Shear Wave Elastography of the Plantar Fascia: Comparison between Patients with Plantar Fasciitis and Healthy Control Subjects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Consideration
2.2. Design and Sampling
2.3. Study Population
2.4. Data Recollection
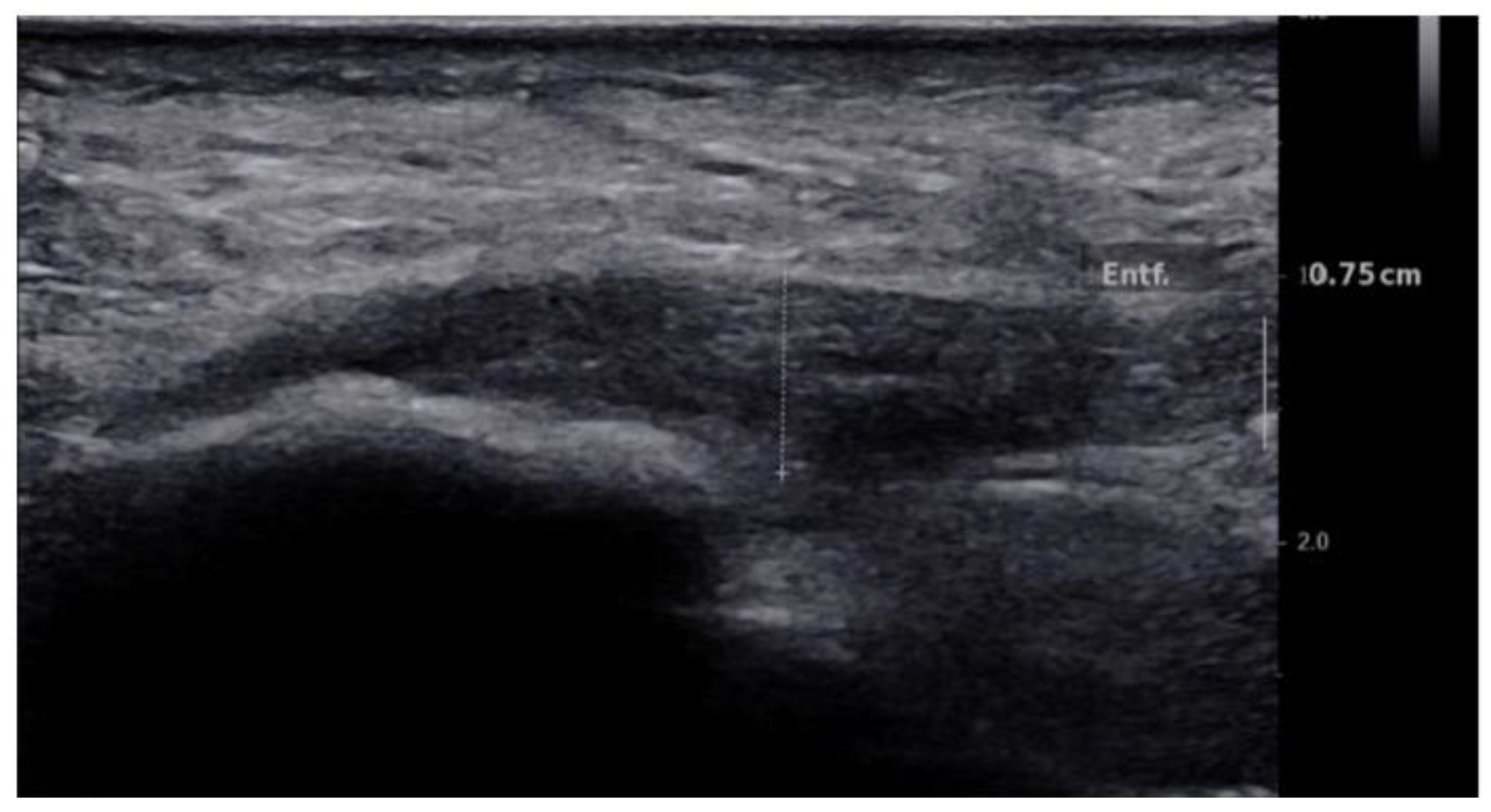
2.4.1. B-Mode US Examination
2.4.2. CDUS Examination
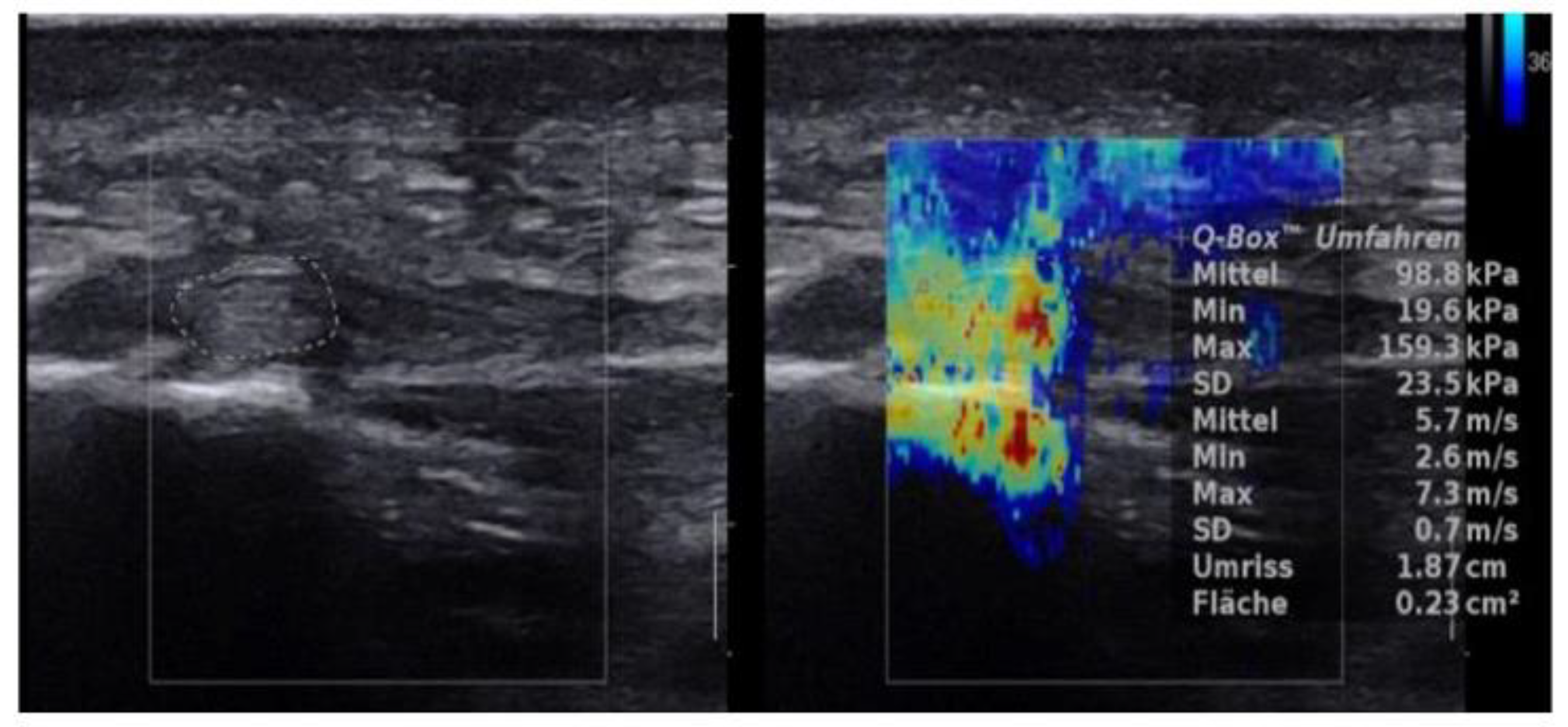
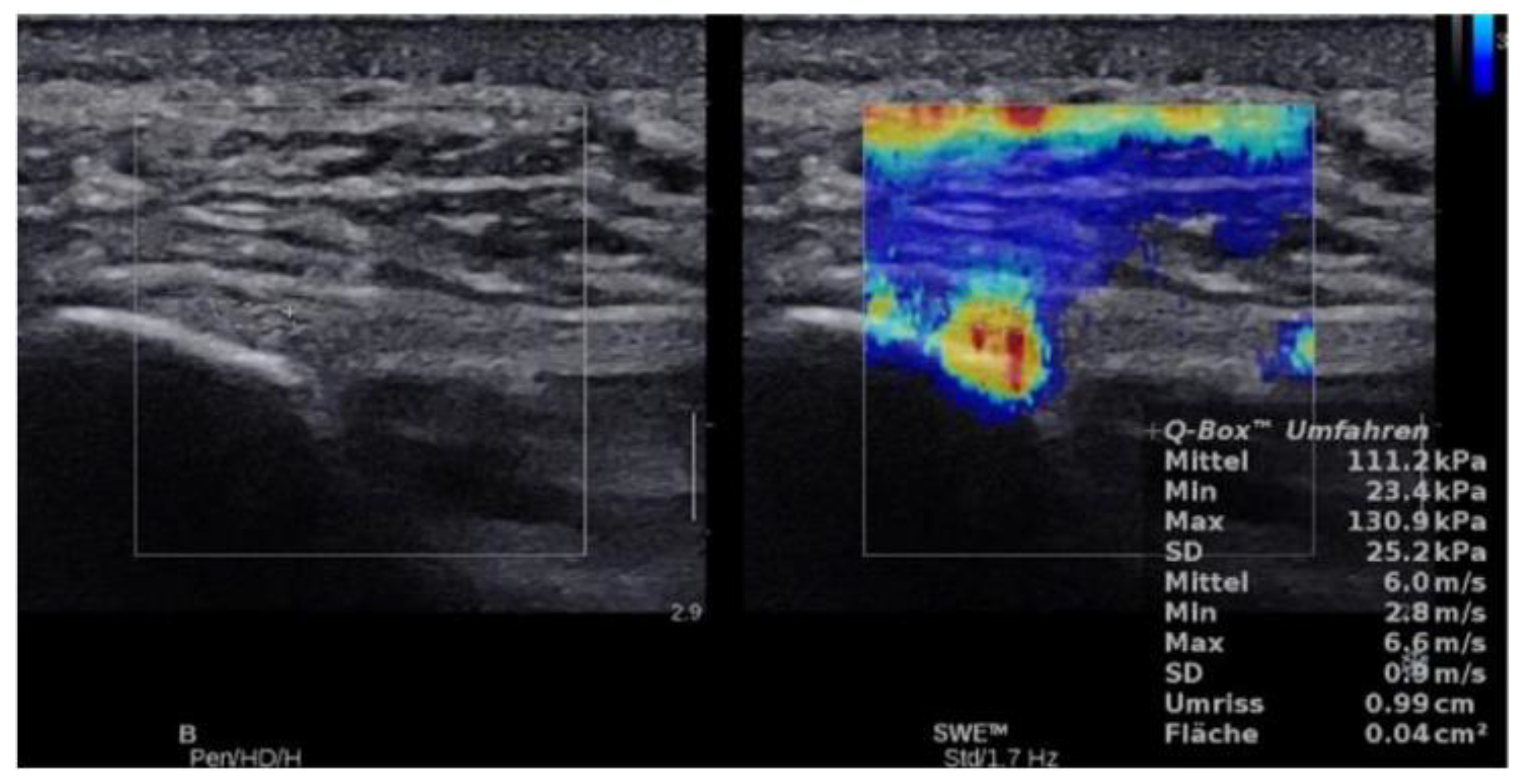
2.4.3. SWE Examination
2.5. Statistical Analysis
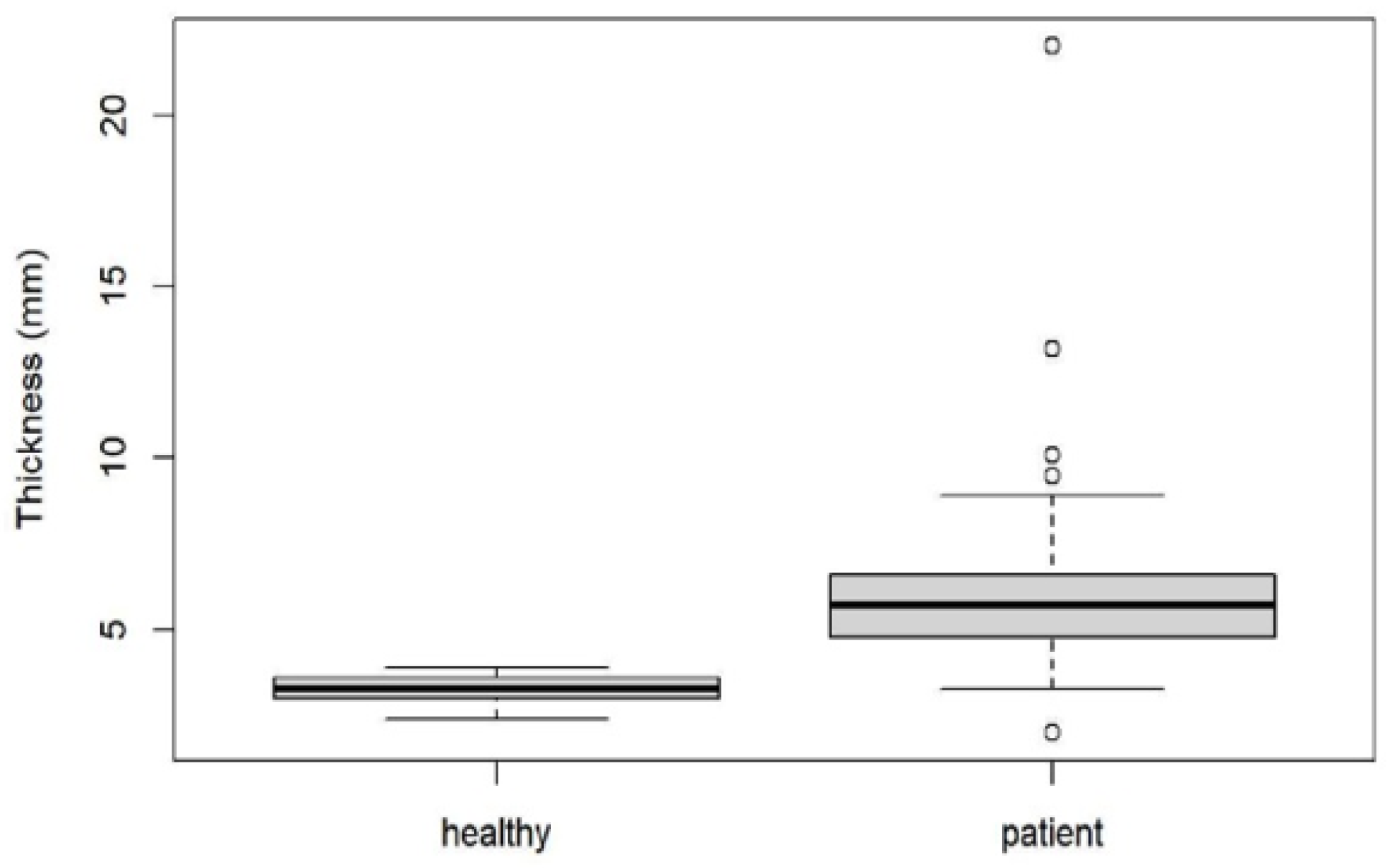
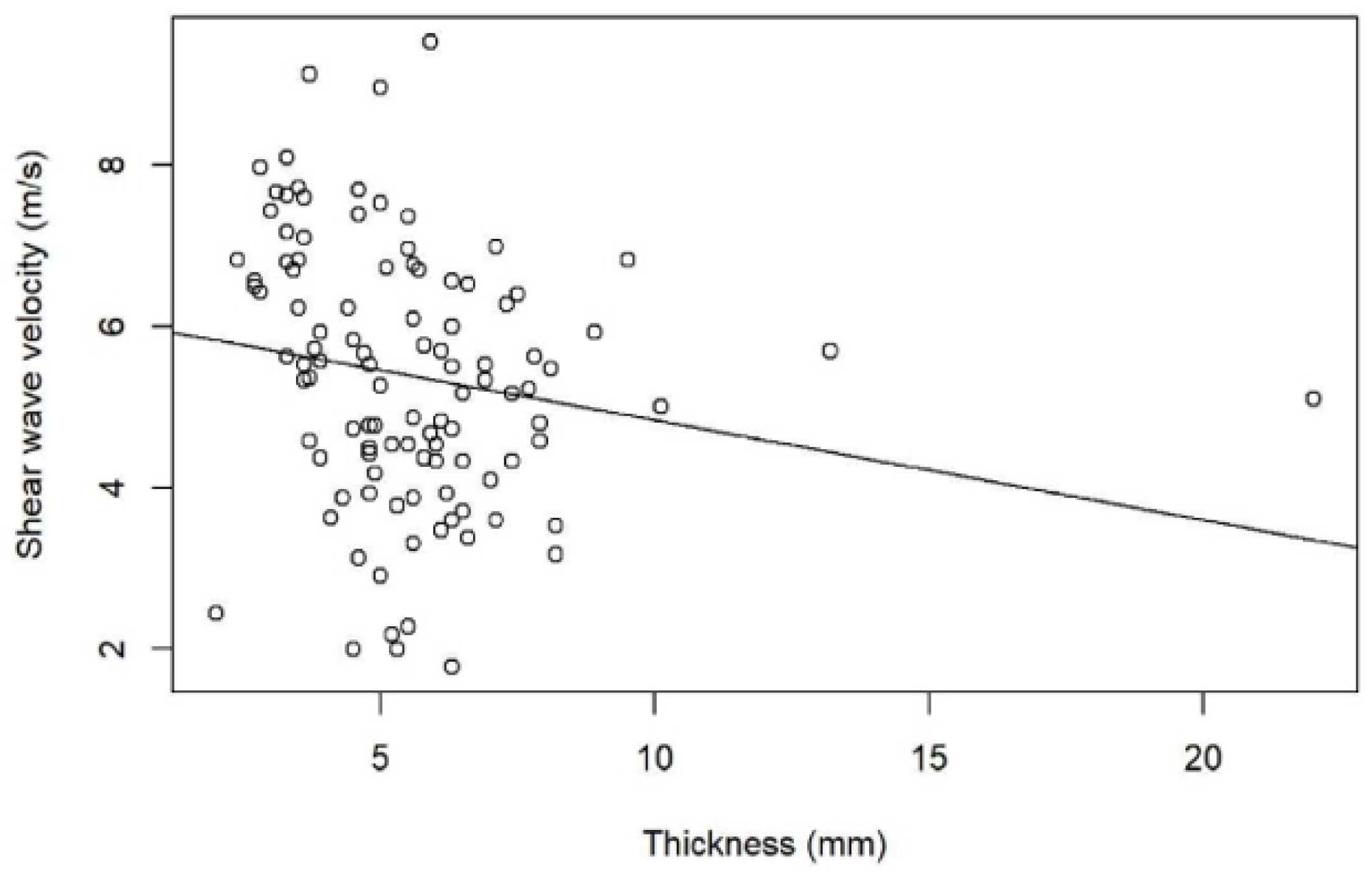
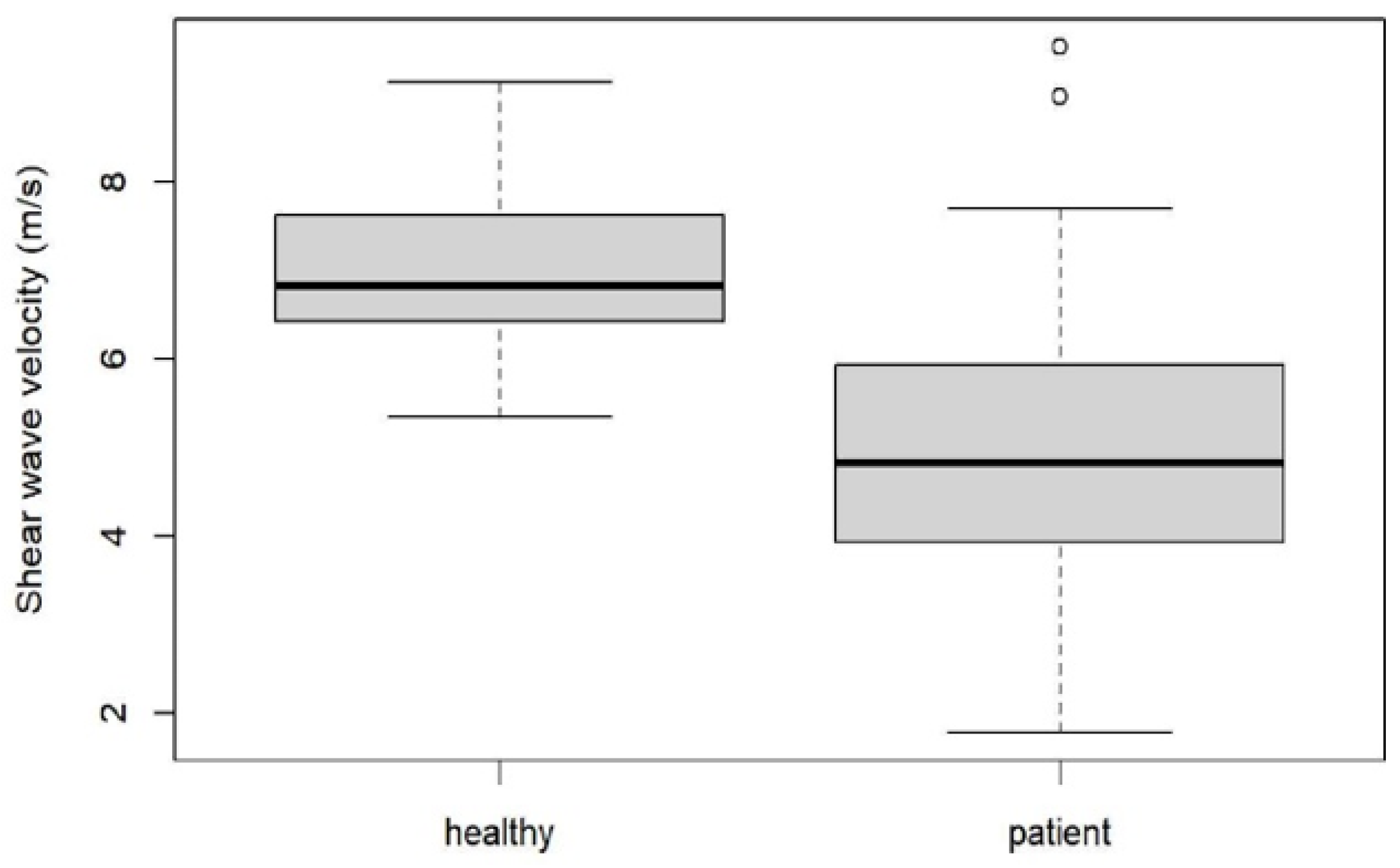
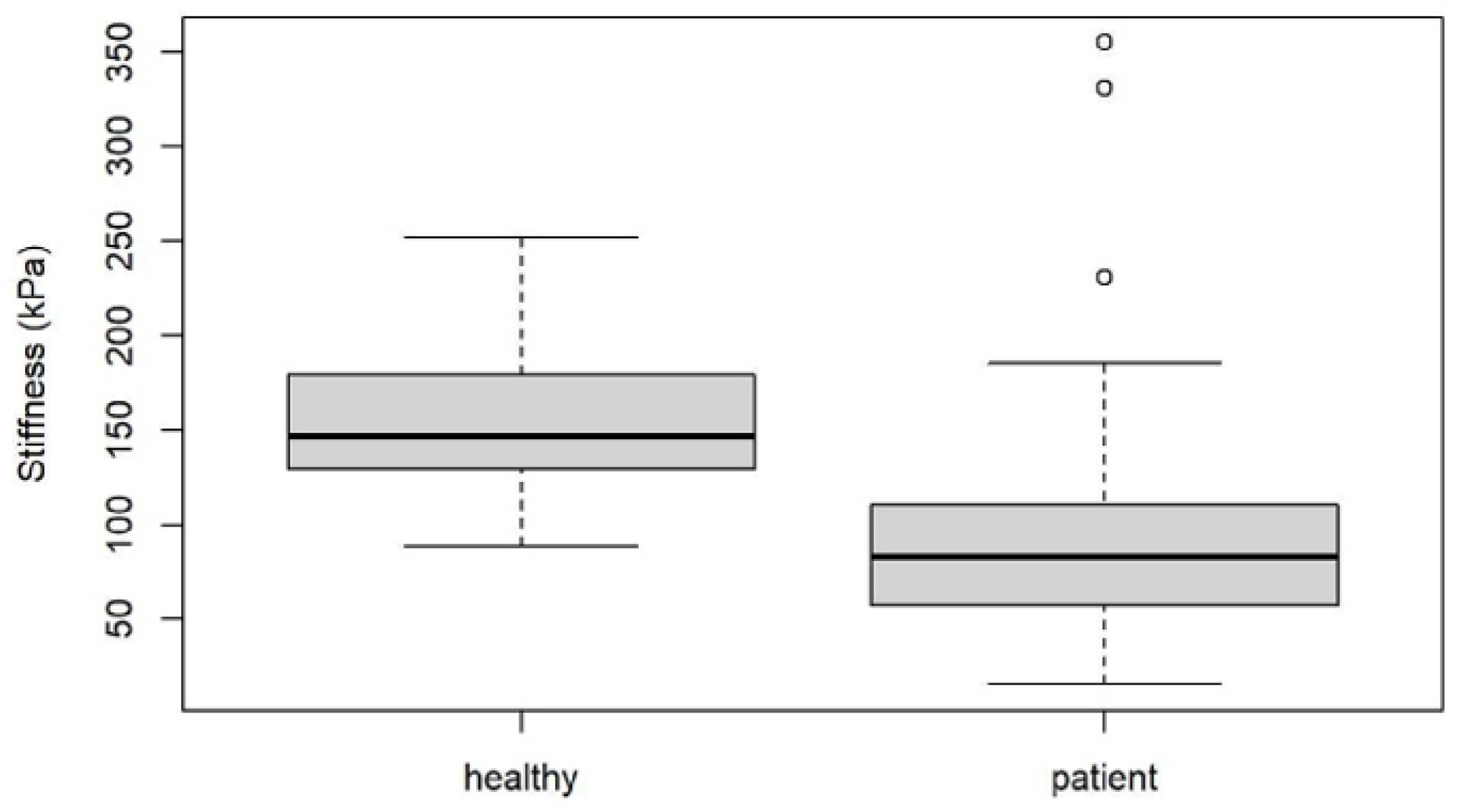
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rompe, J.D. Plantar fasciopathy. Sports Med. Arthrosc. Rev. 2009, 17, 100–104. [Google Scholar] [CrossRef]
- Irving, D.B.; Cook, J.L.; Young, M.A.; Menz, H.B. Impact of chronic plantar heel pain on health-related quality of life. J. Am. Podiatr. Med. Assoc. 2008, 98, 283–289. [Google Scholar] [CrossRef] [PubMed]
