1. Introduction
Pigmented purpuric dermatoses (PPD) represent a group of cutaneous diseases characterized by petechial hemorrhage as a consequence of capillaritis [
1]. Extravasated erythrocytes result in purpura, and hemosiderin-laden macrophages give a red–brown appearance to older lesions. PPD usually present as remitting-relapsing non-palpable flat purpura with bilateral distribution on the legs of elderly. Although adults are more frequently affected, there are numerous reports of childhood PPD in literature [
2]. These conditions have no systemic findings, but occasionally lead to a patient evaluation to exclude thrombocytopenia or vasculitis because of the purpuric (usually petechial) nature of the lesions and clinical misdiagnosis. Based on the clinical appearance, PPD are classified as five major entities: progressive pigmentary dermatosis or Schamberg disease (the commonest), pigmented purpuric lichenoid dermatosis of Gougerot and Blum, purpura annularis telangiectodes of Majocchi, eczematid-like purpura of Doucas and Kapetanakis, and lichen aureus [
3]. Other forms are rare and with an unusual presentation [
1].
2. Aetiology and Pathogenesis
The aetiology is unknown but physical activity; venous hypertension; capillary fragility, especially in lower limbs; and local infections seem to play a role in the pathogenesis [
1]. Some drugs, such as acetaminophen, non-steroidal anti-inflammatory drugs, antibiotic, and oral antidiabetic (
Table 1), may appear as a trigger, in particular in Schamberg disease [
4,
5,
6]. PPD can be associated with systemic diseases [
1], such as diabetes mellitus, rheumatoid arthritis, systemic lupus erythematosus, thyroid dysfunction, mycosis fungoides, hematological and solid neoplasms, liver disease, and hyperlipidemia; however, most of the PPD remain idiopathic. All PPD result from minimal inflammation and hemorrhage of superficial papillary dermal vessels, usually capillaries. The reason for inflammation is unknown, and there is no association with any abnormality of coagulation. Immunopathologic studies suggest that the vascular damage and erythrocyte extravasation are secondary to a localized cell-mediated immunologic event [
7]. Some studies suggest an upregulation of cell adhesion molecules, such as LFA-1 (lymphocyte function antigen-1) and ICAM-1 (intercellular adhesion molecule-1) in inflammatory cells and ICAM-1 and ELAM-1 (endothelial leukocyte adhesion molecule-1) in endothelial cells [
8].
3. Pathology
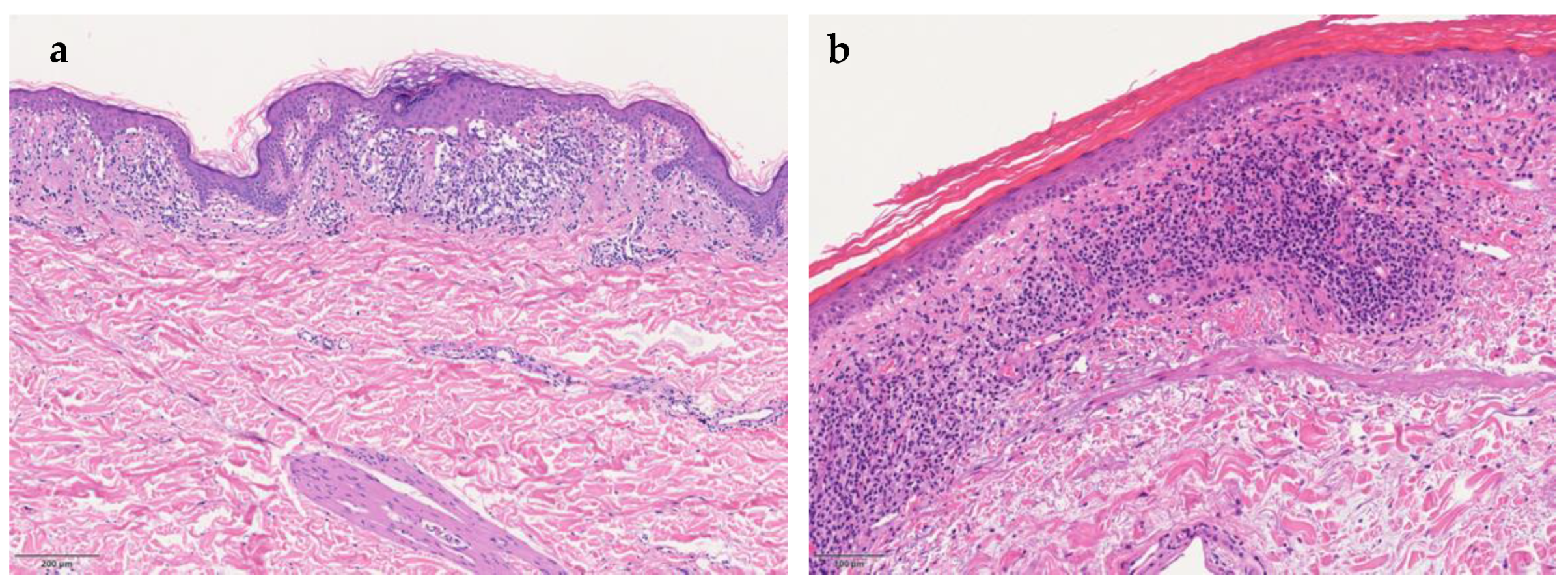
Despite varying clinical features, all PPD share similar histopathology. The four characterizing elements are dilated blood vessels, with endothelial cell swelling, deposit of hemosiderin, red cell extravasation, and perivascular lymphocytic-macrophage infiltration. It is also possible to define the pathological patterns based on the predominant inflammatory presentation: spongiotic, interface/lichenoid, perivascular, or granulomatous pattern (
Figure 1a) [
9]. Epidermal spongiosis and lymphocytic exocytosis are common in all PPD but are more pronounced in eczematid-like purpura of Doucas and Kapetanakis. Lichen aureus (
Figure 1b) is characterized by a band-like dermal infiltrate separate from normal epidermis by grenz zone. Lichenoid infiltrate and spongiosis with patchy parakeratosis are the distinguishing features of dermatosis of Gougerot and Blum. A rare granulomatous form, variant of a chronic pigmented purpura, has also been described [
10].
