Screening Pregnant Women for Bacterial Vaginosis Using a Point-of-Care Test: A Prospective Validation Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
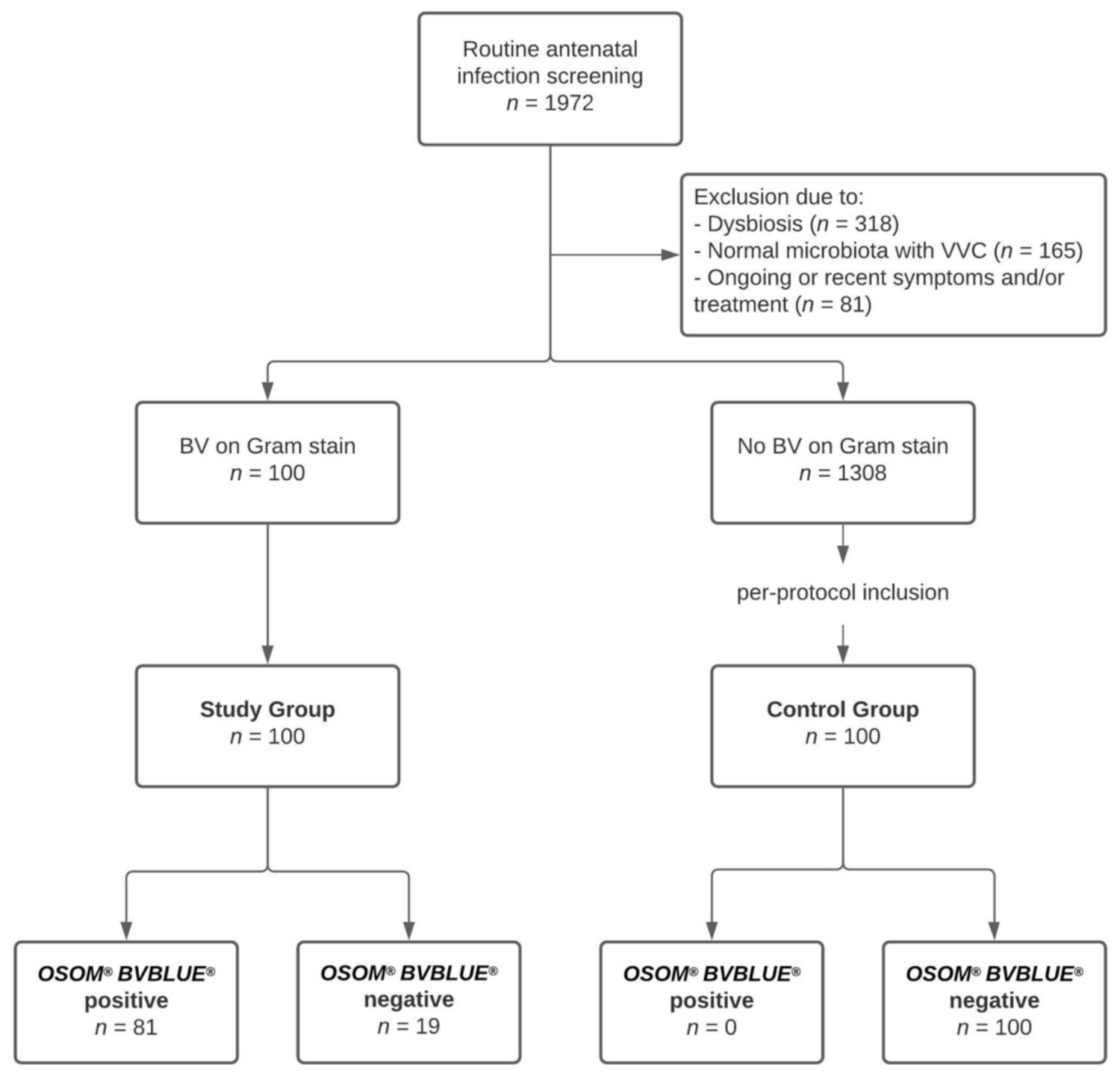
2.2. Setting and Study Population
2.3. Sampling and Gram Staining Procedure
2.4. Study Groups
2.5. Point-of-Care Testing Procedure
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leitich, H.; Bodner-Adler, B.; Brunbauer, M.; Kaider, A.; Egarter, C.; Husslein, P. Bacterial vaginosis as a risk factor for preterm delivery: A meta-analysis. Am. J. Obstet. Gynecol. 2003, 189, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.F.; Nhan-Chang, C.L.; Sobel, J.D.; Workowski, K.; Conde-Agudelo, A.; Romero, R. Treatment of abnormal vaginal flora in early pregnancy with clindamycin for the prevention of spontaneous preterm birth: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2011, 205, 177–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, H.K.; Speechley, K.N.; Macnab, J.; Natale, R.; Campbell, M.K. Neonatal morbidity associated with late preterm and early term birth: The roles of gestational age and biological determinants of preterm birth. Int. J. Epidemiol. 2014, 43, 802–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Foessleitner, P.; Petricevic, L.; Boerger, I.; Steiner, I.; Kiss, H.; Rieger, A.; Touzeau-Roemer, V.; Farr, A. HIV infection as a risk factor for vaginal dysbiosis, bacterial vaginosis, and candidosis in pregnancy: A matched case-control study. Birth 2021. [Google Scholar] [CrossRef]
- Romero, R.; Espinoza, J.; Kusanovic, J.P.; Gotsch, F.; Hassan, S.; Erez, O.; Chaiworapongsa, T.; Mazor, M. The preterm parturition syndrome. Bjog 2006, 113 (Suppl. 3), 17–42. [Google Scholar] [CrossRef]
- Farr, A.; Kiss, H.; Hagmann, M.; Marschalek, J.; Husslein, P.; Petricevic, L. Routine Use of an Antenatal Infection Screen-and-Treat Program to Prevent Preterm Birth: Long-Term Experience at a Tertiary Referral Center. Birth 2015, 42, 173–180. [Google Scholar] [CrossRef]
- Bagnall, P.; Rizzolo, D. Bacterial vaginosis: A practical review. J. Am. Acad. PAs 2017, 30, 15–21. [Google Scholar] [CrossRef]
- Turovskiy, Y.; Sutyak Noll, K.; Chikindas, M.L. The aetiology of bacterial vaginosis. J. Appl. Microbiol. 2011, 110, 1105–1128. [Google Scholar] [CrossRef]
- Hardy, L.; Jespers, V.; Van den Bulck, M.; Buyze, J.; Mwambarangwe, L.; Musengamana, V.; Vaneechoutte, M.; Crucitti, T. The presence of the putative Gardnerella vaginalis sialidase A gene in vaginal specimens is associated with bacterial vaginosis biofilm. PLoS ONE 2017, 12, e0172522. [Google Scholar] [CrossRef] [Green Version]
- Govinden, G.; Parker, J.L.; Naylor, K.L.; Frey, A.M.; Anumba, D.O.C.; Stafford, G.P. Inhibition of sialidase activity and cellular invasion by the bacterial vaginosis pathogen Gardnerella vaginalis. Arch. Microbiol. 2018, 200, 1129–1133. [Google Scholar] [CrossRef] [Green Version]
