Acute and Preventive Management of Migraine during Menstruation and Menopause
Abstract
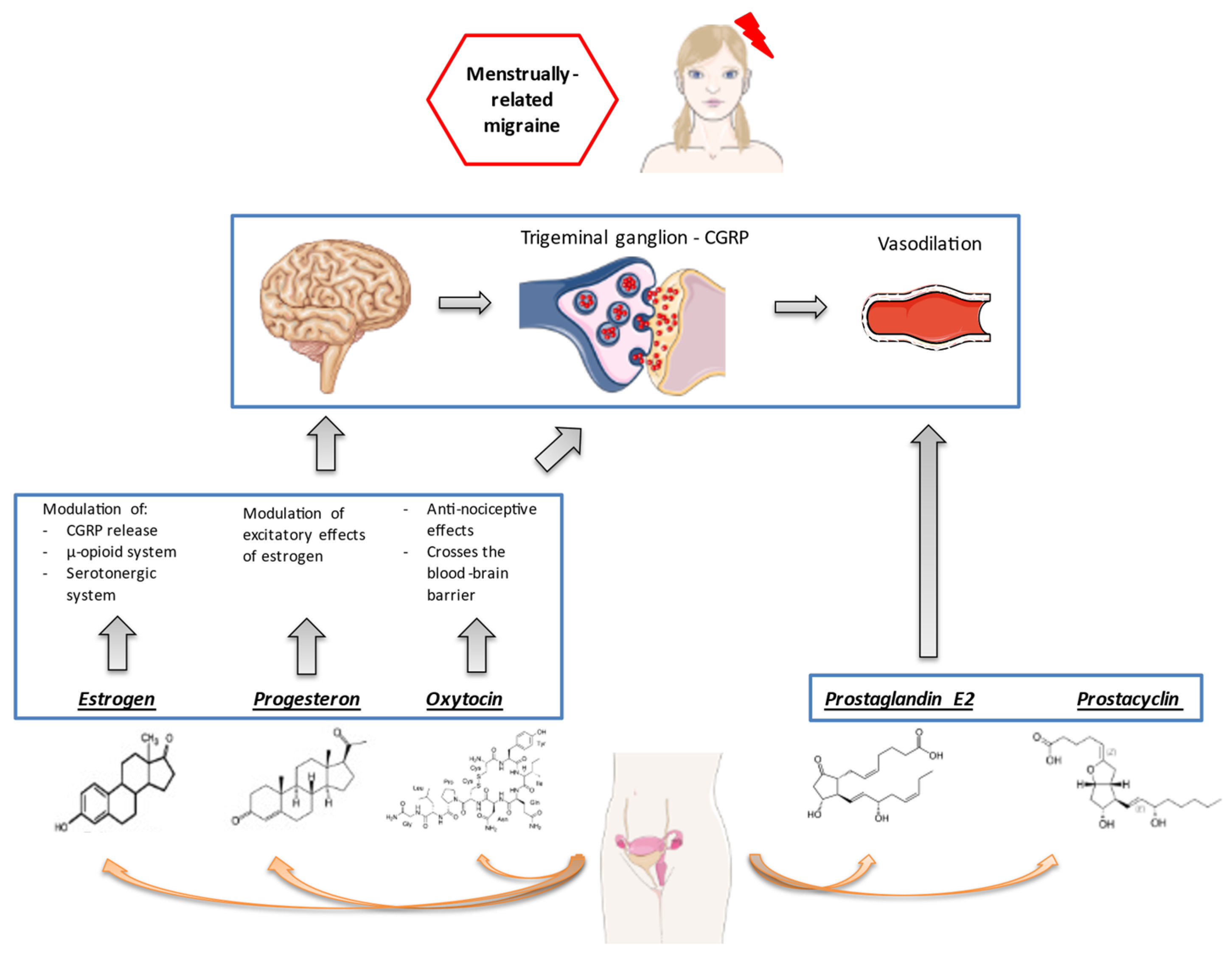
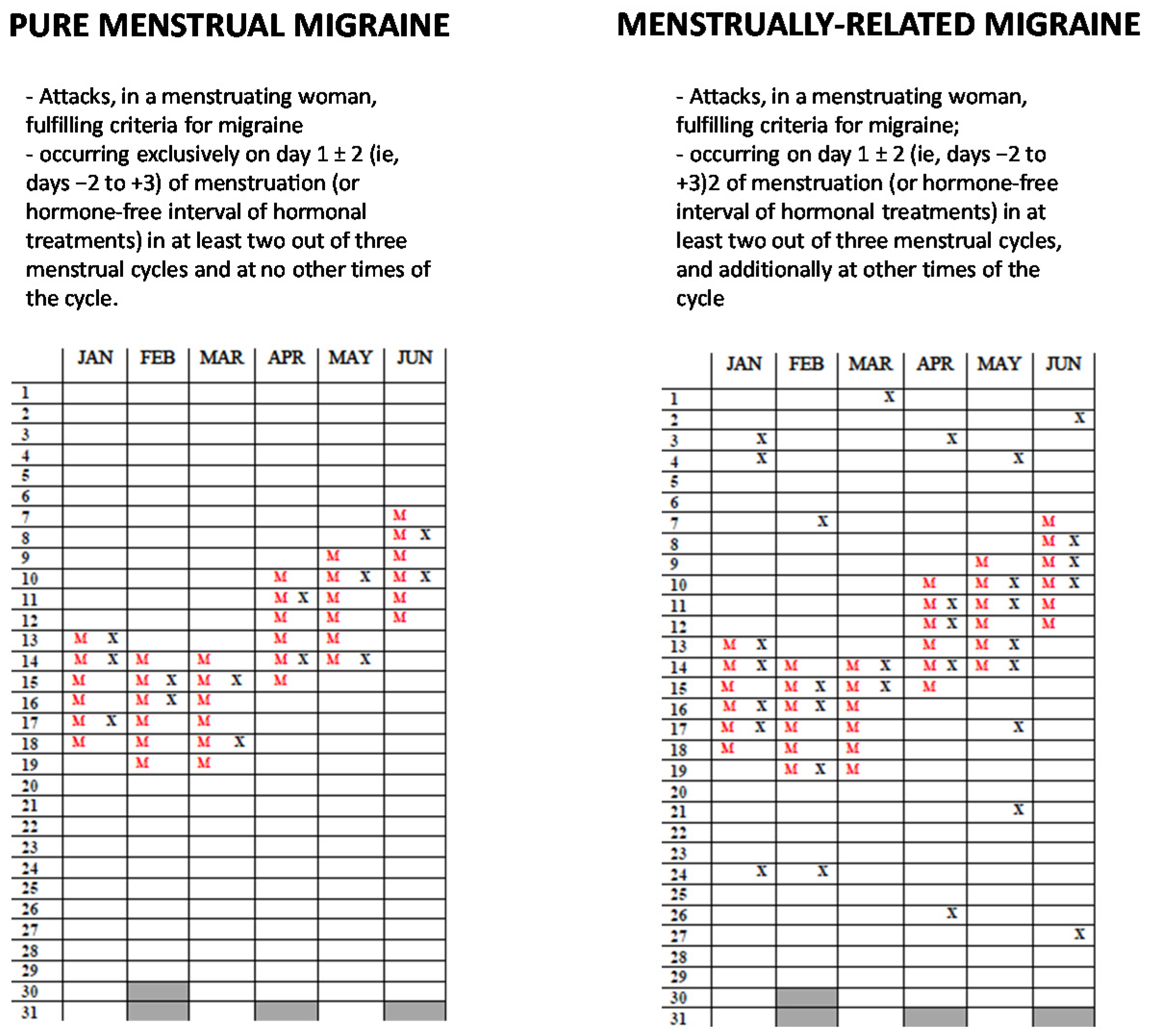
1. Introduction
2. Methods
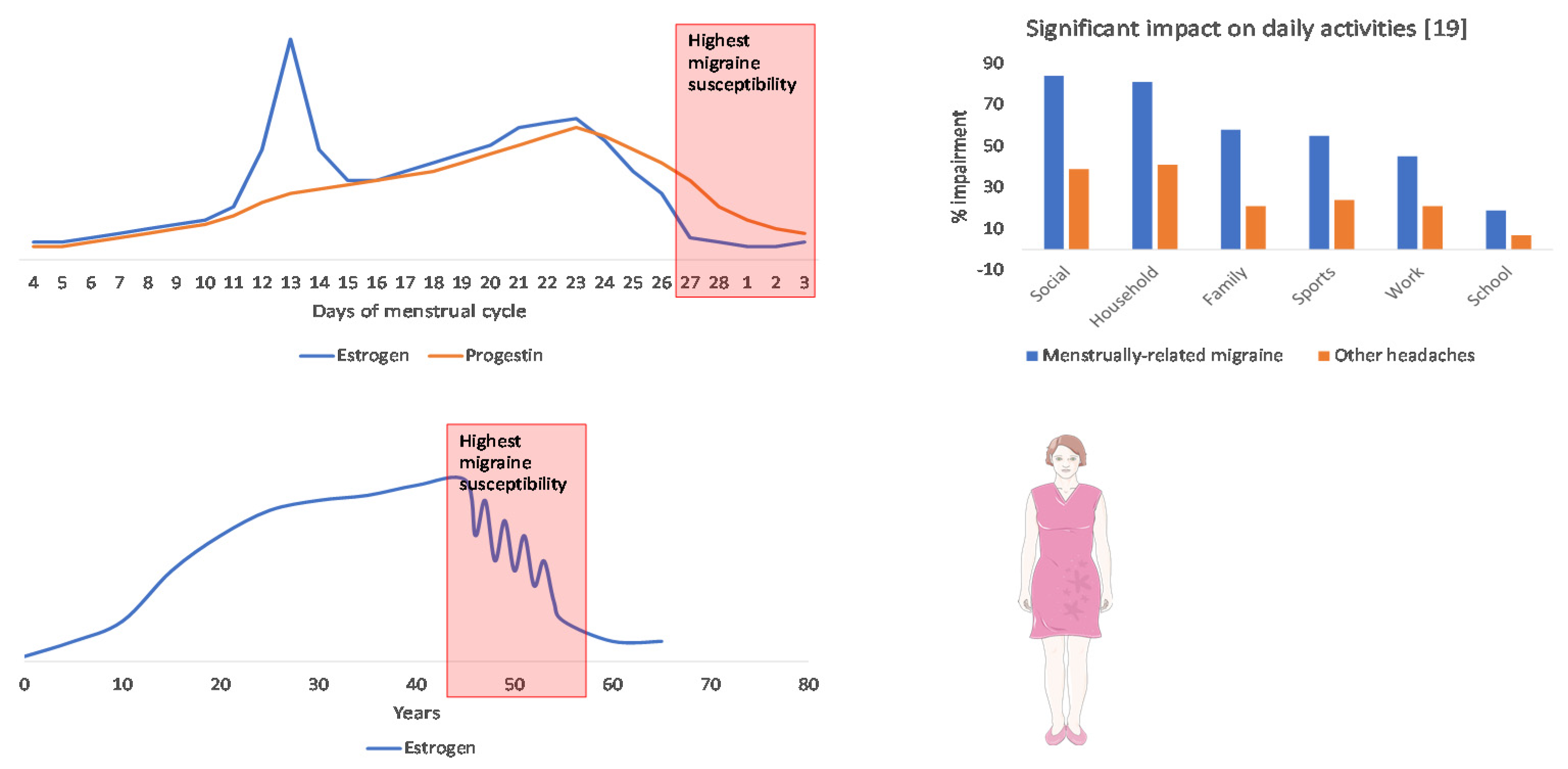
3. The Burden and Unmet Needs of Women with Menstrual and Perimenopausal Migraine
4. Acute Treatment of Menstrual Migraine
4.1. Triptans
4.2. Common Analgesics
4.3. General Considerations on the Acute Treatment of MM
| Drug | Available Studies | Main Findings |
|---|---|---|
| Single drug | ||
| Frovatriptan | 5 RCTs vs. other triptans [40,41,42]; 1 OLS | Early relief: superior to placebo, equivalent to other triptans Sustained relief: superior to almotriptan, rizatriptan, zolmitriptan Non-headache symptoms: effective on nausea and phonophobia, not on other symptoms Adverse events: comparable to placebo Other outcomes: higher patient satisfaction with frovatriptan compared with previous treatments |
| Sumatriptan | 3 RCTs [33,34,35] | Early relief: superior to placebo Sustained relief: comparable to placebo Non-headache symptoms: effective on photophobia and phonophobia Adverse events: comparable to placebo |
| Naratriptan | 1 RCT [37] | Early relief: superior to placebo Sustained relief: superior to placebo Non-headache symptoms: superior to placebo for all symptoms Adverse events: comparable to placebo Other outcomes: superior to placebo in ability to carry on daily activities and patient satisfaction |
| Zolmitriptan | 1 RCT [38] | Early relief: superior to placebo Adverse events: comparable to placebo |
| Almotriptan | 1 RCT [39] | Superior to placebo in pain-free status at 2 and 24 h; significant reduction in nausea and photophobia; adverse events comparable to placebo |
| Combination drugs | ||
| Sumatriptan + naproxen | 5 RCTs [50,51,52] | Early relief: superior to placebo Sustained relief: superior to placebo, especially with comorbid dysmenorrhea Adverse events: comparable to placebo Other outcomes: patient satisfaction, productivity, quality of life |
| Frovatriptan + dexketoprofen | 1 RCT [53] | Early relief: superior to frovatriptan alone Sustained relief: superior to frovatriptan alone Adverse events: comparable to frovatriptan alone |
5. Prevention of Menstrual Migraine
5.1. Short-Term Prevention with Triptans or NSAIDs
5.2. Hormonal Prevention
| Drug | Available Studies | Treatment Protocol | Main Findings |
|---|---|---|---|
| NSAIDs | |||
| Naproxen | 1 RCT [64] | 500 mg twice daily for 14 days for 3 cycles | Significant reduction in number, duration, and severity of attacks compared with placebo only during the 2nd and 3rd cycle |
| Nimesulide | 1 RCT [65] | 100 mg thrice daily for 10 days for 3 cycles | Significant reduction in pain intensity and duration compared with placebo during all the cycles |
| Triptans | |||
| Frovatriptan | 2 RCTs, 1 open-label extension [66,67,68] | 2.5 mg daily or twice daily for 6 days | Significant reduction in headache days, headache intensity, headache duration, and use of rescue medication; twice daily formulation better than daily formulation |
| Naratriptan | 1 RCT [70] | 1 mg or 2.5 mg twice daily for 5 days for 3 cycles | Significant reduction in headache days, headache intensity, headache duration, and use of rescue medication; significant improvement in quality of life; 2.5 mg dose better than 1 mg dose |
| Zolmitriptan | 1 RCT [71] | 2.5 mg twice or thrice daily for 7 days for 3 cycles | Significant reduction in headache days, pain recurrence, and rescue medication with both doses |
| Hormone supplementation | |||
| Percutaneous estradiol | 3 RCTs [75,76,77] | 7–10 days for 2–3 cycles | Significant reduction in headache days and acute medication use, only during the treatment, with subsequent rebound headache; good tolerability profile |
| Transdermal 17 β-estradiol | 1 RCTs [78] | 6 days for 3 cycles | Estradiol effective only if synchronized with menstruation |
| Conjugated equine estrogens | 1 Open-label [74] | 7 days (hormone-free interval of a combined contraceptive) for 2 cycles | At least 50% reduction in monthly headache days in all treated women; improvement in menstrual symptoms |
5.3. Non-Pharmacological Treatments
6. Considerations on the Treatment of Perimenopausal Migraine
Hormonal Treatments
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Collaborators, G.H. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 954–976. [Google Scholar]
