New Treatments for Atopic Dermatitis Targeting Skin Barrier Repair via the Regulation of FLG Expression
Abstract
:1. Introduction
2. Genetic Causes of FLG Deficiency
3. Direct FLG Replacement Therapy
4. Indirect FLG Replacement Therapy
5. Acquired FLG Deficiency–Regulation of FLG Expression
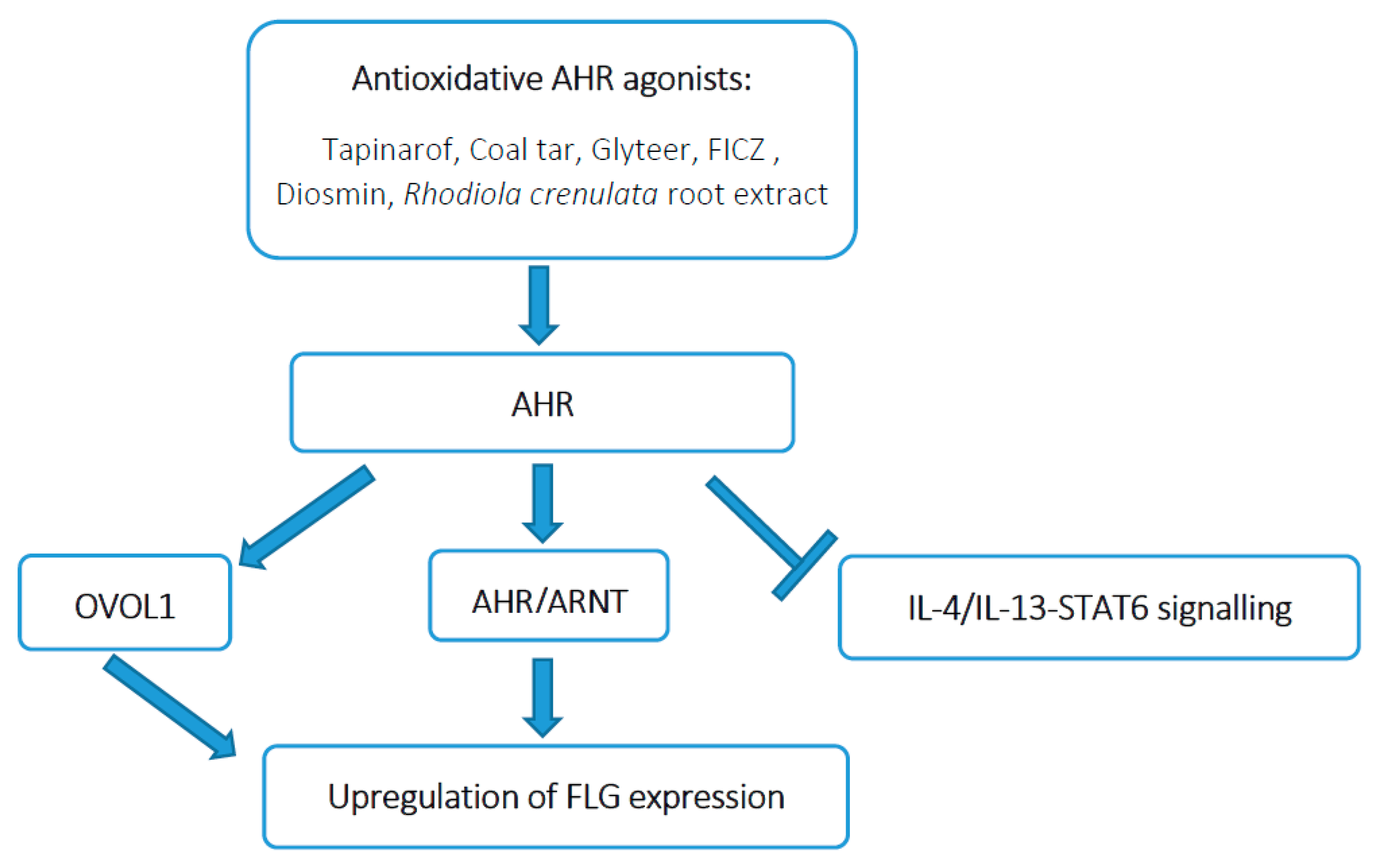
5.1. Upregulation of FLG Expression by AHR Activation
5.2. Downregulation of FLG Expression by IL-4/IL-13
5.2.1. IL-4/IL-13 Inhibitors
5.2.2. IL-13 Inhibitors
5.2.3. JAK Inhibitors
5.3. Downregulation of FLG Expression by IL-22
| Agent | Target(s) | Type of Formulation | Phase | Past Clinical Trial Results | Most Common Side Effects |
|---|---|---|---|---|---|
| Tapinarof | AHR | Topical | 2 | Improvement in EASI, SCORAD, IGA, pruritus scores and reduction in affected BSA [100,101] | No serious side effects |
| 2 | Improvement in EASI-75, IGA and itch scores, 1% was the most effective [102] | Nasopharyngitis, Folliculitis | |||
| 2 | Improvement in EASI-75, EASI-90, IGA, pruritus and POEM scores and reduction in affected BSA [103] | No serious side effects | |||
| Dupilumab | IL-4Rα | Oral | 3 | SOLO1 and SOLO2: Improvement in EASI-75 and IGA scores, as well as in patient-reported outcomes (PROs), symptoms of anxiety/depression, pruritus and DLQI [121] | Injection-site reactions and conjunctivitis |
| 3 | LIBERTY AD CHRONOS: Improvement in EASI and IGA scores for a period of 52 weeks [122] | No significant dupilumab-induced laboratory abnormalities were noted | |||
| 3 | LIBERTY AD PEDS: Improvement in IGA, EASI-75, itch scores and QoL in children aged 6–11 years with severe AD [125] | Injection-site reactions and conjunctivitis | |||
| 3 | LIBERTY AD ADOL: Improvement in IGA and EASI-75 scores in adolescents aged 12–18 years [126] | Injection-site reactions and conjunctivitis | |||
| Lebrikizumab | IL-13 | Oral | 2 | TRIBLE: Improvement in EASI-50, SCORAD and pruritus for dose 125 mg every 4 weeks [137] | No serious side effects |
| 2 | Dose-dependent, significant improvement in EASI, IGA, in the pruritus numeric rating scale (NRS) scores [138] | Upper respiratory tract infections, conjunctivitis and herpesvirus infections | |||
| Tralokinumab | IL-13 | Oral | 2 | Improvement in EASI and IGA, SCORAD, DLQI scores and pruritus numeric rating scale for dose 300 mg, better efficacy in patients with elevated DDP-4 and periostin [140] | Upper respiratory tract infections |
| 3 | ECZTRA1 and ECZTRA2: Improvement in pruritus, sleep interference, DLQI, SCORAD and Patient-Oriented Eczema Measure, monotherapy was effective and was well tolerated up to 52 weeks [141] | Upper respiratory tract infections and conjunctivitis | |||
| Fezakinumab | IL-22 | Intravenous | 2 | Improvement in SCORAD, IGA and BSA [180] | Upper respiratory tract infections |
| Delgocitinib | Pan-JAK (JAK1, JAK2, JAK3, TYK2) | Topical | 3 | Improvement in EASI for 0.5% ointment, very rapid improvement of pruritus [161,162] | No serious side effects |
| 2 | Improvement in EASI score in pediatric patients aged 2–15 years [163] | No serious side effects | |||
| Tofacitinib | Pan-JAK (more selective for JAK1 and JAK3) | Topical | 2 | Significantly decreased EASI, PGA, BSA by 4 weeks and pruritus by 2 days [164] | No serious side effects |
| Ruxolitinib | Selective JAK1, JAK2 | Topical | 2 | Dose-dependent improvement in EASI, IGA, QoL scores and rapid itch reduction. 1.5% was the most effective [165] | No serious sideeffects |
| 3 | Superior efficacy in IGA-TS, EASI-75, and rapid and sustained reduction in itch NRS score [166] | No serious side effects | |||
| Baricitinib | Selective JAK1, JAK2 | Oral | 3 | BREEZE-AD1 and BREEZE-AD2: Improvements in IGA, EASI, itch scores and in night-time awakenings, skin pain and QoL for both 2 mg and 4 mg doses [168] | Nasopharyngitis and headache |
| Upadacitinib | Selective JAK1 | Oral | 2 | Dose-depended improvement in EASI score; the 30-mg once-daily dose showed the greatest clinical benefit [170] | Upper respiratory tract infections |