- López, P.P.; Vallejo, R.B.D.B.; Losa-Iglesias, M.E.; Rodríguez-Sanz, D.; Lobo, C.C.; López, D.L. Impact of plantar fasciitis on the quality of life of male and female patients according to the foot health status questionnaire. J. Pain Res. 2018, 11, 875–880. [Google Scholar] [CrossRef] [Green Version]
- Wearing, S.C.; Smeathers, J.E.; Urry, S.R.; Hennig, E.M.; Hills, A.P. The pathomechanics of plantar fasciitis. Sports Med. 2006, 36, 585–611. [Google Scholar] [CrossRef]
- Lobo, C.C.; Marín, A.G.; Sanz, D.R.; López, D.L.; López, P.P.; Morales, C.R.; Corbalán, I.S. Ultrasound evaluation of intrinsic plantar muscles and fascia in hallux valgus: A case-control study. Medicine 2016, 95, e5243. [Google Scholar] [CrossRef] [PubMed]
- Irving, D.; Cook, J.; Menz, H. Factors associated with chronic plantar heel pain: A systematic review. J. Sci. Med. Sport 2006, 9, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.A.; Tramaglini, D.M.; Levine, R.E.; Ohno, K.; Choi, N.-Y.; Woo, S.L.-Y. Tensile and viscoelastic properties of human patellar tendon. J. Orthop. Res. 1994, 12, 796–803. [Google Scholar] [CrossRef]