- Burch, R.C.; Buse, D.C.; Lipton, R.B. Migraine: Epidemiology, Burden, and Comorbidity. Neurol. Clin. 2019, 37, 631–649. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T.J.; Stovner, L.J.; Jensen, R.; Uluduz, D.; Katsarava, Z. Migraine remains second among the world’s causes of disability, and first among young women: Findings from GBD2019. J. Headache Pain 2020, 21, 137. [Google Scholar] [CrossRef] [PubMed]
- Vetvik, K.G.; MacGregor, E.A. Menstrual migraine: A distinct disorder needing greater recognition. Lancet Neurol. 2021, 20, 304–315. [Google Scholar] [CrossRef]
- Victor, T.W.; Hu, X.; Campbell, J.C.; Buse, D.C.; Lipton, R.B. Migraine prevalence by age and sex in the United States: A life-span study. Cephalalgia 2010, 30, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Delaruelle, Z.; Ivanova, T.A.; Khan, S.; Negro, A.; Ornello, R.; Raffaelli, B.; Terrin, A.; Mitsikostas, D.D.; Reuter, U. Male and female sex hormones in primary headaches. J. Headache Pain. 2018, 19, 117. [Google Scholar] [CrossRef]
- MacGregor, E.A.; Frith, A.; Ellis, J.; Aspinall, L.; Hackshaw, A. Incidence of migraine relative to menstrual cycle phases of rising and falling estrogen. Neurology 2006, 67, 2154–2158. [Google Scholar] [CrossRef]
- Calhoun, A.H. Understanding Menstrual Migraine. Headache 2018, 58, 626–630. [Google Scholar] [CrossRef]
- Sacco, S.; Ripa, P.; Ornello, R.; Degan, D.; Tiseo, C.; Stewart, J.; Pistoia, F.; Carolei, A.; Sacco, S. Migraine in menopausal women: A systematic review. Int. J. Women’s Health 2015, 7, 773–782. [Google Scholar] [CrossRef]
- Ramírez, A.L.; Rubio-Beltrán, E.; Villalón, C.M.; MaassenVanDenBrink, A. Gender aspects of CGRP in migraine. Cephalalgia 2017, 39, 435–444. [Google Scholar] [CrossRef]
- Warfvinge, K.; Krause, D.N.; Maddahi, A.; Edvinsson, J.C.A.; Edvinsson, L.; Haanes, K.A. Estrogen receptors α, β and GPER in the CNS and trigeminal system-molecular and functional aspects. J. Headache Pain 2020, 21, 131. [Google Scholar] [CrossRef]
- Rossetti, M.F.; Cambiasso, M.J.; Holschbach, M.A.; Cabrera, R. Oestrogens and Progestagens: Synthesis and Action in the Brain. J. Neuroendocr. 2016, 28. [Google Scholar] [CrossRef] [PubMed]
- Amandusson, Å.; Blomqvist, A. Estrogenic influences in pain processing. Front. Neuroendocr. 2013, 34, 329–349. [Google Scholar] [CrossRef] [PubMed]
- Tzabazis, A.; Kori, S.; Mechanic, J.; Miller, J.; Pascual, C.; Manering, N.; Carson, D.; Klukinov, M.; Spierings, E.; Jacobs, D.; et al. Oxytocin and Migraine Headache. Headache: J. Head Face Pain 2017, 57 (Suppl. 2), 64–75. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, M.; Puri, V.; Puri, S. Effects of Estrogen on the Serotonergic System and Calcitonin Gene-Related Peptide in Trigeminal Ganglia of Rats. Ann. Neurosci. 2012, 19, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Tzabazis, A.; Mechanic, J.; Miller, J.; Klukinov, M.; Pascual, C.; Manering, N.; Carson, D.S.; Jacobs, A.; Qiao, Y.; Cuellar, J.; et al. Oxytocin receptor: Expression in the trigeminal nociceptive system and potential role in the treatment of headache disorders. Cephalalgia 2016, 36, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Hornung, R.; Benton, W.L.; Tongkhuya, S.; Uphouse, L.; Kramer, P.R.; Averitt, D.L. Progesterone and Allopregnanolone Rapidly Attenuate Estrogen-Associated Mechanical Allodynia in Rats with Persistent Temporomandibular Joint Inflammation. Front. Integr. Neurosci. 2020, 14, 26. [Google Scholar] [CrossRef]
- Olesen, J. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar]
- Couturier, E.G.M.; Bomhof, M.A.M.; Neven, A.K.; Van Duijn, N.P. Menstrual Migraine in A Representative Dutch Population Sample: Prevalence, Disability and Treatment. Cephalalgia 2003, 23, 302–308. [Google Scholar] [CrossRef]
- Vetvik, K.G.; MacGregor, E.A.; Lundqvist, C.; Russell, M.B. Prevalence of menstrual migraine: A population-based study. Cephalalgia 2013, 34, 280–288. [Google Scholar] [CrossRef]
- Granella, F.; Sances, G.; Allais, G.; Nappi, R.E.; Tirelli, A.; Benedetto, C.; Brundu, B.; Facchinetti, F.; Nappi, G. Characteristics of menstrual and nonmenstrual attacks in women with menstrually related migraine referred to headache centres. Cephalalgia 2004, 24, 707–716. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, E.A.; Victor, T.W.; Hu, X.; Xiang, Q.; Puenpatom, R.A.; Chen, W.; Campbell, J.C. Characteristics of Menstrual vs Nonmenstrual Migraine: A Post Hoc, Within-Woman Analysis of the Usual-Care Phase of a Nonrandomized Menstrual Migraine Clinical Trial. Headache J. Head Face Pain 2010, 50, 528–538. [Google Scholar] [CrossRef]
