Evaluation of Effects of Diabetes Mellitus, Hypercholesterolemia and Hypertension on Bell’s Palsy
Abstract
:1. Introduction
2. Materials—Methods
3. Statistic Methods
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, S.Y.; Jung, J.; Byun, J.Y.; Park, M.S.; Kim, S.H.; Yeo, S.G. The effect of metabolic syndrome on Bell’s palsy recovery rate. Acta Otolaryngol. 2018, 138, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jung, J.; Jung, S.Y.; Dong, S.H.; Byun, J.Y.; Park, M.S.; Kim, S.H.; Yeo, S.G. Comparative prognosis in patients with Ramsay-Hunt syndrome and Bell’s palsy. Eur. Arch. Otorhinolaryngol. 2019, 276, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Savadi-Oskouei, D.; Abedi, A.; Sadeghi-Bazargani, H. Independent role of hypertension in Bell’s palsy: A case-control study. Eur. Neurol. 2008, 60, 253–257. [Google Scholar] [CrossRef]
- Yoo, M.C.; Soh, Y.; Chon, J.; Lee, J.H.; Jung, J.; Kim, S.S.; You, M.W.; Byun, J.Y.; Kim, S.H.; Yeo, S.G. Evaluation of factors associated with favorable outcomes in adults with Bell palsy. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 256–263. [Google Scholar] [CrossRef]
- Greco, A.; Gallo, A.; Fusconi, M.; Marinelli, C.; Macri, G.F.; de Vincentiis, M. Bell’s palsy and autoimmunity. Autoimmun. Rev. 2012, 12, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Kariya, S.; Cureoglu, S.; Morita, N.; Nomiya, S.; Nomiya, R.; Schachern, P.A.; Nishizaki, K.; Paparella, M.M. Vascular findings in the facial nerve canal in human temporal bones with diabetes mellitus. Otol. Neurotol. 2009, 30, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Hato, N.; Gyo, K.; Yanagihara, N. Prognostic factors of Bell’s palsy: Prospective patient collected observational study. Eur. Arch. Otorhinolaryngol. 2014, 271, 1891–1895. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, N.; Horii, A.; Sakata, Y.; Inohara, H. Prognostic factors of peripheral facial palsy: Multivariate analysis followed by receiver operating characteristics and Kaplan-Meier analyses. Otol. Neurotol. 2011, 32, 1031–1036. [Google Scholar] [CrossRef]
- Yeo, S.W.; Lee, D.H.; Jun, B.C.; Chang, K.H.; Park, Y.S. Analysis of prognostic factors in Bell’s palsy and Ramsay Hunt syndrome. Auris Nasus Larynx 2007, 34, 159–164. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42 (Suppl. 1), S13–S28. [Google Scholar]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC /AHA /AAPA /ABC /ACPM /AGS /APhA /ASH /ASPC /NMA /PCNA. Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Executive summary of the third report of the National Cholesterol Education Program (NCEP). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Liu, B.; Sun, Y.; Du, Y.; Snetselaar, L.G.; Hu, F.B.; Bao, W. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: Population based study. BMJ 2018, 362, k1497. [Google Scholar] [CrossRef] [Green Version]