- Lewis, G.; Shaw, K.M. Tensile properties of human tendo achillis: Effect of donor age and strain rate. J. Foot Ankle Surg. 1997, 36, 435–445. [Google Scholar] [CrossRef]
- Lee, T.Q.; Dettling, J.; Sandusky, M.D.; McMahon, P.J. Age related biomechanical properties of the glenoid–anterior band of the inferior glenohumeral ligament-humerus complex. Clin. Biomech. 1999, 14, 471–476. [Google Scholar] [CrossRef]
- Wright, D.G.; Rennels, D.C. A study of the elastic properties of plantar fascia. J. Bone Jt. Surg. 1964, 46, 482–492. [Google Scholar] [CrossRef]
- Hall, T.J. AAPM/RSNA physics tutorial for residents: Topics in US: Beyond the basics: Elasticity imaging with US. Radiographics 2003, 23, 1657–1671. [Google Scholar] [CrossRef]
- Garra, B.S. Imaging and estimation of tissue elasticity by ultrasound. Ultrasound Q. 2007, 23, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Klauser, A.S.; Miyamoto, H.; Bellmann-Weiler, R.; Feuchtner, G.M.; Wick, M.C.; Jaschke, W.R. Sonoelastography: Musculoskeletal ap-plications. Radiology 2014, 272, 