- Vetvik, K.G.; Benth, J.Š.; MacGregor, E.A.; Lundqvist, C.; Russell, M.B. Menstrual versus non-menstrual attacks of migraine without aura in women with and without menstrual migraine. Cephalalgia 2015, 35, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Güven, B.; Güven, H.; Çomoğlu, S. Clinical characteristics of menstrually related and non-menstrual migraine. Acta Neurol. Belg. 2017, 117, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.F.; Mercante, J.P.; Guendler, V.Z.; Corchs, F.; Bernik, M.A.; Zukerman, E.; Silberstein, S.D. Cephalalgiaphobia: A possible specific phobia of illness. J. Headache Pain 2007, 8, 56–59. [Google Scholar] [CrossRef]
- Giannini, G.; Zanigni, S.; Grimaldi, D.; Melotti, R.; Pierangeli, G.; Cortelli, P.; Cevoli, S. Cephalalgiaphobia as a feature of high-frequency migraine: A pilot study. J. Headache Pain 2013, 14, 49. [Google Scholar] [CrossRef]
- Martin, V.T.; Pavlovic, J.; Fanning, K.M.; Buse, D.C.; Reed, M.L.; Lipton, R.B. Perimenopause and Menopause Are Associated With High Frequency Headache in Women With Migraine: Results of the American Migraine Prevalence and Prevention Study. Headache J. Head Face Pain 2016, 56, 292–305. [Google Scholar] [CrossRef]
- Wang, S.J.; Fuh, J.L.; Lu, S.R.; Juang, K.D.; Wang, P.H. Migraine prevalence during menopausal transition. Headache 2003, 43, 470–478. [Google Scholar] [CrossRef]
- Makita, K.; Inagaki, M.; Kitamura, S.; Tatsuoka, Y. Changes in migraine before and after menopause in Japanese climacteric women. Cephalalgia 2017, 37, 1088–1092. [Google Scholar] [CrossRef]
- Oh, K.; Jung, K.-Y.; Choi, J.-Y.; Seo, W.-K.; Park, K.-W. Headaches in Middle-Aged Women during Menopausal Transition: A Headache Clinic-Based Study. Eur. Neurol. 2012, 68, 79–83. [Google Scholar] [CrossRef]
- MacGregor, E.A. Menstruation, Sex Hormones, and Migraine. Neurol. Clin. 1997, 15, 125–141. [Google Scholar] [CrossRef]
- Ornello, R.; Caponnetto, V.; Frattale, I.; Sacco, S. Patterns of Migraine in Postmenopausal Women: A Systematic Review. Neuropsychiatr. Dis. Treat. 2021, 17, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Dowson, A.J.; Massiou, H.; Aurora, S.K. Managing migraine headaches experienced by patients who self-report with menstrually related migraine: A prospective, placebo-controlled study with oral sumatriptan. J. Headache Pain 2005, 6, 81–87. [Google Scholar] [CrossRef][Green Version]
- Landy, S.; Savani, N.; Shackelford, S.; Loftus, J.; Jones, M. Efficacy and tolerability of sumatriptan tablets administered during the mild-pain phase of menstrually associated migraine. Int. J. Clin. Pr. 2004, 58, 913–919. [Google Scholar] [CrossRef]
- Nett, R.; Landy, S.; Shackelford, S.; Richardson, M.S.; Ames, M.; Lener, M. Pain-Free Efficacy after Treatment with Sumatriptan in the Mild Pain Phase of Menstrually Associated Migraine. Obstet. Gynecol. 2003, 102, 835–842. [Google Scholar] [PubMed]
- Newman, L.C.; Harper, S.; Jones, B.A.; Campbell, J. Frovatriptan for Acute Treatment of Migraine Associated with Menstruation: Results from an Open-Label Postmarketing Surveillance Study. J. Women’s Health 2009, 18, 1265–1273. [Google Scholar] [CrossRef]
- Massiou, H.; Jamin, C.; Hinzelin, G.; The French Naramig Collaborative Study Group; Bidaut-Mazel, C. Efficacy of oral naratriptan in the treatment of menstrually related migraine. Eur. J. Neurol. 2005, 12, 774–781. [Google Scholar] [CrossRef]
- Loder, E.; Silberstein, S.D.; Abu-Shakra, S.; Mueller, L.; Smith, T. Efficacy and tolerability of oral zolmitriptan in menstrually associated migraine: A randomized, prospective, parallel-group, double-blind, placebo-controlled study. Headache 2004, 44, 120–130. [Google Scholar] [CrossRef]
- Allais, G.; Bussone, G.; D’Andrea, G.; Moschiano, F.; d’Onofrio, F.; Valguarnera, F.; Manzoni, G.C.; Grazzi, L.; Allais, R.; Benedetto, C.; et al. Almotriptan 12.5 mg in menstrually related migraine: A randomized, double-blind, placebo-controlled study. Cephalalgia 2011, 31, 144–151. [Google Scholar] [CrossRef]
- Allais, G.; Tullo, V.; Omboni, S.; Benedetto, C.; Sances, G.; Zava, D.; Ferrari, M.D.; Bussone, G. Efficacy of frovatriptan versus other triptans in the acute treatment of menstrual migraine: Pooled analysis of three double-blind, randomized, crossover, multicenter studies. Neurol. Sci. 2012, 33 (Suppl. 1), S65–S69. [Google Scholar] [CrossRef][Green Version]