- Lacruz, M.E.; Kluttig, A.; Hartwig, S.; Löer, M.; Tiller, D.; Greiser, K.H.; Werdan, K.; Haerting, J. Prevalence and incidence of hypertension in the general adult population: Results of the CARLA-Cohort study. Medicine 2015, 94, e952. [Google Scholar] [CrossRef]
- Carroll, M.D.; Fryar, C.D.; Nguyen, D.T. Total and high lipoprotein cholesterol in adults: United States, 2015–2016. NCHS Data Brief 2017, 290, 1–8. [Google Scholar]
- Riga, M.; Kefalidis, G.; Danielides, V. The role of diabetes mellitus in the clinical presentation and prognosis of Bell’s palsy. J. Am. Board Fam. Med. 2012, 25, 819–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pecket, P.; Schattner, A. Concurrent Bell’s palsy and diabetes mellitus: A diabetic mononeuropathy? J. Neurol. Neurosurg. Psychiatry 1982, 45, 652–655. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, A.; Haginomori, S.; Takamaki, A.; Nonaka, R.; Araki, M.; Takenaka, H. Prognosis for Bell’s palsy: A comparison of diabetic and nondiabetic patients. Acta Otolaryngol. 2007, 127, 888–891. [Google Scholar] [CrossRef]
- Yanagihara, N.; Hyodo, M. Association of diabetes mellitus and hypertension with Bell’s palsy and Ramsay Hunt syndrome. Ann. Rhinol. Laryngol. 1988, 137, 5–7. [Google Scholar] [CrossRef]
- Chiu, Y.N.; Yen, M.F.; Chen, L.S.; Pan, S.L. Increased risk of stroke after Bell’s palsy: A population-based longitudinal follow-up study. J. Neurol. Neurosurg. Psychiatry 2012, 83, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Eliçora, S.S.; Erdem, D. Does type 2 diabetes mellitus affect the healing of Bell’s palsy in adults? Can. J. Diabetes 2018, 42, 433–436. [Google Scholar] [CrossRef]
- Kiziltan, M.E.; Uluduz, D.; Yaman, M.; Uzun, N. Electrophysiological findings of acute peripheral facial palsy in diabetic and non-diabetic patients. Neurosci. Lett. 2007, 418, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Bosco, D.; Plastino, M.; Bosco, F.; Consoli, A.; Labate, A.; Pirritano, D.; Consoli, D.; Fava, A. Bell’s palsy: A manifestation of prediabetes? Acta Neurol. Scand. 2011, 123, 68–72. [Google Scholar] [CrossRef]
- Kudoh, A.; Ebina, M.; Kudo, H.; Matsuki, A. Delayed recovery of patients with Bell’s palsy complicated by non-insulin-dependent diabetes mellitus and hypertension. Eur. Arch. Otorhinolaryngol. 1998, 255, 166–167. [Google Scholar] [CrossRef] [PubMed]
- Peitersen, E. Bell’s palsy: The spontaneous course of 2500 peripheral facial nerve palsies of different etiologies. Acta Otolaryngol. 2002, 549, 4–30. [Google Scholar] [CrossRef]
- Benatar, M.; Edlow, J. The spectrum of cranial neuropathy in patients with Bell’s palsy. Arch. Intern. Med. 2004, 164, 2383–2385. [Google Scholar] [CrossRef]