4. Clinical Variants
4.1. Schamberg Disease or Progressive Purpuric Pigmented Dermatosis
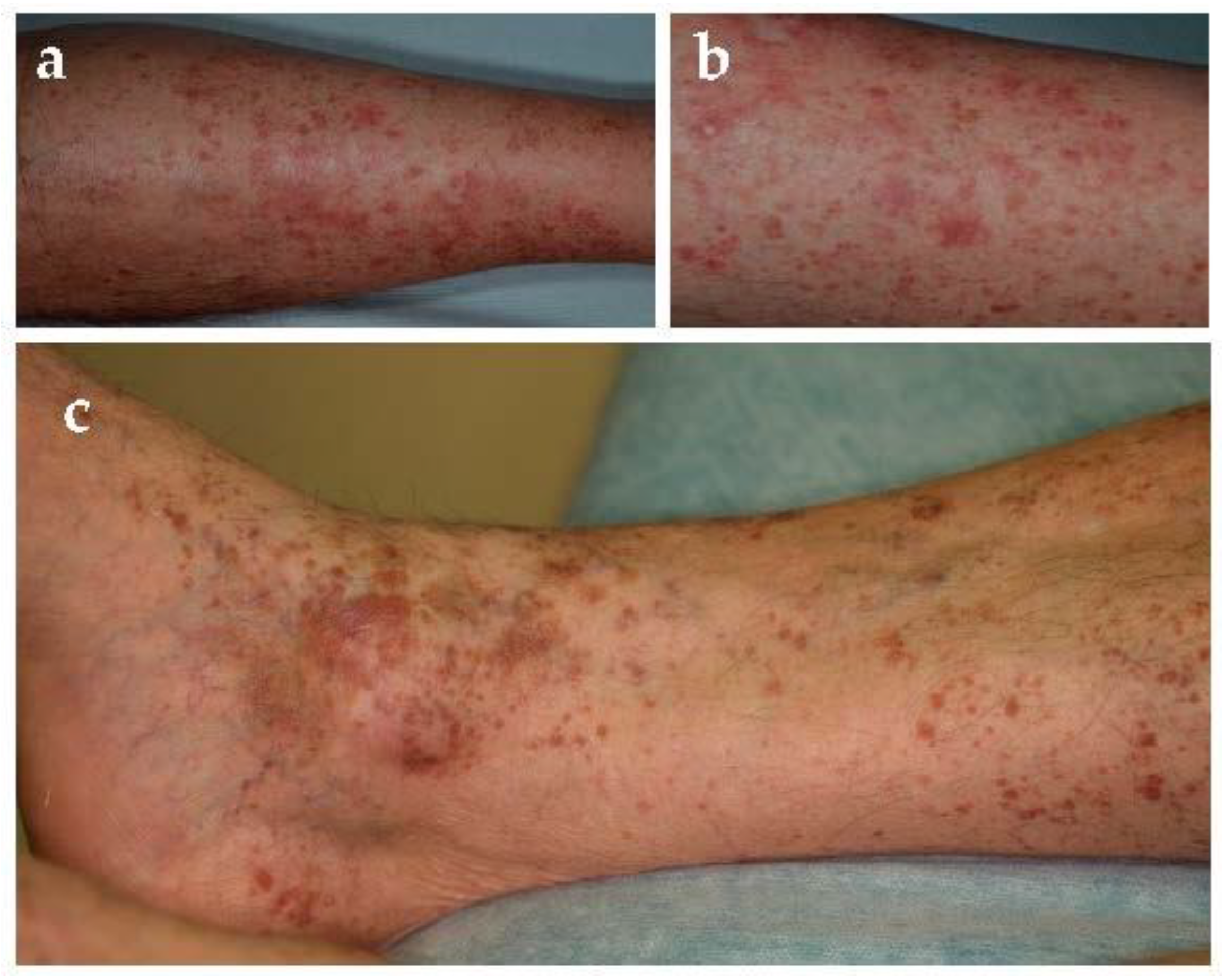
Schamberg disease, first described in 1901 [
11], is the most common form of PPD [
1]. It may affect all ages but commonly occurs in middle-aged to older men and less frequently children [
2,
12]. Typical localizations are lower limbs but can also involve thighs, buttocks, trunk, and arms. Clinical examination usually shows pinpoint petechiae, likened to “cayenne pepper”, that flows in yellow-orange-brown patches with an oval to irregular outline (
Figure 2). It is a chronic-persistent dermatosis that, in some cases, can extend to the proximal areas. Most patients are asymptomatic, whereas some complain of mild itching. Venous insufficiency might play a role in their localization. Moreover, chronic alcohol uptake seems to be related to the development of purpura and Schamberg disease, especially when liver damage is associated [
13].