- Bartolini, M.; Giamberardino, M.A.; Lisotto, C.; Martelletti, P.; Moscato, D.; Panascia, B.; Savi, L.; Pini, L.A.; Sances, G.; Santoro, P.; et al. Frovatriptan versus almotriptan for acute treatment of menstrual migraine: Analysis of a double-blind, randomized, cross-over, multicenter, Italian, comparative study. J. Headache Pain 2012, 13, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Savi, L.; Omboni, S.; Lisotto, C.; Zanchin, G.; Ferrari, M.D.; Zava, D.; Pinessi, L. Efficacy of frovatriptan in the acute treatment of menstrually related migraine: Analysis of a double-blind, randomized, cross-over, multicenter, Italian, comparative study versus rizatriptan. J. Headache Pain 2011, 12, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Durham, P.L.; Vause, C.V.; Derosier, F.; McDonald, S.; Cady, R.; Martin, V. Changes in salivary prostaglandin levels during menstrual migraine with associated dysmenorrhea. Headache 2010, 50, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Mannix, L.K. Menstrual-Related Pain Conditions: Dysmenorrhea and Migraine. J. Women’s Health 2008, 17, 879–891. [Google Scholar] [CrossRef]
- Antonova, M.; Wienecke, T.; Olesen, J.; Ashina, M. Prostaglandin E(2) induces immediate migraine-like attack in migraine patients without aura. Cephalalgia 2012, 32, 822–833. [Google Scholar] [CrossRef]
- Al-Waili, N.S. Treatment of menstrual migraine with prostaglandin synthesis inhibitor mefenamic acid: Double-blind study with placebo. Eur. J. Med. Res. 2000, 5, 176–182. [Google Scholar]
- Vane, J.R.; Botting, R.M. Mechanism of action of nonsteroidal anti-inflammatory drugs. Am. J Med. 1998, 104, 2S–8S, discussion 21S–22S. [Google Scholar] [CrossRef]
- Facchinetti, F.; Allais, G.; Nappi, R.E.; Gabellari, I.C.; Di Renzo, G.C.; Genazzani, A.R.; Bellafronte, M.; Roncolato, M.; Benedetto, C. Sumatriptan (50 mg tablets vs. 25 mg suppositories) in the acute treatment of menstrually related migraine and oral contraceptive-induced menstrual migraine: A pilot study. Gynecol. Endocrinol. 2010, 26, 773–779. [Google Scholar] [CrossRef]
- Allais, G.; Castagnoli Gabellari, I.; Rolando, S.; Benedetto, C. Evaluation of the use of sumatriptan-naproxen sodium for menstrual migraine and dysmenorrhea. Expert Rev. Neurother. 2011, 11, 1383–1387. [Google Scholar] [CrossRef]
- Cady, R.K.; Diamond, M.L.; Diamond, M.P.; Ballard, J.E.; Lener, M.E.; Dorner, D.P.; DeRosier, F.J.; McDonald, S.A.; White, J.; Runken, M.C. Sumatriptan-Naproxen Sodium for Menstrual Migraine and Dysmenorrhea: Satisfaction, Productivity, and Functional Disability Outcomes. Headache 2011, 51, 664–673. [Google Scholar] [CrossRef]
- Martin, V.T.; Ballard, J.; Diamond, M.P.; Mannix, L.K.; DeRosier, F.J.; Lener, S.E.; Krishen, A.; McDonald, S.A. Relief of Menstrual Symptoms and Migraine with a Single-Tablet Formulation of Sumatriptan and Naproxen Sodium. J. Women’s Health 2014, 23, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Mannix, L.K.; Martin, V.T.; Cady, R.K.; Diamond, M.L.; Lener, S.E.; White, J.D.; DeRosier, F.J.; McDonald, S.A. Combination treatment for menstrual migraine and dysmenorrhea using sumatriptan-naproxen: Two randomized controlled trials. Obstet. Gynecol. 2009, 114, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Allais, G.; Bussone, G.; Tullo, V.; Cortelli, P.; Valguarnera, F.; Barbanti, P.; Sette, G.; D’Onofrio, F.; Curone, M.; Benedetto, C. Frovatriptan 2.5 mg plus dexketoprofen (25 mg or 37.5 mg) in menstrually related migraine. Subanalysis from a double-blind, randomized trial. Cephalalgia 2014, 35, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Ornello, R.; Ripa, P.; Pistoia, F.; Degan, D.; Tiseo, C.; Carolei, A.; Sacco, S. Migraine and body mass index categories: A systematic review and meta-analysis of observational studies. J. Headache Pain 2015, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Gelaye, B.; Sacco, S.; Brown, W.J.; Nitchie, H.L.; Ornello, R.; Peterlin, B.L. Body composition status and the risk of migraine: A meta-analysis. Neurology 2017, 88, 1795–1804. [Google Scholar] [CrossRef] [PubMed]
- Peterlin, B.L.; Rosso, A.L.; Williams, M.A.; Rosenberg, J.R.; Haythornthwaite, J.A.; Merikangas, K.R.; Gottesman, R.F.; Bond, D.S.; He, J.P.; Zonderman, A.B. Episodic migraine and obesity and the influence of age, race, and sex. Neurology 2013, 81, 1314–1321. [Google Scholar] [CrossRef]
- Peterlin, B.L.; Sacco, S.; Bernecker, C.; Scher, A.I. Adipokines and Migraine: A Systematic Review. Headache 2016, 56, 622–644. [Google Scholar] [CrossRef]
- Leeners, B.; Geary, N.; Tobler, P.N.; Asarian, L. Ovarian hormones and obesity. Hum. Reprod. Update 2017, 23, 300–321. [Google Scholar] [CrossRef]