- Chaco, J. Subclinical peripheral nerve involvement in unilateral Bell’s palsy. Am. J. Phys. Med. 1973, 52, 195–197. [Google Scholar] [PubMed]
- Jörg, R.; Milani, G.P.; Simonetti, G.D.; Bianchetti, M.G.; Simonetti, B.G. Peripheral facial nerve palsy in severe systemic hypertension: A systematic review. Am. J. Hypertens. 2013, 26, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, E.; Nishimura, H.; Hirono, Y. Occurrence of sequelae in Bell’s palsy. Acta Otolaryngol. 1988, 446, 93–96. [Google Scholar] [CrossRef]

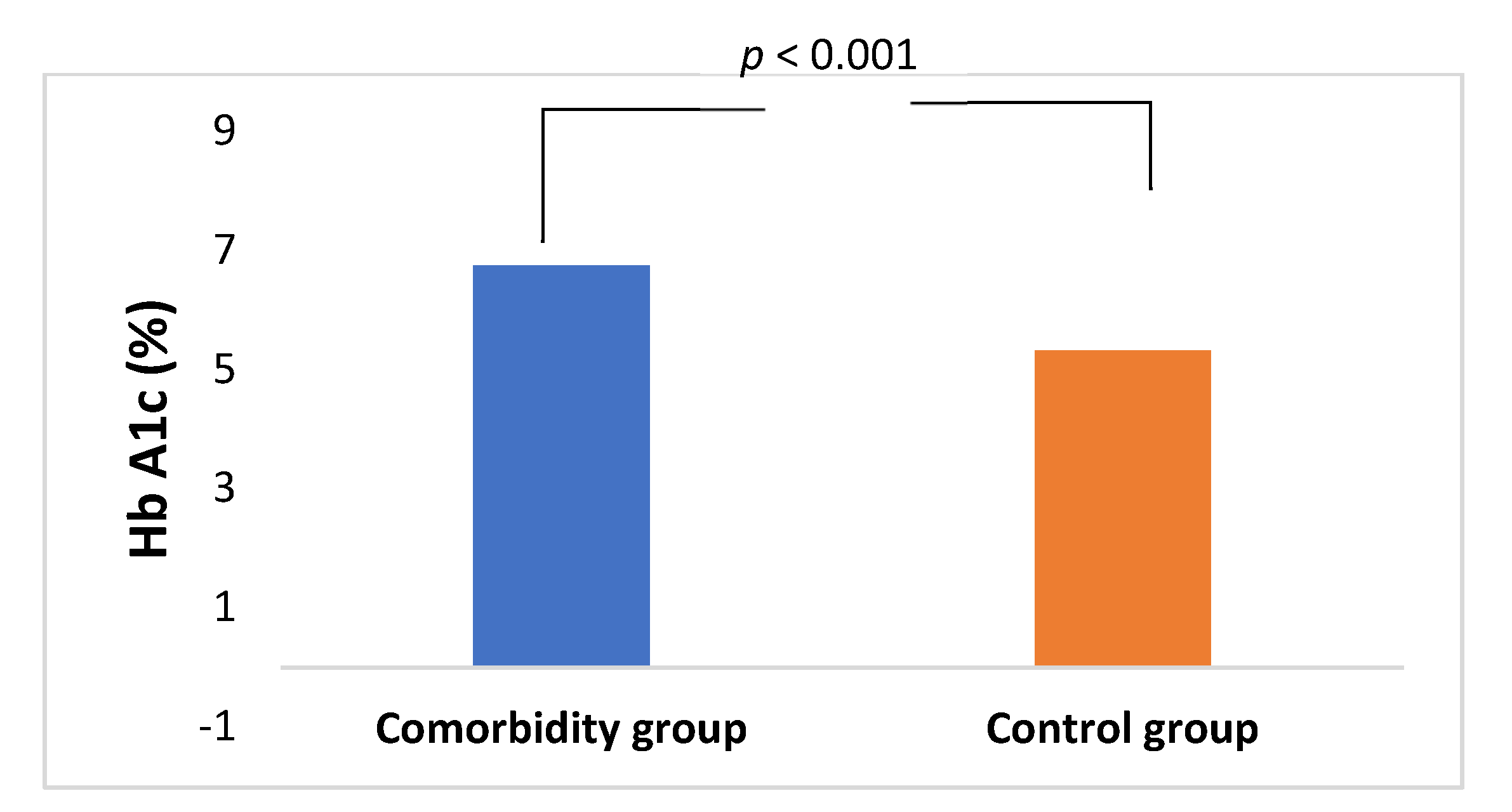
| Mean Age (Years) ± SD | Comorbidity Group | Control Group | Statistic Test |
|---|---|---|---|
| Male | 65.9 ± 9.7 | 59.1 ± 13.3 | t(46) = 1.933, p = 0.059 |
| Female | 64.6 ± 9.9 | 55.4 ± 11.1 | t(46) = 2.983, p < 0.01 |
| Total | 65.1 ± 9.7 | 57.7 ± 12.5 | t(94) = 3.272, p < 0.01 |
| Grade | Description | |
|---|---|---|
| I | Normal | Normal symmetrical function. |
| II | Mild dysfunction | Slight weakness noticeable only on close inspection. Complete eye closure with minimal effort. Slight asymmetry of smile with maximal effort. Synkinesis barely noticeable, contracture or spasm is absent. |
| III | Moderate dysfunction | Obvious weakness, but not disfiguring. May not be able to lift eyebrow. Complete eye closure and asymmetric mouth movement with maximal effort; obvious but not disfiguring synkinesis. |
| IV | Moderate severe dysfunction | Obvious disfiguring weakness; inability to lift brow; incomplete eye closure and asymmetry of mouth with maximal effort; severe synkinesis. |
| V | Severe dysfunction | Motion barely perceptible; incomplete eye closure, slight movement of corner of mouth; synkinesis. |
| VI | Total paralysis | No movement; loss of tone; no synkinesis, contracture or spasm. |
| DM (n = 34) | HT (n = 27) | HC (n = 19) | DM & HT (n = 14) | DM & HC (n = 4) | HT & HC (n = 7) | DM & HT & HC (n = 6) | Control Group (n = 46) | |
|---|---|---|---|---|---|---|---|---|
| Gender (male/female) | 16/18 (Χ2(1) = 1.065, p = 0.302) | 8/19 (Χ2(1) = 5.759 p < 0.05) * | 5/14 (X2(1) = 5.640 p < 0.05) * | 6/8 (X2(1) = 1.088, p = 0.297) | 2/2 (X2(1) = 0.181, p = 0.670) | 1/6 (Χ2(1) = 5.321, p < 0.05) * | 0/6 (Χ2(1) = 7.913, p < 0.05) * | 27/19 |
| Age (Mean ± SD) | 66.82 ± 9.72 (t(78) = 3.137, p < 0.01) | 65.56 ± 8.08 (t(71) = 2.540, p < 0.05) * | 64.84 ± 10.35 (t(63) = 1.887, p = 0.064) | 68.29 ± 7.37 (t(58) = 2.686, p < 0.05) * | 65.25 ± 15.327 (t(48) = 1.147, p = 0.257) | 63.14 ± 7.669 (t(51) = 1.122, p = 0.267) | 68.50 ± 8.503 (t(50) = 2.054, p < 0.05) * | 58.57 ± 12.87 |