- Cauci, S.; Culhane, J.F. High sialidase levels increase preterm birth risk among women who are bacterial vaginosis–positive in early gestation. Am. J. Obstet. Gynecol. 2011, 204, 142.e141–142.e149. [Google Scholar] [CrossRef]
- Kiss, H.; Petricevic, L.; Husslein, P. Prospective randomised controlled trial of an infection screening programme to reduce the rate of preterm delivery. BMJ 2004, 329, 371. [Google Scholar] [CrossRef] [Green Version]
- Amsel, R.; Totten, P.A.; Spiegel, C.A.; Chen, K.C.; Eschenbach, D.; Holmes, K.K. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 1983, 74, 14–22. [Google Scholar] [CrossRef]
- Myziuk, L.; Romanowski, B.; Johnson, S.C. BVBlue test for diagnosis of bacterial vaginosis. J. Clin. Microbiol. 2003, 41, 1925–1928. [Google Scholar] [CrossRef] [Green Version]
- Vardar, E.; Maral, I.; Inal, M.; Ozgüder, O.; Tasli, F.; Postaci, H. Comparison of Gram stain and Pap smear procedures in the diagnosis of bacterial vaginosis. Infect. Dis. Obstet. Gynecol. 2002, 10, 203–207. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, C.S.; Morton, A.N.; Garland, S.M.; Horvath, L.B.; Kuzevska, I.; Fairley, C.K. Evaluation of a point-of-care test, BVBlue, and clinical and laboratory criteria for diagnosis of bacterial vaginosis. J. Clin. Microbiol. 2005, 43, 1304–1308. [Google Scholar] [CrossRef] [Green Version]
- Foessleitner, P.; Kiss, H.; Deinsberger, J.; Ott, J.; Zierhut, L.; Farr, A. Validation of the SavvyCheck™ Vaginal Yeast Test for Screening Pregnant Women for Vulvovaginal Candidosis: A Prospective, Cross-Sectional Study. J. Fungi 2021, 7, 233. [Google Scholar] [CrossRef]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.; et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015, 351, h5527. [Google Scholar] [CrossRef] [Green Version]
- Hillier, S.L.; Krohn, M.A.; Nugent, R.P.; Gibbs, R.S. Characteristics of three vaginal flora patterns assessed by gram stain among pregnant women. Vaginal Infections and Prematurity Study Group. Am. J. Obstet. Gynecol. 1992, 166, 938–944. [Google Scholar] [CrossRef]
- Petricevic, L.; Witt, A. The role of Lactobacillus casei rhamnosus Lcr35 in restoring the normal vaginal flora after antibiotic treatment of bacterial vaginosis. BJOG 2008, 115, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.B.; Narwal, R.; Adler, A.; Vera Garcia, C.; Rohde, S.; Say, L.; et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 2012, 379, 2162–2172. [Google Scholar] [CrossRef] [Green Version]
- Vogel, J.P.; Chawanpaiboon, S.; Moller, A.B.; Watananirun, K.; Bonet, M.; Lumbiganon, P. The global epidemiology of preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Johnson, H.L.; Cousens, S.; Perin, J.; Scott, S.; Lawn, J.E.; Rudan, I.; Campbell, H.; Cibulskis, R.; Li, M.; et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 2012, 379, 2151–2161. [Google Scholar] [CrossRef]