4.2. Purpura Annularis Telangiectodes of Majocchi
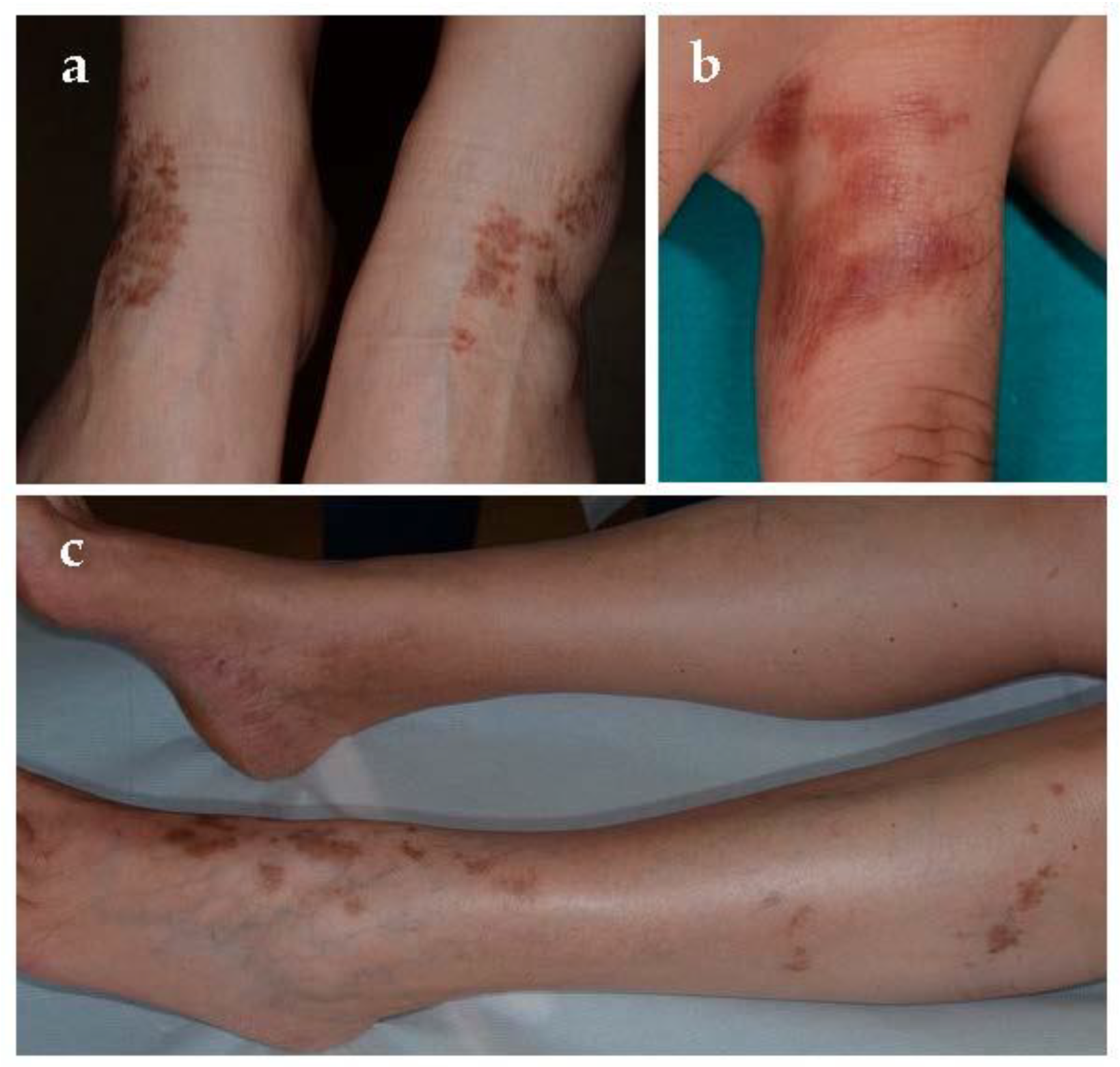
Purpura annularis telangiectodes of Majocchi was first described in 1896 [
11]. It is an uncommon PPD that mainly affects adolescents and young adults, especially women [
14]. Early lesions are bluish to red annular macules in which dark-red telangiectatic puncta appear; subsequently, they have a centrifugal extension with central progressive resolution, and slight atrophy, giving them an annular configuration (
Figure 3). The eruption begins bilaterally on the lower limbs and then extends to the upper extremities, but it is also described as a rare unilateral form [
15]. Lesions may vary from few in number to innumerable; patients can be asymptomatic or complain of mild itch or burning. As far as the trigger factors, in addition to those already mentioned above, there is sclerotherapy due to direct endothelial damage with resultant red blood cell extravasation and hemosiderin deposition [
16]. Touraine [
17] described the “arciform telangiectatic variant” characterized by a less number of lesions with lower size and arciform and irregular edges.