| Arocitinib | Selective JAK1 | Oral | 2 | Dose-depended improvement in IGA and EASI scores; rapid reduction in pruritus NRS scores by 2 days after the initiation of treatment [171] | Upper respiratory tract infection, headache, nausea, diarrhea |
| 3 | Improvement in IGA, EASI-75, EASI-90, itch NRS and DLQI [172,173] | Nausea, nasopharyngitis, headache, atopic dermatitis | |||
| Gusacitinib | Pan-JAK (JAK1, JAK2, JAK3, TYK2) and SYK | Oral | 1 | Dose-dependent improvement in IGA, EASI-50, EASI-75 and pruritus NRS scores with highest efficacy for 40 mg dose [174] | No serious side effects |
| Cis-urocanic acid | Replacement therapy | Topical | 1/2b | Improvement in EASI and IGA scores, reduce TEWL and cutaneous erythema [63] | No serious side effects |
| L-histidine | Replacement therapy | Oral | pilot | Improvement in EASI and SCORAD scores [64] | No serious side effects |
5.4. Downregulation of FLG Expression by IL-17A
5.5. Downregulation of FLG Expression by IL-24
5.6. FLG Enhancement Therapy
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Dalgard, F.J.; Gieler, U.; Tomas-Aragones, L.; Lien, L.; Poot, F.; Jemec, G.B.E.; Misery, L.; Szabo, C.; Linder, D.; Sampogna, F.; et al. The psychological burden of skin diseases: A cross-sectional multicenter study among dermatological out-patients in 13 European countries. J. Investig. Dermatol. 2015, 135, 984–991. [Google Scholar] [CrossRef] [Green Version]
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.W.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.; et al. The global burden of skin disease in 2010: An analysis of the prevalence and impact of skin conditions. J. Investig. Dermatol. 2014, 134, 1527–1534. [Google Scholar] [CrossRef] [Green Version]
- Deckers, I.A.; McLean, S.; Linssen, S.; Mommers, M.; van Schayck, C.P.; Sheikh, A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990–2010: A systematic review of epidemiological studies. PLoS ONE 2012, 7, e39803. [Google Scholar] [CrossRef] [Green Version]
- Silverberg, N.B. A practical overview of pediatric atopic dermatitis, part 1: Epidemiology and pathogenesis. Cutis 2016, 97, 267–271. [Google Scholar] [PubMed]
- Bieber, T.; D’Erme, A.M.; Akdis, C.A.; Traidl-Hoffmann, C.; Lauener, R.; Schäppi, G.; Schmid-Grendelmeier, P. Clinical phenotypes and endophenotypes of atopic dermatitis: Where are we, and where should we go? J. Allergy Clin. Immunol. 2017, 139, S58–S64. [Google Scholar] [CrossRef] [Green Version]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primers 2018, 21, 1. [Google Scholar] [CrossRef]
- Grobe, W.; Bieber, T.; Novak, N. Pathophysiology of atopic dermatitis. J. Dtsch. Dermatol. Ges. 2019, 17, 433–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakahara, T.; Kido-Nakahara, M.; Tsuji, G.; Furue, M. Basics and recent advances in the pathophysiology of atopic dermatitis. J. Dermatol. 2020, 48, 130–139. [Google Scholar] [CrossRef]
- Novak, N.; Leung, D.Y. Advances in atopic dermatitis. Curr. Opin. Immunol. 2011, 23, 778–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Seok, J.K.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Skin Barrier Abnormalities and Immune Dysfunction in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 2867. [Google Scholar] [CrossRef] [Green Version]
- Elias, M.S.; Long, H.A.; Newman, C.F.; Wilson, P.A.; West, A.; McGill, P.J.; Wu, K.C.; Donaldson, M.J.; Reynolds, N.J. Proteomic analysis of filaggrin deficiency identifies molecular signatures characteristic of atopic eczema. J. Allergy Clin. Immunol. 2017, 140, 1299–1309. [Google Scholar] [CrossRef] [Green Version]
- Saunders, S.P.; Moran, T.; Floudas, A.; Wurlod, F.; Kaszlikowska, A.; Salimi, M.; Quinn, E.M.; Oliphant, C.J.; Núñez, G.; McManus, R.; et al. Spontaneous atopic dermatitis is mediated by innate immunity, with the secondary lung inflammation of the atopic march requiring adaptive immunity. J. Allergy Clin. Immunol. 2016, 137, 482–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, P.M.; Hatano, Y.; Williams, M.L. Basis for the barrier abnormality in atopic dermatitis: Outside-inside-outside pathogenic mechanisms. J. Allergy Clin. Immunol. 2008, 121, 1337–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, P.M. Primary role of barrier dysfunction in the pathogenesis of atopic dermatitis. Exp. Dermatol. 2018, 27, 847–851. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.E.; Leung, D.Y.M. Significance of Skin Barrier Dysfunction in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Egawa, G.; Kabashima, K. Multifactorial skin barrier deficiency and atopic dermatitis: Essential topics to prevent the atopic march. J. Allergy Clin. Immunol. 2016, 138, 350–358.e1. [Google Scholar] [CrossRef] [Green Version]