622–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandrin, L.; Tanter, M.; Gennisson, J.L.; Catheline, S.; Fink, M. Shear elasticity probe for soft tissues with 1-D transient elas-tography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2002, 49, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Taljanovic, M.S.; Gimber, L.H.; Becker, G.W.; Latt, L.D.; Klauser, A.; Melville, D.M.; Gao, L.; Witte, R.S. Shear-wave elastography: Basic physics and musculoskeletal applications. Radiographics 2017, 37, 855–870. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Park, H.J.; Lee, S.Y. Usefulness of strain elastography of the musculoskeletal system. Ultrasonography 2016, 35, 104–109. [Google Scholar] [CrossRef]
- Davis, L.C.; Baumer, T.G.; Bey, M.J.; Van Holsbeeck, M.T. Clinical utilization of shear wave elastography in the musculoskeletal system. Ultrasonography 2019, 38, 2–12. [Google Scholar] [CrossRef]
- Gatz, M.; Bejder, L.; Quack, V.; Schrading, S.; Dirrichs, T.; Tingart, M.; Kuhl, C.; Betsch, M. Shear wave elastography (SWE) for the evaluation of patients with plantar fasciitis. Acad. Radiol. 2020, 27, 363–370. [Google Scholar] [CrossRef]
- De Zordo, T.; Lill, S.R.; Fink, C.; Feuchtner, G.M.; Jaschke, W.; Bellmann-Weiler, R.; Klauser, A.S. Real-time sonoelastography of lateral epi-condylitis: Comparison of findings between patients and healthy volunteers. Am. J. Roentgenol. 2009, 193, 180–185. [Google Scholar] [CrossRef]
- De Zordo, T.; Fink, C.; Feuchtner, G.M.; Smekal, V.; Reindl, M.; Klauser, A.S. Real-time sonoelastography findings in healthy Achilles tendons. Am. J. Roentgenol. 2009, 193, W134–W138. [Google Scholar] [CrossRef]
- Drakonaki, E.; Allen, G.; Wilson, D. Real-time ultrasound elastography of the normal Achilles tendon: Reproducibility and pattern description. Clin. Radiol. 2009, 64, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Chen, W.-S.; Wang, T.-G. Plantar fascia softening in plantar fasciitis with normal B-mode sonography. Skelet. Radiol. 2015, 44, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Sconfienza, L.M.; Silvestri, E.; Orlandi, D.; Fabbro, E.; Ferrero, G.; Martini, C.; Sardanelli, F.; Cimmino, M.A. Real-time sonoelastography of the