4.3. Pigmented Purpuric Lichenoid Dermatitis of Gougerot and Blum
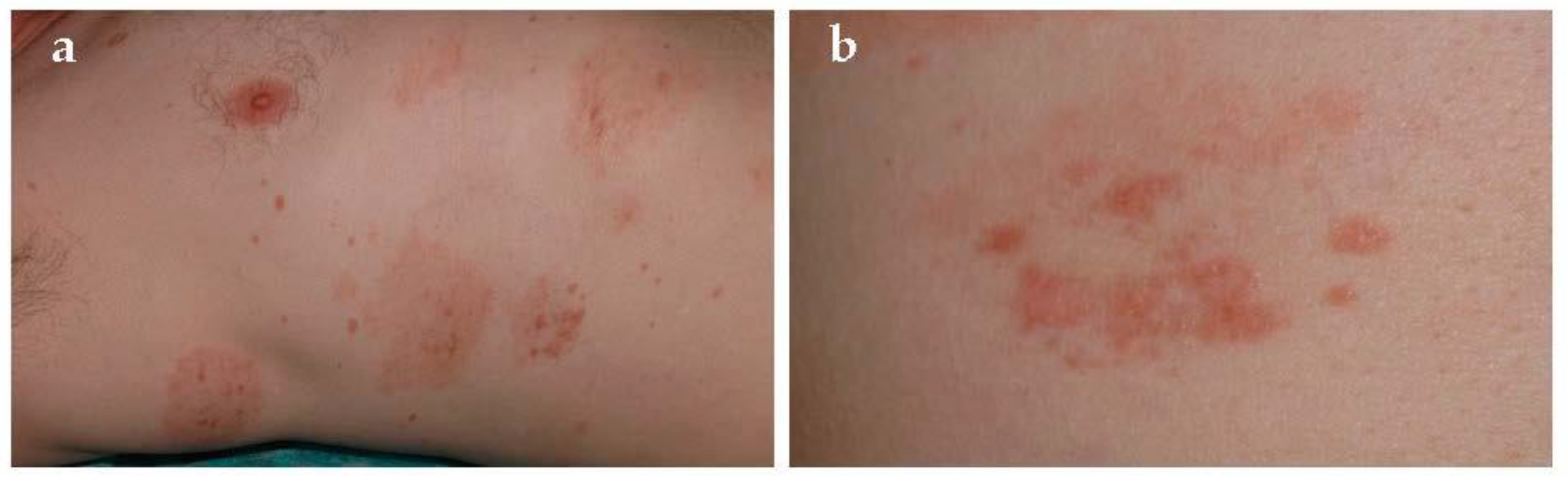
Gougerot and Blum defined this entity in 1925 [
11]. It is a rare form characterized by two type of lesions [
18]: purpuric red–brown lichenoid papules and Schamberg-like purpura (
Figure 4). Lesions are usually located on limbs and not uncommonly involved oral mucosa [
19]. This disease usually affects middle-aged to older men, has a chronic course, and occasionally presents with pruritus. However, PPD of Gougerot-Blum might clinically resemble Kaposi’s sarcoma, mycosis fungoides, cutaneous vasculitis, and traumatic purpura.
4.4. Lichen Aureus
First described as lichen purpuricus in 1958, it was successively named lichen aureus by Calnan in 1960 [
11]. It is a more localized, persistent, intensely purpuric eruption, rarely painful [
20]. It appears as a purpuric lesion associated with lichenoid papule, usually located on the lower extremities, often over a perforator vein, occasionally on the trunk, upper extremities, buttocks, or face (
Figure 5). The color varies from golden to rust to purple–brown and justifies the term “lichen aureus” as well as the pathologic pattern of a lichenoid dermatitis. Unlike with other forms, young men are the most affected.
4.5. Eczematid-Like Purpura of Doucas and Kapetanakis
This form is considered an inflammatory and more extensive variety of Schamberg disease, presented for the first time in 1953 [
11]. In fact, it tends to spread cranially from lower limb up to the abdomen, and it is characterized by intense itching and scaly petechial or purpuric macules, papules, and patches [
21] (
Figure 6). In confirmation of this, pathology shows spongiosis and intense lymphocytic perivascular infiltration. Resolution is always spontaneous but relapses are frequent. Association with allergic contact dermatitis to rubber and clothing has been described.