- Czarnowicki, T.; Krueger, J.G.; Guttman-Yassky, E. Novel concepts of prevention and treatment of atopic dermatitis through barrier and immune manipulations with implications for the atopic march. J. Allergy Clin. Immunol. 2017, 139, 1723–1734. [Google Scholar] [CrossRef] [Green Version]
- Tsakok, T.; Woolf, R.; Smith, C.H.; Weidinger, S.; Flohr, C. Atopic dermatitis: The skin barrier and beyond. Br. J. Dermatol. 2019, 180, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Halling-Overgaard, A.S.; Kezic, S.; Jakasa, I.; Engebretsen, K.A.; Maibach, H.; Thyssen, J.P. Skin absorption through atopic dermatitis skin: A systematic review. Br. J. Dermatol. 2017, 177, 84–106. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Woodfolk, J.A. Skin barrier defects in atopic dermatitis. Curr. Allergy Asthma Rep. 2014, 14, 433. [Google Scholar] [CrossRef] [PubMed]
- Zaniboni, M.C.; Samorano, L.P.; Orfali, R.L.; Aoki, V. Skin barrier in atopic dermatitis: Beyond filaggrin. Bras. Dermatol. 2016, 91, 472–478. [Google Scholar] [CrossRef]
- Pellerin, L.; Henry, J.; Hsu, C.Y.; Balica, S.; Jean-Decoster, C.; Méchin, M.C.; Hansmann, B.; Rodriguez, E.; Weindinger, S.; Schmitt, A.M.; et al. Defects of filaggrin-like proteins in both lesional and nonlesional atopic skin. J. Allergy Clin. Immunol. 2013, 131, 1094–1102. [Google Scholar] [CrossRef]
- Drislane, C.; Irvine, A.D. The role of filaggrin in atopic dermatitis and allergic disease. Ann. Allergy Asthma Immunol. 2020, 124, 36–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Lim, K.M. Skin barrier dysfunction and filaggrin. Arch. Pharm. Res. 2021, 44, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; McLean, W.H. One remarkable molecule: Filaggrin. J. Investig. Dermatol. 2012, 132, 751–762. [Google Scholar] [CrossRef] [Green Version]
- Harding, C.R.; Aho, S.; Bosko, C.A. Filaggrin—Rrevisited. Int. J. Cosmet. Sci. 2013, 35, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Scharschmidt, T.C.; Man, M.Q.; Hatano, Y.; Crumrine, D.; Gunathilake, R.; Sundberg, J.P.; Silva, K.A.; Mauro, T.M.; Hupe, M.; Cho, S.; et al. Filaggrin deficiency confers a paracellular barrier abnormality that reduces inflammatory thresholds to irritants and haptens. J. Allergy Clin. Immunol. 2009, 124, 496–506.e5066. [Google Scholar] [CrossRef] [Green Version]
- Man, M.Q.; Hatano, Y.; Lee, S.H.; Man, M.; Chang, S.; Feingold, K.R.; Leung, D.Y.; Holleran, W.; Uchida, Y.; Elias, P.M. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: Structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J. Investig. Dermatol. 2008, 128, 79–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasaki, H.; Nagao, K.; Kubo, A.; Hata, T.; Shimizu, A.; Mizuno, H.; Yamada, T.; Amagai, M. Altered stratum corneum barrier and enhanced percutaneous immune responses in filaggrin-null mice. J. Allergy Clin. Immunol. 2012, 129, 1538–1546. [Google Scholar] [CrossRef] [Green Version]
- Thyssen, J.P.; Kezic, S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 792–799. [Google Scholar] [CrossRef]
- Kezic, S.; Kemperman, P.M.; Koster, E.S.; de Jongh, C.M.; Thio, H.B.; Campbell, L.E.; Irvine, A.D.; McLean, W.H.; Puppels, G.J.; Caspers, P.J. Loss-of-function mutations in the filaggrin gene lead to reduced level of natural moisturizing factor in the stratum corneum. J. Investig. Dermatol. 2008, 128, 2117–2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flohr, C.; England, K.; Radulovic, S.; McLean, W.H.; Campbel, L.E.; Barker, J.; Perkin, M.; Lack, G. Filaggrin loss-of-function mutations are associated with early-onset eczema, eczema severity and transepidermal water loss at 3 months of age. Br. J. Dermatol. 2010, 163, 1333–1336. [Google Scholar] [CrossRef]