| Initial HB grade (Mean ± SD) | 4.82 ± 1.29 (t(78) = 4.097, p < 0.001) | 4.26 ± 1.51 (t(71) = 2.090, p < 0.05) * | 4.74 ± 1.41 (t(63) = 3.040, p < 0.01) | 4.71 ± 1.44 (t(58) = 2.641, p < 0.05) * | 5.75 ± 0.500 (t(48) = 2.876, p < 0.01) | 3.86 ± 1.773 (t(51) = 0.589, p = 0.559) | 5.33 ± 0.516 (t(50) = 2.871, p < 0.01) | 3.48 ± 1.56 |
| Final HΒ grade (HB I/HB II-VI) | 20/14 (Χ2(1) = 3.517, p = 0.061) | 19/8 (Χ2(1) = 0.570, p = 0.450) | 14/5 (Χ2(1) = 0.159, p = 0.690) | 8/6 (Χ2(1) = 2.448, p = 0.118) | 2/2 (Χ2(1) = 1.611, p = 0.204) | 7/0 (Χ2(1) = 1.876, p = 0.171) | 4/2 (Χ2(1) = 0.402, p = 0.526) | 36/10 |
| HB Grade | Comorbidity Group (n = 50) | Control Group (n = 46) | |
|---|---|---|---|
| I-II | 36 (72%) | 42 (91.3%) | X2(1) = 5.861, p < 0.05 |
| III-VI | 14 (28%) | 4 (8.7%) |
| A | Final HB (Comorbidity Group, n = 50) | B | Final HB (Control Group, n = 46) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial HB | I | II | III | IV | V | VI | Initial HB | I | II | III | IV | V | VI |
| II | 5 | II | 11 | 1 | |||||||||
| III | 11 | III | 15 | 1 | |||||||||
| IV | 2 | 1 | IV | 2 | 1 | ||||||||
| V | 8 | 2 | 5 | 1 | V | 6 | 2 | 1 | |||||
| VI | 6 | 2 | 3 | 3 | 1 | VI | 2 | 1 | 3 | ||||
| Final House–Brackmann Grade | I–II | III–VI | |
|---|---|---|---|
| HbA1c > 6.7% | 9 | 7 | p = 0.004 *, OR = 3.5 (1.2–13.5) |
| Hypertension | 22 | 7 | p = 0.2, OR = 1.2 (0.2–5.1) |
| Hypercholesterolemia | 16 | 3 | p = 0.3, OR = 0.3 (0.4–7.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psillas, G.; Dimas, G.G.; Sarafidou, A.; Didangelos, T.; Perifanis, V.; Kaiafa, G.; Mirkopoulou, D.; Tegos, T.; Savopoulos, C.; Constantinidis, J. Evaluation of Effects of Diabetes Mellitus, Hypercholesterolemia and Hypertension on Bell’s Palsy. J. Clin. Med. 2021, 10, 2357. https://doi.org/10.3390/jcm10112357
Psillas G, Dimas GG, Sarafidou A, Didangelos T, Perifanis V, Kaiafa G, Mirkopoulou D, Tegos T, Savopoulos C, Constantinidis J. Evaluation of Effects of Diabetes Mellitus, Hypercholesterolemia and Hypertension on Bell’s Palsy. Journal of Clinical Medicine. 2021; 10(11):2357. https://doi.org/10.3390/jcm10112357
Chicago/Turabian StylePsillas, George, Grigorios G. Dimas, Anastasia Sarafidou, Triantafyllos Didangelos, Vasilios Perifanis, Georgia Kaiafa, Daphne Mirkopoulou, Thomas Tegos, Christos Savopoulos, and Jiannis Constantinidis. 2021. "Evaluation of Effects of Diabetes Mellitus, Hypercholesterolemia and Hypertension on Bell’s Palsy" Journal of Clinical Medicine 10, no. 11: 2357. https://doi.org/10.3390/jcm10112357
APA StylePsillas, G., Dimas, G. G., Sarafidou, A., Didangelos, T., Perifanis, V., Kaiafa, G., Mirkopoulou, D., Tegos, T., Savopoulos, C., & Constantinidis, J. (2021). Evaluation of Effects of Diabetes Mellitus, Hypercholesterolemia and Hypertension on Bell’s Palsy. Journal of Clinical Medicine, 10(11), 2357. https://doi.org/10.3390/jcm10112357