plantar fascia: Comparison between patients with plantar fasciitis and healthy control subjects. Radiology 2013, 267, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Chino, K.; Lacourpaille, L.; Sasahara, J.; Suzuki, Y.; Hug, F. Effect of toe dorsiflexion on the regional distribution of plantar fascia shear wave velocity. Clin. Biomech. 2019, 61, 11–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-H.; Chang, K.-V.; Mio, S.; Chen, W.-S.; Wang, T.-G. Sonoelastography of the plantar fascia. Radiology 2011, 259, 502–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, J.L.; Christensen, J.C.; Kravitz, S.R.; Mendicino, R.W.; Schuberth, J.M.; Vanore, J.V.; Weil, L.S., Sr.; Zlotoff, H.J.; Bouché, R.; Baker, J. The diagnosis and treatment of heel pain: A clinical practice guideline-revision 2010. J. Foot Ankle Surg. 2010, 49 (Suppl. S3), S1–S19. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, A.S.; Hazleman, B.L.; Riley, G.P. The vasculature and its role in the damaged and healing tendon. Arthritis Res. 2002, 4, 252–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granado, M.J.; Lohman, E.B.; Daher, N.S., III; Gordon, K.E. Effect of gender, toe extension position, and plantar fasciitis on plantar fascia thickness. Foot Ankle Int. 2019, 40, 439–446. [Google Scholar] [CrossRef]
- McMillan, A.M.; Landorf, K.B.; Gregg, J.M.; De Luca, J.; Cotchett, M.P.; Menz, H.B. Hyperemia in plantar fasciitis determined by power doppler ultrasound. J. Orthop. Sports Phys. Ther. 2013, 43, 875–880. [Google Scholar] [CrossRef] [Green Version]
- Putz, F.J.; Hautmann, M.G.; Banas, M.C.; Jung, E.M. Investigation of the acute plantar fasciitis with contrast-enhanced ultrasound and shear wave elastography—First results. Clin. Hemorheol. Microcirc. 2017, 67, 415–423. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baur, D.; Schwabl, C.; Kremser, C.; Taljanovic, M.S.; Widmann, G.; Sconfienza, L.M.; Sztankay, J.; Feuchtner, G.; Klauser, A.S. Shear Wave Elastography of the Plantar Fascia: Comparison between Patients with Plantar Fasciitis and Healthy Control Subjects. J. Clin. Med. 2021, 10, 2351. https://doi.org/10.3390/jcm10112351