- Mwaniki, M.K.; Atieno, M.; Lawn, J.E.; Newton, C.R. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: A systematic review. Lancet 2012, 379, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Meis, P.J.; Goldenberg, R.L.; Mercer, B.; Moawad, A.; Das, A.; McNellis, D.; Johnson, F.; Iams, J.D.; Thom, E.; Andrews, W.W. The preterm prediction study: Significance of vaginal infections. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am. J. Obstet. Gynecol. 1995, 173, 1231–1235. [Google Scholar] [CrossRef]
- Hillier, S.L.; Nugent, R.P.; Eschenbach, D.A.; Krohn, M.A.; Gibbs, R.S.; Martin, D.H.; Cotch, M.F.; Edelman, R.; Pastorek, J.G., 2nd; Rao, A.V.; et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N. Engl. J. Med. 1995, 333, 1737–1742. [Google Scholar] [CrossRef]
- Hay, P.E.; Lamont, R.F.; Taylor-Robinson, D.; Morgan, D.J.; Ison, C.; Pearson, J. Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage. BMJ 1994, 308, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Goldenberg, R.L.; Hauth, J.C.; Andrews, W.W. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000, 342, 1500–1507. [Google Scholar] [CrossRef]
- Ugwumadu, A.; Manyonda, I.; Reid, F.; Hay, P. Effect of early oral clindamycin on late miscarriage and preterm delivery in asymptomatic women with abnormal vaginal flora and bacterial vaginosis: A randomised controlled trial. Lancet 2003, 361, 983–988. [Google Scholar] [CrossRef]
- Witt, A.; Petricevic, L.; Kaufmann, U.; Gregor, H.; Kiss, H. DNA hybridization test: Rapid diagnostic tool for excluding bacterial vaginosis in pregnant women with symptoms suggestive of infection. J. Clin. Microbiol. 2002, 40, 3057–3059. [Google Scholar] [CrossRef] [Green Version]
- Huppert, J.S.; Hesse, E.; Kim, G.; Kim, M.; Agreda, P.; Quinn, N.; Gaydos, C. Adolescent women can perform a point-of-care test for trichomoniasis as accurately as clinicians. Sex. Transm. Infect. 2010, 86, 514–519. [Google Scholar] [CrossRef] [Green Version]
- Chatwani, A.J.; Mehta, R.; Hassan, S.; Rahimi, S.; Jeronis, S.; Dandolu, V. Rapid testing for vaginal yeast detection: A prospective study. Am. J. Obstet. Gynecol. 2007, 196, 309.e301–309.e304. [Google Scholar] [CrossRef]
- Dan, M.; Leshem, Y.; Yeshaya, A. Performance of a rapid yeast test in detecting Candida spp. in the vagina. Diagn Microbiol. Infect. Dis. 2010, 67, 52–55. [Google Scholar] [CrossRef]
- Hoyme, U.B.; Saling, E. Efficient prematurity prevention is possible by pH-self measurement and immediate therapy of threatening ascending infection. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 115, 148–153. [Google Scholar] [CrossRef]
- Shujatullah, F.; Khan, H.M.; Khatoon, R.; Rabbani, T.; Malik, A. An evaluation of OSOM BV blue test in the diagnosis of bacterial vaginosis. Asian Pac. J. Trop. Med. 2010, 3, 574–576. [Google Scholar] [CrossRef] [Green Version]