- Allais, G.; Del Rio, M.S.; Diener, H.-C.; Benedetto, C.; Pfeil, J.; Schäuble, B.; von Oene, J. Perimenstrual migraines and their response to preventive therapy with topiramate. Cephalalgia 2010, 31, 152–160. [Google Scholar] [CrossRef]
- Pavlovic, J.M.; Paemeleire, K.; Göbel, H.; Bonner, J.; Rapoport, A.; Kagan, R.; Zhang, F.; Picard, H.; Mikol, D.D.; Rapoport, A. Efficacy and safety of erenumab in women with a history of menstrual migraine. J. Headache Pain 2020, 21, 95. [Google Scholar] [CrossRef]
- Ornello, R.; Frattale, I.; Caponnetto, V.; De Matteis, E.; Pistoia, F.; Sacco, S. Menstrual Headache in Women with Chronic Migraine Treated with Erenumab: An Observational Case Series. Brain Sci. 2021, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Schwedt, T.J. Preventive Therapy of Migraine. Contin. Lifelong Learn. Neurol. 2018, 24, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, E.A. Perimenstrual headaches: Unmet needs. Curr. Pain Headache Rep. 2008, 12, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Sances, G.; Martignoni, E.; Fioroni, L.; Blandini, F.; Facchinetti, F.; Nappi, G. Naproxen sodium in menstrual migraine prophylaxis: A double-blind placebo controlled study. Headache 1990, 30, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Giacovazzo, M.; Gallo, M.F.; Guidi, V.; Rico, R.; Scaricabarozzi, I. Nimesulide in the Treatment of Menstrual Migraine. Drugs 1993, 46 (Suppl. 1), 140–141. [Google Scholar] [CrossRef]
- Brandes, J.L.; Poole, A.; Kallela, M.; Schreiber, C.P.; MacGregor, E.A.; Silberstein, S.D.; Tobin, J.; Shaw, R. Short-term frovatriptan for the prevention of difficult-to-treat menstrual migraine attacks. Cephalalgia 2009, 29, 1133–1148. [Google Scholar] [CrossRef]
- MacGregor, E.A.; Brandes, J.L.; Silberstein, S.; Jeka, S.; Czapinski, P.; Shaw, B.; Pawsey, S. Safety and Tolerability of Short-Term Preventive Frovatriptan: A Combined Analysis. Headache J. Head Face Pain 2009, 49, 1298–1314. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.D.; Elkind, A.H.; Schreiber, C.; Keywood, C. A randomized trial of frovatriptan for the intermittent prevention of menstrual migraine. Neurology 2004, 63, 261–269. [Google Scholar] [CrossRef]
- Mannix, L.K.; Savani, N.; Landy, S.; Valade, M.; Shackelford, S.; Ames, M.H.; Jones, M.W. Efficacy and Tolerability of Naratriptan for Short-Term Prevention of Menstrually Related Migraine: Data from Two Randomized, Double-Blind, Placebo-Controlled Studies. Headache J. Head Face Pain 2007, 47, 1037–1049. [Google Scholar] [CrossRef]
- Newman, L.; Mannix, L.K.; Landy, S.; Silberstein, S.; Lipton, R.B.; Putnam, D.G.; Watson, C.; Jöbsis, M.; Batenhorst, A.; O’Quinn, S. Naratriptan as short-term prophylaxis of menstrually associated migraine: A randomized, double-blind, placebo-controlled study. Headache 2001, 41, 248–256. [Google Scholar] [CrossRef]
- Tuchman, M.M.; Hee, A.; Emeribe, U.; Silberstein, S. Oral zolmitriptan in the short-term prevention of menstrual migraine: A randomized, placebo-controlled study. CNS Drugs 2008, 22, 877–886. [Google Scholar] [CrossRef]
- Silberstein, S.; Patel, S. Menstrual migraine: An updated review on hormonal causes, prophylaxis and treatment. Expert Opin. Pharmacother. 2014, 15, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Sacco, S.; Merki-Feld, G.S.; Ægidius, K.L.; Bitzer, J.; Canonico, M.; Gantenbein, A.R.; Kurth, T.; Lampl, C.; Lidegaard, Ø.; MacGregor, E.A.; et al. Effect of exogenous estrogens and progestogens on the course of migraine during reproductive age: A consensus statement by the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESCRH). J. Headache Pain 2018, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, A.H. A Novel Specific Prophylaxis for Menstrual-associated Migraine. South Med. J. 2004, 97, 819–822. [Google Scholar] [CrossRef]
- De Lignières, B.; Vincens, M.; Mauvais-Jarvis, P.; Mas, J.L.; Touboul, P.J.; Bousser, M.G. Prevention of menstrual migraine by percutaneous oestradiol. Br. Med. J. Clin. Res. Ed. 1986, 293, 1540. [Google Scholar] [CrossRef]
- Dennerstein, L.; Morse, C.; Burrows, G.; Oats, J.; Brown, J.; Smith, M. Menstrual migraine: A double-blind trial of percutaneous estradiol. Gynecol. Endocrinol. 1988, 2, 113–120. [Google Scholar] [CrossRef]
- MacGregor, E.A.; Frith, A.; Ellis, J.; Aspinall, L.; Hackshaw, A. Prevention of menstrual attacks of migraine: A double-blind placebo-controlled crossover study. Neurology 2006, 67, 2159–2163. [Google Scholar] [CrossRef]