- Tenn, M.W.; Ellis, A.K. The clinical relevance of filaggrin mutations: Effect on allergic disease. Ann. Allergy Asthma Immunol. 2016, 117, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Margolis, D.J. Filaggrin gene mutations with special reference to atopic dermatitis. Curr Treat. Options Allergy 2020, 7, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.; Baurecht, H.; Herberich, E.; Wagenpfeil, S.; Brown, S.J.; Cordell, H.J.; Irvine, A.D.; Weidinger, S. Meta-analysis of filaggrin polymorphisms in eczema and asthma: Robust risk factors in atopic disease. J. Allergy Clin. Immunol. 2009, 123, 1361–1370.e7. [Google Scholar] [CrossRef]
- Palmer, C.N.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.; et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Illi, S.; von Mutius, E.; Lau, S.; Nickel, R.; Grüber, C.; Niggemann, B.; Wahn, U.; Multicenter Allergy Study Group. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J. Allergy Clin. Immunol. 2004, 113, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, A.; Kono, M.; Tsujiuchi, H.; Kobayashi, T.; Nomura, T.; Kitakawa, M.; Suzuki, N.; Yamanaka, K.; Sueki, H.; McLean, W.H.; et al. Compound heterozygotes for filaggrin gene mutations do not always show severe atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Suárez-Fariñas, M.; Tintle, S.J.; Shemer, A.; Chiricozzi, A.; Nograles, K.; Cardinale, I.; Duan, S.; Bowcock, A.M.; Krueger, J.G.; Guttman-Yassky, E. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J. Allergy. Clin. Immunol. 2011, 127, 954–964. [Google Scholar] [CrossRef] [Green Version]
- Howell, M.D.; Kim, B.E.; Gao, P.; Grant, A.V.; Boguniewicz, M.; DeBenedetto, A.; Schneider, L.; Beck, L.A.; Barnes, K.C.; Leung, D.Y. Cytokine modulation of atopic dermatitis filaggrin skin expression. J. Allergy Clin. Immunol. 2009, 124 (Suppl. 2), R7–R12. [Google Scholar] [CrossRef]
- Kezic, S.; O’Regan, G.M.; Yau, N.; Sandilands, A.; Chen, H.; Campbell, L.E.; Kroboth, K.; Watson, R.; Rowland, M.; McLean, W.H.; et al. Levels of filaggrin degradation products are influenced by both filaggrin genotype and atopic dermatitis severity. Allergy 2011, 66, 934–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mischke, D.; Korge, B.P.; Marenholz, I.; Volz, A.; Ziegler, A. Gene encoding structural proteins of epidermal cornification and S100 calcium-binding proteins form a gene complex („epidermal differentiation complex”) on human chromosome 1q21. J. Investig. Dermatol. 1996, 106, 989–992. [Google Scholar] [CrossRef] [Green Version]
- Kypriotou, M.; Huber, M.; Hohl, D. The human epidermal differentiation complex: Cornified envelope precursors, S100 proteins and the ‘fused genes’ family. Exp. Dermatol. 2012, 21, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Irvine, A.D.; McLean, W.H.; Leung, D.Y. Filaggrin mutations associated with skin and allergic diseases. N. Engl. J. Med. 2011, 365, 1315–1327. [Google Scholar] [CrossRef] [Green Version]
- Bin, L.; Leung, D.Y. Genetic and epigenetic studies of atopic dermatitis. Allergy Asthma Clin. Immunol. 2016, 12, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stemmler, S.; Hoffjan, S. Trying to understand the genetics of atopic dermatitis. Mol. Cell Probes 2016, 30, 374–385. [Google Scholar] [CrossRef]
- Irvine, A.D.; McLean, W.H. Breaking the (un)sound barrier: Filaggrin is a major gene for atopic dermatitis. J. Investig. Dermatol. 2006, 126, 1200–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thyssen, J.P.; Godoy-Gijon, E.; Elias, P.M. Ichthyosis vulgaris: The filaggrin mutation disease. Br. J. Dermatol. 2013, 168, 1155–1166. [Google Scholar] [CrossRef]
- Nath, S.; Kumari, N.; Bandyopadhyay, D. Disbiotic lesional microbiome with filaggrin missence variants associate with atopic dermatitis in India. Front. Cell Infect. Microbiol. 2020, 10, 570423. [Google Scholar] [CrossRef] [PubMed]