4.6. Other Forms
Granulomatous pigmented purpura is a rare variety of PPD that seems to affect Asian people more frequently than Caucasians. It was first described in 1997 by Saito et al. [
22]. It generally occurs in middle-aged to elderly patients and commonly follows a chronic waxing and waning course. A correlation with dyslipidemia was reported in literature [
23]. Clinical examination often shows brown patches with superimposed hemorrhagic papules on the lower legs. Histologic specimen is characterized by hemorrhagic granulomatous infiltrate, including histiocytes, located in the upper dermis.
Linear pigmented purpura is an uncommon form that can occur in children and adolescence. The terms quadrantic capillaropathy and unilateral linear capillaritis have been used to describe this entity in the past [
24]. It is characterized by lesions that are similar in appearance to lichen aureus or Schamberg disease but are unilateral and linear [
11,
15]. It must be differentiated from other dermatoses such as lichen aureus (segmental variant), unilateral nevoid telangiectasia, and angioma serpiginosum, which have linear aspects such as linear purpura.
Itching purpura of Loewenthal should be considered as a symptomatic variant of Schamberg disease that affects almost exclusively adults [
25]. It is characterized by abrupt onset and severe itching on lower extremities.
5. Differential Diagnosis
Clinical presentation is always sufficient for diagnosis (
Table 2). PPD must be differentiated from purpura caused by platelet alteration and non-thrombocytopenic purpura.
Platelet alteration could result from thrombocytopenic, thrombocytopatic, or thrombocytosis syndromes. These forms are usually associated with bleeding in other locations, or in case of thrombocytosis with a prothrombotic state. Thus blood count test and peripheral blood smear could help in doubtful cases. On the contrary, there is no platelet alteration in non-thrombocytopenic purpura. Some examples are “exercise-induced purpura”, which occurs on lower legs after unusual or major muscular activity [
26], or “solar purpura”, which appears acutely after sun exposure [
27]. Pigmented purpuric eruptions on the legs must be also differentiated from dermal hemorrhage secondary to venous hypertension, which presents with petechiae superimposed on diffuse hemosiderosis, especially in elderly people with stasis dermatitis.
Hypergammaglobulinemic purpura of Waldenström is characterized by hypergammaglobulinemia; recurring purpura, especially on the legs; elevated erythrocyte sedimentation rate; and the presence of rheumatoid factor indicative of circulating immune complexes. It is often preceded by slight itch, mild tingling, or burning, and it may be aggravated by tight-fitting garments, prolonged standing, and heat [
28].
Sometimes biopsy is required to distinguish the lichenoid variant from cutaneous small vessel vasculitis. Leukocytoclastic vasculitis/cutaneous venulitis are characterized by palpable erythematous papules that do not blanch on diascopy.
Finally, purpuric clothing dermatitis, hyperglobulinemic purpura, early mycosis fungoides, stasis pigmentation, scurvy, leukocytoclastic vasculitis, and drug-hypersensitivity reactions can mimic Schamberg disease.
6. Diagnosis
Diagnosis is clinical in the majority of cases: orange-red-brown macules with purpuric spots associated with specific features of variants mentioned above are decisive. Doubtful forms benefit from biopsy. All subtypes share dilated blood vessels, endothelial cell swelling, deposit of hemosiderin, red cell extravasation, and perivascular lymphocytic-macrophage infiltration. Moreover, epiluminescence may address the correct diagnosis: coppery-red pigmentation, red dots, and globules, due to the extravasation of red blood cells and to hemosiderin in the histiocytes and dotted vessels, are the main findings. Brown dots and globules result from melanocytes or melanophages at the dermo-epidermal junction [
29].
7. Investigation
Blood count test and peripheral blood smear are necessary to exclude thrombocytopenia; coagulation screen can help to exclude other possible causes of purpura. Sometimes, tests for rheumatoid factor, anti-nuclear antibodies, anti-Hepatitis B Virus, and anti-Hepatitis C Virus antibodies can be useful to identify purpura associated with autoimmune diseases or secondary to hepatitis.
8. Therapy
Standard guidelines of treatment are not available for PPD [
3]. They are usually persistent and resistant to therapies. Thus, conservative or non-pharmacologic management may be considered in the case of asymptomatic patients [
1]. Therapeutic options include topical and oral drugs, photo-therapy, and laser-therapy (
Table 3).
In the pruritic forms or evident erythema, as in purpuric lichenoid dermatitis of Gougerot-Blum, topical corticosteroids, often associated with moisturizing cream, are helpful [
30]. Duration of therapy and steroids potency are variable [
11,
31]. Prolonged steroid use should be discouraged due to the risk of local atrophy. Topical pimecrolimus 0.1% was effectively used in lichen aureus in a child: color improvement was noticed after three months of daily treatment, while a complete resolution after one year [
32]. Unlike steroids, calcineurin-inhibitors are not responsible for skin atrophy.