Baur D, Schwabl C, Kremser C, Taljanovic MS, Widmann G, Sconfienza LM, Sztankay J, Feuchtner G, Klauser AS. Shear Wave Elastography of the Plantar Fascia: Comparison between Patients with Plantar Fasciitis and Healthy Control Subjects. Journal of Clinical Medicine. 2021; 10(11):2351. https://doi.org/10.3390/jcm10112351
Chicago/Turabian StyleBaur, Daniel, Christoph Schwabl, Christian Kremser, Mihra S. Taljanovic, Gerlig Widmann, Luca Maria Sconfienza, Judith Sztankay, Gudrun Feuchtner, and Andrea S. Klauser. 2021. "Shear Wave Elastography of the Plantar Fascia: Comparison between Patients with Plantar Fasciitis and Healthy Control Subjects" Journal of Clinical Medicine 10, no. 11: 2351. https://doi.org/10.3390/jcm10112351
APA StyleBaur, D., Schwabl, C., Kremser, C., Taljanovic, M. S., Widmann, G., Sconfienza, L. M., Sztankay, J., Feuchtner, G., & Klauser, A. S. (2021). Shear Wave Elastography of the Plantar Fascia: Comparison between Patients with Plantar Fasciitis and Healthy Control Subjects. Journal of Clinical Medicine, 10(11), 2351. https://doi.org/10.3390/jcm10112351