- Margolis, D.J.; Mitra, N.; Gochnauer, H.; Wubbenhorst, B.; D’Andrea, K.; Kraya, A.; Hoffstad, O.; Gupta, J.; Kim, B.; Yan, A.; et al. Uncommon Filaggrin Variants Are Associated with Persistent Atopic Dermatitis in African Americans. J. Investig. Dermatol. 2018, 138, 1501–1506. [Google Scholar] [CrossRef] [Green Version]
- Pigors, M.; Common, J.; Wong, X.; Malik, S.; Scott, C.A.; Tabarra, N.; Liany, H.; Liu, J.; Limviphuvadh, V.; Maurer-Stroh, S.; et al. Exome Sequencing and Rare Variant Analysis Reveals Multiple Filaggrin Mutations in Bangladeshi Families with Atopic Eczema and Additional Risk Genes. J. Investig. Dermatol. 2018, 138, 2674–2677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Common, J.E.; Haines, R.L.; Balakrishnan, A.; Brown, S.J.; Goh, C.S.; Cordell, H.J.; Sandilands, A.; Campbell, L.E.; Kroboth, K.; et al. Wide spectrum of filaggrin-null mutations in atopic dermatitis highlights differences between Singaporean Chinese and European populations. Br. J. Dermatol. 2011, 165, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Czarnowicki, T.; He, H.; Krueger, J.G.; Guttman-Yassky, E. Atopic dermatitis endotypes and implications for targeted therapeutics. J. Allergy Clin. Immunol. 2019, 143, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Paternoster, L.; Savenije, O.; Heron, J.; Evans, D.M.; Vonk, J.M.; Brunekreef, B.; Wijga, A.H.; Henderson, A.J.; Koppelman, G.H.; Brown, S.J. Identification of atopic dermatitis subgroups in children from 2 longitudinal birth cohorts. J. Allergy Clin. Immunol. 2018, 141, 964–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, S.J.; Kroboth, K.; Sandilands, A.; Campbell, L.E.; Pohler, E.; Kezic, S.; Cordell, H.J.; McLean, W.H.; Irvine, A.D. Intragenic copy number variation within filaggrin contributes to the risk of atopic dermatitis with a dose-dependent effect. J. Investig. Dermatol. 2012, 132, 98–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, K.; Asad, S.; Taylan, F.; Wahlgren, C.F.; Bilcha, K.D.; Nordenskjöld, M.; Winge, M.; Bradley, M. Intragenic Copy Number Variation in the Filaggrin Gene in Ethiopian Patients with Atopic Dermatitis. Pediatr. Dermatol. 2017, 34, e140–e141. [Google Scholar] [CrossRef] [PubMed]
- Quiggle, A.M.; Goodwin, Z.A.; Marfatia, T.R.; Kumar, M.G.; Ciliberto, H.; Bayliss, S.J.; de Guzman Strong, C. Low filaggrin monomer repeats in African American pediatric patients with moderate to severe atopic dermatitis. JAMA Dermatol. 2015, 151, 557–559. [Google Scholar] [CrossRef] [Green Version]
- Nagel-Wolfrum, K.; Möller, F.; Penner, I.; Baasov, T.; Wolfrum, U. Targeting Nonsense Mutations in Diseases with Translational Read-Through-Inducing Drugs (TRIDs). BioDrugs 2016, 30, 49–74. [Google Scholar] [CrossRef]
- Campofelice, A.; Lentini, L.; Di Leonardo, A.; Melfi, R.; Tutone, M.; Pace, A.; Pibiri, I. Strategies against Nonsense: Oxadiazoles as Translational Readthrough-Inducing Drugs (TRIDs). Int. J. Mol. Sci. 2019, 20, 3329. [Google Scholar] [CrossRef] [Green Version]
- Irvine, A.D. Crossing barriers; restoring barriers? Filaggrin protein replacement takes a bow. J. Investig. Dermatol. 2014, 134, 313–314. [Google Scholar] [CrossRef] [Green Version]
- Stout, T.E.; McFarland, T.; Mitchell, J.C.; Appukuttan, B.; Timothy Stout, J. Recombinant filaggrin is internalized and processed to correct filaggrin deficiency. J. Investig. Dermatol. 2014, 134, 423–429. [Google Scholar] [CrossRef] [Green Version]
- Peltonen, J.M.; Pylkkänen, L.; Jansén, C.T.; Volanen, I.; Lehtinen, T.; Laihia, J.K.; Leino, L. Three randomised phase I/IIa trials of 5% cis-urocanic acid emulsion cream in healthy adult subjects and in patients with atopic dermatitis. Acta Dermatol. Venereol. 2014, 94, 415–420. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.P.; Brown, S.B.; Griffiths, C.E.; Weller, R.B.; Gibbs, N.K. Feeding filaggrin: Effects of l-histidine supplementation in atopic dermatitis. Clin. Cosmet. Investig. Dermatol. 2017, 10, 403–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbs, N.K. l-Histidine Supplementation in Adults and Young Children with Atopic Dermatitis (Eczema). J. Nutr. 2020, 150 (Suppl. 1), 2576S–2579S. [Google Scholar] [CrossRef] [PubMed]