Oral rutoside (50 mg twice a day) and ascorbic acid (500 mg twice a day), through the increase capillary resistance and with antioxidative radical scavenging activities, cleared three patients in a trial within 4 weeks [
33,
34]. Pentoxifylline at different dosages (300 mg daily [
35]; 400 mg bid [
36]) has proven to be a satisfactory drug in these conditions. It seems to act at level of T-cell adherence to endothelial cells and keratinocytes. A therapy with daily oral flavonoids (diosmin 450 mg, and hesperidin 50 mg, and euphorbia prostata extract 100 mg) and calcium dobesilate 500 mg successfully treated a case of Schamberg disease in 14 days [
37]. Colchicine 0.5 mg twice a day for two months was also adopted successfully by Cavalcante et al. for treating Schamberg disease [
38]. Tamaki et al. reported a dramatic improvement within 7–14 days of treatment in five patients receiving 500 to 750 mg griseofulvin daily. Therapeutic response seems to come from local inflammation reduction [
39]. The efficacy of oral cyclosporine in chronic PPD is worth mentioning, given that it supports the immune-mediated pathogenesis of some of these forms [
40], although, due to the benign nature of PPD and the serious adverse-reaction profile of cyclosporine A, it is not recommended as a first-line option. Other treatments reported in literature included tranexamic acid and heparinoid gel [
9]. Associated venous stasis should be treated by compression, and prolonged leg dependency should be avoided [
7].
PUVA-treatment has been successfully used in extended forms of Gougerot-Blum dermatitis [
41], lichen aureus [
42], and Schamberg disease [
43]. Other reports suggest the potential role of narrowband UVB [
44,
45].
Finally, another valid option is laser-therapy. In particular, 595-vascular laser proved to be effective in Schamberg disease [
46]. Other reports suggest the positive role of fractional non-ablative 1540 nm erbium with 4 monthly sessions in Schamberg disease [
47]. Dye laser was also adopted for treating lichen aureus [
48,
49].
9. Conclusions
PPD include a group of skin disorders characterized by petechial hemorrhage, deposit of hemosiderin, red cell extravasation, and dilated blood vessels. They are classified on the basis of clinical presentation since histopathology is almost superimposable. Sometimes PPD are associated with venous and capillary dysfunction, which explain the frequent involvement of lower legs. Although in the majority of cases they are idiopathic, routine blood tests and coagulation tests are recommended to exclude a possible association with thrombocytopaenia or blood clotting disorder. As far as the treatment, most cases are resistant and usually relapse thus non-pharmacological measures must be always considered. Topic steroids might be helpful for inflammatory and itching variants.
Author Contributions
Conceptualization, C.B.S., S.G. and G.N.; methodology, C.B.S., S.G. and G.N.; validation, C.B.S., S.G. and G.N.; formal analysis, C.B.S. and S.G.; resources, C.B.S., S.G. and G.N.; data curation, C.B.S., S.G. and G.N.; writing—original draft preparation, C.B.S.; writing—review and editing, C.B.S., S.G. and G.N.; supervision, G.N.; project administration, C.B.S., S.G. and G.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sardana, K.; Sarkar, R.; Sehgal, V.N. Pigmented Purpuric Dermatoses: An Overview. Int. J. Dermatol. 2004, 43, 482–488. [Google Scholar] [CrossRef]
- Tristani-Firouzi, P.; Meadows, K.P.; Vanderhooft, S. Pigmented Purpuric Eruptions of Childhood: A Series of Cases and Review of Literature. Pediatric Dermatol. 2001, 18, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Pallás, M.; Del Mazo, R.C.; Biosca, V.L. Pigmented Purpuric Dermatosis: A Review of the Literature. Available online: https://pubmed.ncbi.nlm.nih.gov/31983388/ (accessed on 4 February 2021).
- Adams, B.B.; Gadenne, A.S. Glipizide-Induced Pigmented Purpuric Dermatosis. J. Am. Acad. Dermatol. 1999, 41, 827–829. [Google Scholar] [CrossRef]
- Nishioka, K.; Katayama, I.; Masuzawa, M.; Yokozeki, H.; Nishiyama, S. Drug-Induced Chronic Pigmented Purpura. J. Dermatol. 1989, 16, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.J.; Lee, C.W. Figurate Purpuric Eruptions on the Trunk: Acetaminophen-Induced Rashes. J. Dermatol. 1998, 25, 756–758. [Google Scholar] [CrossRef]