- Akobeng, A.K. Understanding diagnostic tests 1: Sensitivity, specificity and predictive values. Acta Paediatr. 2007, 96, 338–341. [Google Scholar] [CrossRef]
- Trevethan, R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front. Public Health 2017, 5, 307. [Google Scholar] [CrossRef]
- Janulaitiene, M.; Paliulyte, V.; Grinceviciene, S.; Zakareviciene, J.; Vladisauskiene, A.; Marcinkute, A.; Pleckaityte, M. Prevalence and distribution of Gardnerella vaginalis subgroups in women with and without bacterial vaginosis. BMC Infect. Dis. 2017, 17, 394. [Google Scholar] [CrossRef]

| Characteristic | Study Group (n = 100) | Control Group (n = 100) | All (n = 200) | p-Value |
|---|---|---|---|---|
| Participant Age (years) | 32.0 ± 5.7 | 33.1 ± 5.1 | 32.6 ± 5.4 | 0.16 |
| Gravidity | 2 (1–11) | 2 (1–7) | 2 (1–11) | 0.36 |
| Parity | 1 (0–8) | 1 (0–6) | 1 (0–8) | 0.22 |
| Smoking | 0.05 | |||
| Yes | 21 (21.0%) | 10 (10.0%) | 31 (15.5%) | |
| No | 79 (79.0%) | 90 (90.0%) | 169 (84.5%) | |
| PTB in the previous pregnancy | 1.00 | |||
| Yes | 6 (6.0%) | 5 (5.0%) | 11 (5.5%) | |
| No | 94 (94.0%) | 95 (95.0%) | 189 (94.5%) | |
| Gestational weeks at sampling | 17.1 ± 7.0 | 15.8 ± 6.2 | 16.4 ± 6.7 | 0.17 |
| Candida colonization | <0.001 | |||
| Yes | 14 (14.0%) | 0 (0.0%) | 14 (7.0%) | |
| No | 86 (86.0%) | 100 (100.0%) | 186 (93.0%) |
| Parameter | Study Group (n = 100) | Control Group (n = 100) | Total (n = 200) |
|---|---|---|---|
| Positive test | 81 | 0 | 81 |
| Negative test | 19 | 100 | 119 |
| Total | 100 | 100 | 200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foessleitner, P.; Kiss, H.; Deinsberger, J.; Ott, J.; Zierhut, L.; Rosta, K.; Falcone, V.; Farr, A. Screening Pregnant Women for Bacterial Vaginosis Using a Point-of-Care Test: A Prospective Validation Study. J. Clin. Med. 2021, 10, 2275. https://doi.org/10.3390/jcm10112275
Foessleitner P, Kiss H, Deinsberger J, Ott J, Zierhut L, Rosta K, Falcone V, Farr A. Screening Pregnant Women for Bacterial Vaginosis Using a Point-of-Care Test: A Prospective Validation Study. Journal of Clinical Medicine. 2021; 10(11):2275. https://doi.org/10.3390/jcm10112275
Chicago/Turabian StyleFoessleitner, Philipp, Herbert Kiss, Julia Deinsberger, Julia Ott, Lorenz Zierhut, Klara Rosta, Veronica Falcone, and Alex Farr. 2021. "Screening Pregnant Women for Bacterial Vaginosis Using a Point-of-Care Test: A Prospective Validation Study" Journal of Clinical Medicine 10, no. 11: 2275. https://doi.org/10.3390/jcm10112275
APA StyleFoessleitner, P., Kiss, H., Deinsberger, J., Ott, J., Zierhut, L., Rosta, K., Falcone, V., & Farr, A. (2021). Screening Pregnant Women for Bacterial Vaginosis Using a Point-of-Care Test: A Prospective Validation Study. Journal of Clinical Medicine, 10(11), 2275. https://doi.org/10.3390/jcm10112275