- Furue, M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 5382. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, G.; Hashimoto-Hachiya, A.; Kiyomatsu-Oda, M.; Takemura, M.; Ohno, F.; Ito, T.; Morino-Koga, S.; Mitoma, C.; Nakahara, T.; Uchi, H.; et al. Aryl hydrocarbon receptor activation restores filaggrin expression via OVOL1 in atopic dermatitis. Cell Death Dis. 2017, 8, e2931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furue, M.; Hashimoto-Hachiya, A.; Tsuji, G. Antioxidative Phytochemicals Accelerate Epidermal Terminal Differentiation via the AHR-OVOL1 Pathway: Implications for Atopic Dermatitis. Acta Dermatol. Venereol. 2018, 98, 918–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunner, P.M.; Guttman-Yassky, E.; Leung, D.Y. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J. Allergy Clin. Immunol. 2017, 139, S65–S76. [Google Scholar] [CrossRef] [Green Version]
- Gutowska-Owsiak, D.; Schaupp, A.L.; Salimi, M.; Taylor, S.; Ogg, G.S. Interleukin-22 downregulates filaggrin expression and affects expression of profilaggrin processing enzymes. Br. J. Dermatol. 2011, 165, 492–498. [Google Scholar] [CrossRef]
- Cornelissen, C.; Marquardt, Y.; Czaja, K.; Wenzel, J.; Frank, J.; Lüscher-Firzlaff, J.; Lüscher, B.; Baron, J.M. IL-31 regulates differentiation and filaggrin expression in human organotypic skin models. J. Allergy Clin. Immunol. 2012, 129, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Gutowska-Owsiak, D.; Ogg, G.S. Cytokine regulation of the epidermal barrier. Clin. Exp. Allergy 2013, 43, 586–598. [Google Scholar] [CrossRef]
- Hvid, M.; Vestergaard, C.; Kemp, K.; Christensen, G.B.; Deleuran, B.; Deleuran, M. IL-25 in atopic dermatitis: A possible link between inflammation and skin barrier dysfunction? J. Investig. Dermatol. 2011, 131, 150–157. [Google Scholar] [CrossRef] [Green Version]
- Seltmann, J.; Roesner, L.M.; von Hesler, F.W.; Wittmann, M.; Werfel, T. IL-33 impacts on the skin barrier by downregulating the expression of filaggrin. J. Allergy Clin. Immunol. 2015, 135, 1659–1661. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y. Interleukin-33 in atopic dermatitis. J. Dermatol. Sci. 2019, 96, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, S.H.; Choi, D.; Chun, Y.J.; Noh, M. Keratinocyte-derived IL-24 plays a role in the positive feedback regulation of epidermal inflammation in response to environmental and endogenous toxic stressors. Toxicol. Appl. Pharmacol. 2014, 280, 199–206. [Google Scholar] [CrossRef]
- Esser, C.; Bargen, I.; Weighardt, H.; Haarmann-Stemmann, T.; Krutmann, J. Functions of the aryl hydrocarbon receptor in the skin. Semin. Immunopathol. 2013, 35, 677–691. [Google Scholar] [CrossRef]
- Furue, M.; Takahara, M.; Nakahara, T.; Uchi, H. Role of AhR/ARNT system in skin homeostasis. Arch. Dermatol. Res. 2014, 306, 769–779. [Google Scholar] [CrossRef] [Green Version]
- Furue, M.; Tsuji, G.; Mitoma, C.; Nakahara, T.; Chiba, T.; Morino-Koga, S.; Uchi, H. Gene regulation of filaggrin and other skin barrier proteins via aryl hydrocarbon receptor. J. Dermatol. Sci. 2015, 80, 83–88. [Google Scholar] [CrossRef]
- van den Bogaard, E.H.; Podolsky, M.A.; Smits, J.P.; Cui, X.; John, C.; Gowda, K.; Desai, D.; Amin, S.G.; Schalkwijk, J.; Perdew, G.H.; et al. Genetic and pharmacological analysis identifies a physiological role for the AHR in epidermal differentiation. J. Investig. Dermatol. 2015, 135, 1320–1328. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, L.H.; Sutter, C.H.; Leon Carrion, S.; Tran, Q.T.; Bodreddigari, S.; Kensicki, E.; Mohney, R.P.; Sutter, T.R. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated production of reactive oxygen species is an essential step in the mechanism of action to accelerate human keratinocyte differentiation. Toxicol. Sci. 2013, 132, 235–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magiatis, P.; Pappas, P.; Gaitanis, G.; Mexia, N.; Melliou, E.; Galanou, M.; Vlachos, C.; Stathopoulou, K.; Skaltsounis, A.L.; Marselos, M.; et al. Malassezia yeasts produce a collection of exceptionally potent activators of the Ah (dioxin) receptor detected in diseased human skin. J. Investig. Dermatol. 2013, 133, 2023–2030. [Google Scholar] [CrossRef] [Green Version]