- Smoller, B.R.; Kamel, O.W. Pigmented Purpuric Eruptions: Immunopathologic Studies Supportive of a Common Immunophenotype. J. Cutan. Pathol. 1991, 18, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Ghersetich, I.; Lotti, T.; Bacci, S.; Comacchi, C.; Campanile, G.; Romagnoli, P. Cell Infiltrate in Progressive Pigmented Purpura (Schamberg’s Disease): Immunophenotype, Adhesion Receptors, and Intercellular Relationships. Int. J. Dermatol. 1995, 34, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-K.; Lin, C.-K.; Wu, Y.-H. The Pathological Spectrum and Clinical Correlation of Pigmented Purpuric Dermatosis—A Retrospective Review of 107 Cases. J. Cutan. Pathol. 2018, 45, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Kerns, M.J.J.; Mallatt, B.D.; Shamma, H.N. Granulomatous Pigmented Purpura: An Unusual Histological Variant. Am. J. Dermatopathol. 2009, 31, 77–80. [Google Scholar] [CrossRef]
- Taketuchi, Y.; Chinen, T.; Ichikawa, Y.; Ito, M. Two Cases of Unilateral Pigmented Purpuric Dermatosis. J. Dermatol. 2001, 28, 493–498. [Google Scholar] [CrossRef]
- Torrelo, A.; Requena, C.; Mediero, I.G.; Zambrano, A. Schamberg’s Purpura in Children: A Review of 13 Cases. J. Am. Acad. Dermatol. 2003, 48, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, U.; Selle, C.; Isbruch, K.; Isbruch, K. Recurrent Purpura Due to Alcohol-Related Schamberg’s Disease and Its Association with Serum Immunoglobulins: A Longitudinal Observation of a Heavy Drinker. J. Med. Case Rep. 2016, 10, 301. [Google Scholar] [CrossRef] [Green Version]
- Hoesly, F.J.; Huerter, C.J.; Shehan, J.M. Purpura Annularis Telangiectodes of Majocchi: Case Report and Review of the Literature. Int. J. Dermatol. 2009, 48, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Shuja, F.; Chan, A.; Wasko, C. Unilateral Purpura Annularis Telangiectodes of Majocchi in an Elderly Male: An Atypical Presentation. Dermatol. Online J. 2013, 19, 19263. [Google Scholar] [PubMed]
- Spaulding, R.T.; Malone, J.C. Sclerotherapy-Induced Purpura Annularis Telangiectodes of Majocchi-like Eruption. JAAD Case Rep. 2019, 5, 704–705. [Google Scholar] [CrossRef]
- Brehm, G. Zur Purpura teleangiectatica arciformis (Touraine) [To the Purpura Teleangiectatica Arciformis (Touraine)]. Z. Haut Geschlechtskr. 1954, 17, 331–336. [Google Scholar] [PubMed]
- Park, M.-Y.; Shim, W.-H.; Kim, J.-M.; Kim, G.-W.; Kim, H.-S.; Ko, H.-C.; Kim, M.-B.; Kim, B.-S. Dermoscopic Finding in Pigmented Purpuric Lichenoid Dermatosis of Gougerot-Blum: A Useful Tool for Clinical Diagnosis. Ann. Dermatol. 2018, 30, 245–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scully, C.; Eveson, J.W. Pigmented Purpuric Stomatitis. Oral Surg. Oral Med. Oral Pathol. 1992, 74, 780–782. [Google Scholar] [CrossRef]
- Mahajan, V.K.; Chauhan, P. Lichen Aureus. Indian J. Pediatr. 2014, 81, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Doucas, C.; Kapetanakis, J. Eczematid-like Purpura. Dermatologica 1953, 106, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Matsuoka, Y. Granulomatous Pigmented Purpuric Dermatosis. J. Dermatol. 1996, 23, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Allan, A.; Altman, D.A.; Su, W. Granulomatous Pigmented Purpuric Dermatosis. Cutis 2017, 100, 256–258. [Google Scholar] [PubMed]
- Yoon, T.Y.; Lee, D.Y.; Kim, Y.J.; Lee, J.Y.; Kim, M.K. Unilateral Linear Capillaropathy Limited to the Upper Extremity in an Infant. J. Dermatol. 2013, 40, 71–75. [Google Scholar] [CrossRef]
- Loewenthal, L.J. Itching Purpura. Br. J. Dermatol. 1954, 66, 95–103. [Google Scholar] [CrossRef]
- Ramelet, A.-A. Exercise-Induced Purpura. Dermatology 2004, 208, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Waters, A.J.; Sandhu, D.R.; Green, C.M.; Ferguson, J. Solar Capillaritis as a Cause of Solar Purpura. Clin. Exp. Dermatol. 2009, 34, e821–e824. [Google Scholar] [CrossRef]
- Finder, K.A.; McCollough, M.L.; Dixon, S.L.; Majka, A.J.; Jaremko, W. Hypergammaglobulinemic Purpura of Waldenström. J. Am. Acad. Dermatol. 1990, 23, 669–676. [Google Scholar] [CrossRef]