- Smits, M.G.; Van Der Meer, Y.G.; Pfeil, J.; Rijnierse, J.; Vos, A.; Smite, M. Perimenstrual Migraine: Effect of Estraderm TTS(r) and the Value of Contingent Negative Variation and Exteroceptive Temporalis Muscle Suppression Test. Headache 1994, 34, 103–106. [Google Scholar] [CrossRef]
- Almén-Christensson, A.; Hammar, M.; Lindh-Åstrand, L.; Landtblom, A.-M.; Brynhildsen, J. Prevention of menstrual migraine with perimenstrual transdermal 17-β-estradiol: A randomized, placebo-controlled, double-blind crossover study. Fertil. Steril. 2011, 96, 498–500.e1. [Google Scholar] [CrossRef]
- Nappi, R.E.; Terreno, E.; Sances, G.; Martini, E.; Tonani, S.; Santamaria, V.; Tassorelli, C.; Spinillo, A. Effect of a contraceptive pill containing estradiol valerate and dienogest (E2V/DNG) in women with menstrually-related migraine (MRM). Contracept 2013, 88, 369–375. [Google Scholar] [CrossRef]
- Göretzlehner, G.; Waldmann-Rex, S.; Schramm, G.A. Extended cycles with the combined oral contraceptive chlormadinone acetate 2 mg/ethinylestradiol 0.03 mg: Pooled analysis of data from three large-scale, non-interventional, observational studies. Clin. Drug Investig. 2011, 31, 269–277. [Google Scholar] [CrossRef]
- MacGregor, E.A.; Guillebaud, J. The 7-day contraceptive hormone-free interval should be consigned to history. BMJ Sex. Reprod. Health 2018, 44, 214–220. [Google Scholar] [CrossRef]
- Calhoun, A.; Ford, S.; Pruitt, A. The Impact of Extended-Cycle Vaginal Ring Contraception on Migraine Aura: A Retrospective Case Series. Headache 2012, 52, 1246–1253. [Google Scholar] [CrossRef]
- Burke, B.E.; Olson, R.D.; Cusack, B.J. Randomized, controlled trial of phytoestrogen in the prophylactic treatment of menstrual migraine. Biomed. Pharmacother. 2002, 56, 283–288. [Google Scholar] [CrossRef]
- Ferrante, F.; Fusco, E.; Calabresi, P.; Cupini, L.M. Phyto-oestrogens in the Prophylaxis of Menstrual Migraine. Clin. Neuropharmacol. 2004, 27, 137–140. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Y.; Tang, S.; Huang, X.; Chen, T. Current use of oral contraceptives and the risk of first-ever ischemic stroke: A meta-analysis of observational studies. Thromb. Res. 2015, 136, 52–60. [Google Scholar] [CrossRef]
- Ornello, R.; Canonico, M.; Merki-Feld, G.S.; Kurth, T.; Lidegaard, Ø.; MacGregor, E.A.; Lampl, C.; Nappi, R.E.; Martelletti, P.; Sacco, S. Migraine, low-dose combined hormonal contraceptives, and ischemic stroke in young women: A systematic review and suggestions for future research. Expert Rev. Neurother. 2020, 2020, 1–5. [Google Scholar] [CrossRef]
- Granella, F.; Sances, G.; Pucci, E.; Nappi, R.E.; Ghiotto, N.; Napp, G. Migraine with aura and reproductive life events: A case control study. Cephalalgia 2000, 20, 701–707. [Google Scholar] [CrossRef]
- MMacGregor, E.A. Oestrogen and attacks of migraine with and without aura. Lancet Neurol. 2004, 3, 354–361. [Google Scholar] [CrossRef]
- Sandweiss, A.J.; Cottier, K.E.; McIntosh, M.I.; Dussor, G.; Davis, T.P.; Vanderah, T.W.; Largent-Milnes, T.M. 17-β-Estradiol induces spreading depression and pain behavior in alert female rats. Oncotarget 2017, 8, 114109–114122. [Google Scholar] [CrossRef]
- Sacco, S.; Merki-Feld, G.S.; Egidius, K.L.; Bitzer, J.; Canonico, M.; Kurth, T.; Lampl, C.; Lidegaard, Ø.; MacGregor, E.A.; MaassenVanDenBrink, A. Hormonal contraceptives and risk of ischemic stroke in women with migraine: A consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J. Headache Pain 2017, 18, 108. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Wernke, S.; Mandell, K.; Zoma, W.; Bean, J.; Pinney, S.; Liu, J.; Ramadan, N.; Rebar, R. Medical oophorectomy with and without estrogen add-back therapy in the prevention of migraine headache. Headache: J. Head Face Pain 2003, 43, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Sances, G.; Borella, P.; Genazzani, A.R.; Nappi, G. Magnesium prophylaxis of menstrual migraine: Effects on intracellular magnesium. Headache 1991, 31, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Ziaei, S.; Kazemnejad, A.; Sedighi, A. The effect of vitamin E on the treatment of menstrual migraine. Med. Sci. Monit. 2009, 15, CR16-9. [Google Scholar] [PubMed]
- Allais, G.; Gabellari, I.C.; Burzio, C.; Rolando, S.; De Lorenzo, C.; Mana, O.; Benedetto, C. Premenstrual syndrome and migraine. Neurol. Sci. 2012, 33 (Suppl. 1), 111–115. [Google Scholar] [CrossRef]
- Ambrosini, A.; Di Lorenzo, C.; Coppola, G.; Pierelli, F. Use of Vitex agnus-castus in migrainous women with premenstrual syndrome: An open-label clinical observation. Acta Neurol. Belg. 2012, 113, 25–29. [Google Scholar] [CrossRef]