- Furue, M.; Uchi, H.; Mitoma, C.; Hashimoto-Hachiya, A.; Tanaka, Y.; Ito, T.; Tsuji, G. Implications of tryptophan photoproduct FICZ in oxidative stress and terminal differentiation of keratinocytes. G. Ital. Dermatol. Venereol. 2019, 154, 37–41. [Google Scholar] [CrossRef]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Mitoma, C.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Uchi, H.; Yan, X.; Hachisuka, J.; Chiba, T.; Esaki, H.; et al. Antioxidant Opuntia ficus-indica Extract Activates AHR-NRF2 Signaling and Upregulates Filaggrin and Loricrin Expression in Human Keratinocytes. J. Med. Food 2015, 18, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Hirano, A.; Goto, M.; Mitsui, T.; Hashimoto-Hachiya, A.; Tsuji, G.; Furue, M. Antioxidant Artemisia princeps extract enhances the expression of filaggrin and loricrin via the AHR/OVOL1 pathway. Int. J. Mol. Sci. 2017, 18, E1948. [Google Scholar] [CrossRef]
- Sutter, C.H.; Bodreddigari, S.; Campion, C.; Wible, R.S.; Sutter, T.R. 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases the expression of genes in the human epidermal differentiation complex and accelerates epidermal barrier formation. Toxicol. Sci. 2011, 124, 128–137. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, G.; Takahara, M.; Uchi, H.; Takeuchi, S.; Mitoma, C.; Moroi, Y.; Furue, M. An environmental contaminant, benzo(a)pyrene, induces oxidative stress-mediated interleukin-8 production in human keratinocytes via the aryl hydrocarbon receptor signaling pathway. J. Dermatol. Sci. 2011, 62, 42–49. [Google Scholar] [CrossRef] [PubMed]
- van den Bogaard, E.H.; Bergboer, J.G.; Vonk-Bergers, M.; van Vlijmen-Willems, I.M.; Hato, S.V.; van der Valk, P.G.; Schröder, J.M.; Joosten, I.; Zeeuwen, P.L.; Schalkwijk, J. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J. Clin. Investig. 2013, 123, 917–927. [Google Scholar] [CrossRef] [Green Version]
- Takei, K.; Mitoma, C.; Hashimoto-Hachiya, A.; Uchi, H.; Takahara, M.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T.; Furue, M. Antioxidant soybean tar Glyteer rescues T-helper-mediated downregulation of filaggrin expression via aryl hydrocarbon receptor. J. Dermatol. 2015, 42, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Mimura, J.; Fujii-Kuriyama, Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochim. Biophys. Acta 2003, 1619, 263–268. [Google Scholar] [CrossRef]
- Furue, K.; Ito, T.; Tsuji, G.; Ulzii, D.; Vu, Y.H.; Kido-Nakahara, M.; Nakahara, T.; Furue, M. The IL-13-OVOL1-FLG axis in atopic dermatitis. Immunology 2019, 158, 281–286. [Google Scholar] [CrossRef] [Green Version]
- Furue, M. Regulation of Skin Barrier Function via Competition between AHR Axis versus IL-13/IL-4‒JAK‒STAT6/STAT3 Axis: Pathogenic and Therapeutic Implications in Atopic Dermatitis. J. Clin. Med. 2020, 9, 3741. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Hashimoto-Hachiya, A.; Tsuji, G. Aryl Hydrocarbon Receptor in Atopic Dermatitis and Psoriasis. Int. J. Mol. Sci 2019, 20, 5424. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Song, K.M.; Jung, C.H. Diosmin restores the skin barrier by targeting the aryl hydrocarbon receptor in atopic dermatitis. Phytomedicine 2021, 81, 153418. [Google Scholar] [CrossRef] [PubMed]
- Kiyomatsu-Oda, M.; Uchi, H.; Morino-Koga, S.; Furue, M. Protective role of 6-formylindolo[3,2-b]carbazole (FICZ), an endogenous ligand for arylhydrocarbon receptor, in chronic mite-induced dermatitis. J. Dermatol. Sci. 2018, 90, 284–294. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto-Hachiya, A.; Tsuji, G.; Murai, M.; Yan, X.; Furue, M. Upregulation of FLG, LOR, and IVL Expression by Rhodiola crenulata Root Extract via Aryl Hydrocarbon Receptor: Differential Involvement of OVOL1. Int. J. Mol. Sci. 2018, 19, 1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.H.; Jayawickreme, C.; Rickard, D.J.; Nicodeme, E.; Bui, T.; Simmons, C.; Coquery, C.M.; Neil, J.; Pryor, W.M.; Mayhew, D.; et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J. Investig. Dermatol. 2017, 137, 2110–2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furue, M.; Nakahara, T. Revival of AHR Agonist for the Treatment of Atopic Dermatitis: Tapinarof. Curr. Treat. Options Allergy 2020, 7, 414–421. [Google Scholar] [CrossRef]