- Ozkaya, D.B.; Emiroglu, N.; Su, O.; Cengiz, F.P.; Bahali, A.G.; Yildiz, P.; Demirkesen, C.; Onsun, N. Dermatoscopic Findings of Pigmented Purpuric Dermatosis. An. Bras. Dermatol. 2016, 91, 584–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risikesan, J.; Sommerlund, M.; Ramsing, M.; Kristensen, M.; Koppelhus, U. Successful Topical Treatment of Pigmented Purpuric Lichenoid Dermatitis of Gougerot-Blum in a Young Patient: A Case Report and Summary of the Most Common Pigmented Purpuric Dermatoses. Case Rep. Dermatol. 2017, 9, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, J.; Burgin, S.; Sepehr, A. Granulomatous Pigmented Purpura: Report of a Case and Review of the Literature. J. Cutan. Pathol. 2011, 38, 984–989. [Google Scholar] [CrossRef]
- Böhm, M.; Bonsmann, G.; Luger, T.A. Resolution of Lichen Aureus in a 10-Year-Old Child after Topical Pimecrolimus. Br. J. Dermatol. 2004, 151, 519–520. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, U.; Seiter, S.; Ugurel, S.; Tilgen, W. Treatment of Progressive Pigmented Purpura with Oral Bioflavonoids and Ascorbic Acid: An Open Pilot Study in 3 Patients. J. Am. Acad. Dermatol. 1999, 41, 207–208. [Google Scholar] [CrossRef]
- Schober, S.M.; Peitsch, W.K.; Bonsmann, G.; Metze, D.; Thomas, K.; Goerge, T.; Luger, T.A.; Schneider, S.W. Early Treatment with Rutoside and Ascorbic Acid Is Highly Effective for Progressive Pigmented Purpuric Dermatosis. J. Dtsch. Dermatol. Ges. JDDG 2014, 12, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y.; Hirayama, K.; Orihara, M.; Shiohara, T. Successful Treatment of Schamberg’s Disease with Pentoxifylline. J. Am. Acad. Dermatol. 1997, 36, 827–830. [Google Scholar] [CrossRef]
- Mun, J.-H.; Jwa, S.-W.; Song, M.; Kim, H.-S.; Ko, H.-C.; Kim, B.-S.; Kim, M.-B. Extensive Pigmented Purpuric Dermatosis Successfully Treated with Pentoxifylline. Ann. Dermatol. 2012, 24, 363–365. [Google Scholar] [CrossRef]
- Gupta, A.; Sardana, K.; Kishan Gautam, R. Venoprotective Drugs in Pigmented Purpuric Dermatoses: A Case Report. J. Cosmet. Dermatol. 2019. [Google Scholar] [CrossRef]
- Cavalcante, M.L.L.L.; Masuda, P.Y.; de Brito, F.F.; Pinto, A.C.V.D.; Itimura, G.; Nunes, A.J.F. Schamberg’s Disease: Case Report with Therapeutic Success by Using Colchicine. An. Bras. Dermatol. 2017, 92, 246–248. [Google Scholar] [CrossRef] [Green Version]
- Tamaki, K.; Yasaka, N.; Osada, A.; Shibagaki, N.; Furue, M. Successful Treatment of Pigmented Purpuric Dermatosis with Griseofulvin. Br. J. Dermatol. 1995, 132, 159–160. [Google Scholar] [CrossRef]
- Okada, K.; Ishikawa, O.; Miyachi, Y. Purpura Pigmentosa Chronica Successfully Treated with Oral Cyclosporin A. Br. J. Dermatol. 1996, 134, 180–181. [Google Scholar] [CrossRef]
- Krizsa, J.; Hunyadi, J.; Dobozy, A. PUVA Treatment of Pigmented Purpuric Lichenoid Dermatitis (Gougerot-Blum). J. Am. Acad. Dermatol. 1992, 27, 778–780. [Google Scholar] [CrossRef]
- Ling, T.C.; Goulden, V.; Goodfield, M.J. PUVA Therapy in Lichen Aureus. J. Am. Acad. Dermatol. 2001, 45, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Seckin, D.; Yazici, Z.; Senol, A.; Demircay, Z. A Case of Schamberg’s Disease Responding Dramatically to PUVA Treatment. Photodermatol. Photoimmunol. Photomed. 2008, 24, 95–96. [Google Scholar] [CrossRef]
- Kocaturk, E.; Kavala, M.; Zindanci, I.; Zemheri, E.; Sarigul, S.; Sudogan, S. Narrowband UVB Treatment of Pigmented Purpuric Lichenoid Dermatitis (Gougerot-Blum). Photodermatol. Photoimmunol. Photomed. 2009, 25, 55–56. [Google Scholar] [CrossRef]
- Gudi, V.S.; White, M.I. Progressive Pigmented Purpura (Schamberg’s Disease) Responding to TL01 Ultraviolet B Therapy. Clin. Exp. Dermatol. 2004, 29, 683–684. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosia, R.A.; Rajpara, V.S.; Glogau, R.G. The Successful Treatment of Schamberg’s Disease with the 595 Nm Vascular Laser. Dermatol. Surg. 2011, 37, 100–101. [Google Scholar] [CrossRef] [PubMed]
- Hilerowicz, Y.; Sprecher, E.; Gat, A.; Artzi, O. Successful Treatment of Schamberg’s Disease with Fractional Non-Ablative 1540 Nm Erbium:Glass Laser. J. Cosmet. Laser Ther. 2018, 20, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Arreola Jauregui, I.E.; López Zaldo, J.B.; Huerta Rivera, G.; Soria Orozco, M.; Bonnafoux Alcaraz, M.; Paniagua Santos, J.E.; Vázquez Huerta, M. A Case of Lichen Aureus Successfully Treated with 595 Nm Wavelength Pulsed-Dye Laser. J. Cosmet. Dermatol. 2020, 19, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Chang, I.-K.; Lee, Y.; Seo, Y.-J.; Kim, C.-D.; Lee, J.-H.; Im, M. Treatment of Segmental Lichen Aureus with a Pulsed-Dye Laser: New Treatment Options for Lichen Aureus. Eur. J. Dermatol. EJD 2013, 23, 891–892. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).