- Wickmann, F.; Stephani, C.; Czesnik, D.; Klinker, F.; Timäus, C.; Chaieb, L.; Paulus, W.; Antal, A. Prophylactic treatment in menstrual migraine: A proof-of-concept study. J. Neurol. Sci. 2015, 354, 103–109. [Google Scholar] [CrossRef]
- Harlow, S.D.; Gass, M.; Hall, J.E.; Lobo, R.; Maki, P.; Rebar, R.W.; Sherman, S.; Sluss, P.M.; de Villiers, T.J.; STRAW + 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: Addressing the unfinished agenda of staging reproductive aging. Menopause 2012, 19, 387–395. [Google Scholar] [CrossRef]
- Carturan, P.; Scorcine, C.; Fragoso, Y.D. Migraine in the post-menopausal period is associated with higher levels of mood disorders, disability, and more menopausal symptoms. Arq. Neuropsiquiatr. 2016, 74, 999–1002. [Google Scholar] [CrossRef]
- Stuenkel, C.A.; Davis, S.R.; Gompel, A.; Lumsden, M.A.; Murad, M.H.; Pinkerton, J.V.; Santen, R.J. Treatment of Symptoms of the Menopause: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2015, 100, 3975–4011. [Google Scholar] [CrossRef]
- Hamoda, H.; Panay, N.; Pedder, H.; Arya, R.; Savvas, M. The British Menopause Society & Women’s Health Concern 2020 recommendations on hormone replacement therapy in menopausal women. Post Reprod. Health 2020, 26, 181–209. [Google Scholar] [PubMed]
- Misakian, A.L.; Langer, R.D.; Bensenor, I.M.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Rexrode, K.M. Postmenopausal hormone therapy and migraine headache. J. Women’s Health 2003, 12, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Aegidius, K.L.; Zwart, J.A.; Hagen, K.; Schei, B.; Stovner, L.J. Hormone replacement therapy and headache prevalence in postmenopausal women. The Head-HUNT study. Eur. J. Neurol. 2007, 14, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Nappi, R.E.; Tirelli, A.; Polatti, F.; Nappi, G. Hormone Supplementation Differently Affects Migraine in Postmenopausal Women. Headache J. Head Face Pain 2002, 42, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Nappi, R.E.; Cagnacci, A.; Granella, F.; Piccinini, F.; Polatti, F.; Facchinetti, F. Course of primary headaches during hormone replacement therapy. Maturitas 2001, 38, 157–163. [Google Scholar] [CrossRef]
- Nappi, R.E.; Sances, G.; Sommacal, A.; Detaddei, S.; Facchinetti, F.; Cristina, S.; Polatti, F.; Nappi, G. Different effects of tibolone and low-dose EPT in the management of postmenopausal women with primary headaches. Menopause 2006, 13, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-N.; Lin, C.-C.; Liu, C.-F. Efficacy of phytoestrogens for menopausal symptoms: A meta-analysis and systematic review. Climacteric 2015, 18, 260–269. [Google Scholar] [CrossRef]
- MacGregor, A. Estrogen replacement and migraine aura. Headache J. Head Face Pain 1999, 39, 674–678. [Google Scholar] [CrossRef]
- Silberstein, S.D.; Merriam, G.R. Estrogens, progestins, and headache. Neurology 1991, 41, 786–793. [Google Scholar] [CrossRef]
- Panay, N.; Studd, J. Progestogen intolerance and compliance with hormone replacement therapy in menopausal women. Hum. Reprod. Update 1997, 3, 159–171. [Google Scholar] [CrossRef]
- Nappi, R.; Sances, G.; Detaddei, S.; Ornati, A.; Chiovato, L.; Polatti, F. Hormonal management of migraine at menopause. Menopause Int. 2009, 15, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Sacco, S.; Cerone, D.; Carolei, A. Comorbid neuropathologies in migraine: An update on cerebrovascular and cardiovascular aspects. J. Headache Pain 2008, 9, 237–248. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sacco, S.; Ricci, S.; Degan, D.; Carolei, A. Migraine in women: The role of hormones and their impact on vascular diseases. J. Headache Pain 2012, 13, 177–189. [Google Scholar] [CrossRef]
- North American Menopause Society. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause 2015, 22, 1155–1172. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, E.A. Migraine, menopause and hormone replacement therapy. Post Reprod. Health 2017, 24, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Joffe, H.; Guthrie, K.A.; LaCroix, A.Z.; Reed, S.D.; Ensrud, K.E.; Manson, J.E.; Newton, K.M.; Freeman, E.W.; Anderson, G.L.; Larson, J.C.; et al. Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: A randomized clinical trial. JAMA Intern. Med. 2014, 174, 1058–1066. [Google Scholar] [CrossRef]
