0 | 0.667 | 0.280–1.586 | 0.36 | 1.098 | 0.434–2.777 | 0.84 |
| Quartile 3 | 126.0–140.7 | 0.558 | 0.228–1.367 | 0.20 | 1.004 | 0.382–2.635 | 0.99 |
| Quartile 4 | >140.7 | 2.240 | 1.103–4.547 | <0.05 | 2.449 | 1.156–5.190 | <0.05 |
| Model fit (χ2): 18.4 p < 0.001 | Model fit (χ2): 40.3 p < 0.001 | ||||||
| Ambulatory SBP | Unadjusted Analysis | Adjusted Analysis * | |||||
|---|---|---|---|---|---|---|---|
| Daytime | Range (mmHg) | HR | 95% CI | p Value | HR | 95% CI | p Value |
| Quartile 1 | <114.2 | 1 | 1 | ||||
| Quartile 2 | 114.2–126.5 | 0.503 | 0.198–1.280 | 0.15 | 0.854 | 0.313–2.328 | 0.76 |
| Quartile 3 | 126.5–142.7 | 0.651 | 0.281–1.508 | 0.32 | 1.061 | 0.427–2.637 | 0.89 |
| Quartile 4 | >142.7 | 2.646 | 1.292–5.422 | <0.01 | 2.631 | 1.247–5.535 | 0.01 |
| Model fit (χ2): 25.0 p < 0.001 | Model fit (χ2): 43.9 p < 0.001 | ||||||
| Nighttime | |||||||
| Quartile 1 | <108.2 | 1 | 1 | ||||
| Quartile 2 | 108.2–123.0 | 0.357 | 0.135–0.939 | <0.05 | 0.594 | 0.214–1.650 | 0.32 |
| Quartile 3 | 123.0–140.0 | 0.619 | 0.271–1.413 | 0.26 | 1.555 | 0.575–4.206 | 0.38 |
| Quartile 4 | >140.0 | 1.886 | 0.947–3.759 | 0.07 | 2.305 | 1.047–5.072 | <0.05 |
| Model fit (χ2): 19.5 p < 0.001 | Model fit (χ2): 40.6 p < 0.001 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgianos, P.I.; Vaios, V.; Zebekakis, P.E.; Liakopoulos, V. The Relation of Clinic and Ambulatory BP with the Risk of Cardiovascular Events and All-Cause Mortality among Patients on Peritoneal Dialysis. J. Clin. Med. 2021, 10, 2232. https://doi.org/10.3390/jcm10112232
Georgianos PI, Vaios V, Zebekakis PE, Liakopoulos V. The Relation of Clinic and Ambulatory BP with the Risk of Cardiovascular Events and All-Cause Mortality among Patients on Peritoneal Dialysis. Journal of Clinical Medicine. 2021; 10(11):2232. https://doi.org/10.3390/jcm10112232
Chicago/Turabian StyleGeorgianos, Panagiotis I., Vasilios Vaios, Pantelis E. Zebekakis, and Vassilios Liakopoulos. 2021. "The Relation of Clinic and Ambulatory BP with the Risk of Cardiovascular Events and All-Cause Mortality among Patients on Peritoneal Dialysis" Journal of Clinical Medicine 10, no. 11: 2232. https://doi.org/10.3390/jcm10112232
APA StyleGeorgianos, P. I., Vaios, V., Zebekakis, P. E., & Liakopoulos, V. (2021). The Relation of Clinic and Ambulatory BP with the Risk of Cardiovascular Events and All-Cause Mortality among Patients on Peritoneal Dialysis. Journal of Clinical Medicine, 10(11), 2232. https://doi.org/10.3390/jcm10112232