- Ozyalcin, S.N.; Talu, G.K.; Kiziltan, E.; Yucel, B.; Ertas, M.; Disci, R. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache 2005, 45, 144–152. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.T.; Shim, J.Y.; Kim, B.; Hwang, S.H.; Kim, S.H.; Park, J.E.; Park, J.H.; Jung, S.H.; Ahn, J.Y.; et al. The Effect of Paroxetine on the Reduction of Migraine Frequency is Independent of Its Anxiolytic Effect. J. Clin. Neurol. 2006, 2, 246–251. [Google Scholar] [CrossRef]
- Tarlaci, S. Escitalopram and Venlafaxine for the Prophylaxis of Migraine Headache Without Mood Disorders. Clin. Neuropharmacol. 2009, 32, 254–258. [Google Scholar] [CrossRef]
- Linde, M.; Mulleners, W.M.; Chronicle, E.P.; McCrory, D.C. Gabapentin or pregabalin for the prophylaxis of episodic migraine in adults. Cochrane Database Syst. Rev. 2013, 2013, CD010609. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.W.; Guthrie, K.A.; Caan, B.; Sternfeld, B.; Cohen, L.S.; Joffe, H.; Carpenter, J.S.; Anderson, G.L.; Larson, J.C.; Ensrud, K.E.; et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: A randomized controlled trial. JAMA 2011, 305, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Butt, D.A.; Lock, M.; Lewis, J.E.; Ross, S.; Moineddin, R. Gabapentin for the treatment of menopausal hot flashes: A randomized controlled trial. Menopause 2008, 15, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Orleans, R.J.; Li, L.; Kim, M.-J.; Guo, J.; Sobhan, M.; Soule, L.; Joffe, H.V. FDA Approval of Paroxetine for Menopausal Hot Flushes. N. Engl. J. Med. 2014, 370, 1777–1779. [Google Scholar] [CrossRef]
- Lauritsen, C.G.; Chua, A.L.; Nahas, S.J. Current Treatment Options: Headache Related to Menopause-Diagnosis and Management. Curr. Treat. Options Neurol. 2018, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, E.; Guglielmetti, M.; Ornello, R.; Spuntarelli, V.; Martelletti, P.; Sacco, S. Targeting CGRP for migraine treatment: Mechanisms, antibodies, small molecules, perspectives. Expert Rev. Neurother. 2020, 2, 1–15. [Google Scholar] [CrossRef]
- Croop, R.; Goadsby, P.J.; Stock, D.; Conway, C.M.; Forshaw, M.; Stock, E.G.; Coric, V.; Lipton, R.B. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: A randomised, phase 3, double-blind, placebo-controlled trial. Lancet 2019, 394, 737–745. [Google Scholar] [CrossRef]
- Croop, R.; Lipton, R.B.; Kudrow, D.; Stock, D.A.; Kamen, L.; Conway, C.M.; Stock, E.G.; Coric, V.; Goadsby, P.J. Oral rimegepant for preventive treatment of migraine: A phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet 2021, 397, 51–60. [Google Scholar] [CrossRef]
- Lipton, R.B.; Croop, R.; Stock, E.G.; Stock, D.A.; Morris, B.A.; Frost, M.; Dubowchik, G.M.; Conway, C.M.; Coric, V.; Goadsby, P.J. Rimegepant, an Oral Calcitonin Gene–Related Peptide Receptor Antagonist, for Migraine. N. Engl. J. Med. 2019, 381, 142–149. [Google Scholar] [CrossRef]
- Ho, T.W.; Ho, A.P.; Ge, Y.J.; Assaid, C.; Gottwald, R.; MacGregor, E.A.; Mannix, L.K.; van Oosterhout, W.P.J.; Koppenhaver, J.; Lines, C.; et al. Randomized controlled trial of the CGRP receptor antagonist telcagepant for prevention of headache in women with perimenstrual migraine. Cephalalgia 2016, 36, 148–161. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Wietecha, L.A.; Dennehy, E.B.; Kuca, B.; Case, M.G.; Aurora, S.K.; Gaul, C. Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine. Brain 2019, 142, 1894–1904. [Google Scholar] [CrossRef]
- Kuca, B.; Silberstein, S.D.; Wietecha, L.; Berg, P.H.; Dozier, G.; Lipton, R.B.; COL MIG-301 Study Group. Lasmiditan is an effective acute treatment for migraine: A phase 3 randomized study. Neurology 2018, 91, e2222–e2232. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ornello, R.; De Matteis, E.; Di Felice, C.; Caponnetto, V.; Pistoia, F.; Sacco, S. Acute and Preventive Management of Migraine during Menstruation and Menopause. J. Clin. Med. 2021, 10, 2263. https://doi.org/10.3390/jcm10112263
Ornello R, De Matteis E, Di Felice C, Caponnetto V, Pistoia F, Sacco S. Acute and Preventive Management of Migraine during Menstruation and Menopause. Journal of Clinical Medicine. 2021; 10(11):2263. https://doi.org/10.3390/jcm10112263
Chicago/Turabian StyleOrnello, Raffaele, Eleonora De Matteis, Chiara Di Felice, Valeria Caponnetto, Francesca Pistoia, and Simona Sacco. 2021. "Acute and Preventive Management of Migraine during Menstruation and Menopause" Journal of Clinical Medicine 10, no. 11: 2263. https://doi.org/10.3390/jcm10112263
APA StyleOrnello, R., De Matteis, E., Di Felice, C., Caponnetto, V., Pistoia, F., & Sacco, S. (2021). Acute and Preventive Management of Migraine during Menstruation and Menopause. Journal of Clinical Medicine, 10(11), 2263. https://doi.org/10.3390/jcm10112263