- Bissonnette, R.; Chen, G.; Bolduc, C.; Maari, C.; Lyle, M.; Tang, L.; Webster, J.; Zhou, Y. Efficacy and safety of topical WBI-1001 in the treatment of atopic dermatitis: Results from a phase 2A, randomized, placebo-controlled clinical trial. Arch. Dermatol. 2010, 146, 446–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bissonnette, R.; Poulin, Y.; Zhou, Y.; Tan, J.; Hong, H.C.; Webster, J.; Ip, W.; Tang, L.; Lyle, M. Efficacy and safety of topical WBI-1001 in patients with mild to severe atopic dermatitis: Results from a 12-week, multicentre, randomized, placebo-controlled double-blind trial. Br. J. Dermatol. 2012, 166, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Peppers, J.; Paller, A.S.; Maeda-Chubachi, T.; Wu, S.; Robbins, K.; Gallagher, K.; Kraus, J.E. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J. Am. Acad. Dermatol. 2019, 80, 89–98.e3. [Google Scholar] [CrossRef]
- Paller, A.S.; Stein Gold, L.; Soung, J.; Tallman, A.M.; Rubenstein, D.S.; Gooderham, M. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J. Am. Acad. Dermatol. 2021, 84, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Werfel, T.; Allam, J.P.; Biedermann, T.; Eyerich, K.; Gilles, S.; Guttman-Yassky, E.; Hoetzenecker, W.; Knol, E.; Simon, H.U.; Wollenberg, A.; et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2016, 138, 336–349. [Google Scholar] [CrossRef] [Green Version]
- Gittler, J.K.; Shemer, A.; Suárez-Fariñas, M.; Fuentes-Duculan, J.; Gulewicz, K.J.; Wang, C.Q.; Mitsui, H.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J. Allergy Clin. Immunol. 2012, 130, 1344–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, D.Y.; Guttman-Yassky, E. Deciphering the complexities of atopic dermatitis: Shifting paradigms in treatment approaches. J. Allergy Clin. Immunol. 2014, 134, 769–779. [Google Scholar] [CrossRef] [Green Version]
- Tsoi, L.C.; Rodriguez, E.; Stolzl, D.; Wehkamp, U.; Sun, J.; Gerdes, S.; Sarkar, M.K.; Hübenthal, M.; Zeng, C.; Uppala, R.; et al. Progression of acute-to-chronic atopic dermatitis is associated with quantitative rather than qualitative changes in cytokine responses. J. Allergy Clin. Immunol. 2020, 145, 1406–1415. [Google Scholar] [CrossRef] [Green Version]
- Chan, L.S.; Robinson, N.; Xu, L. Expression of interleukin-4 in the epidermis of transgenic mice results in a pruritic inflammatory skin disease: An experimental animal model to study atopic dermatitis. J. Investig. Dermatol. 2001, 117, 977–983. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Martinez, O.; Overbergh, L.; Mathieu, C.; Prabhakar, B.S.; Chan, L.S. Early up-regulation of Th2 cytokines and late surge of Th1 cytokines in an atopic dermatitis model. Clin. Exp. Immunol. 2004, 138, 375–387. [Google Scholar] [CrossRef]
- Zheng, T.; Oh, M.H.; Oh, S.Y.; Schroeder, J.T.; Glick, A.B.; Zhu, Z. Transgenic expression of interleukin-13 in the skin induces a pruritic dermatitis and skin remodeling. J. Investig. Dermatol. 2009, 129, 742–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novak, N.; Kruse, S.; Kraft, S.; Geiger, E.; Klüken, H.; Fimmers, R.; Deichmann, K.A.; Bieber, T. Dichotomic nature of atopic dermatitis reflected by combined analysis of monocyte immunophenotyping and single nucleotide polymorphisms of the interleukin-4/interleukin-13 receptor gene: The dichotomy of extrinsic and intrinsic atopic dermatitis. J. Investig. Dermatol. 2002, 119, 870–875. [Google Scholar] [CrossRef] [Green Version]
- He, J.Q.; Chan-Yeung, M.; Becker, A.B.; Dimich-Ward, H.; Ferguson, A.C.; Manfreda, J.; Watson, W.T.; Sandford, A.J. Genetic variants of the IL13 and IL4 genes and atopic diseases in at-risk children. Genes Immun. 2003, 4, 385–389. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.J.; Estravís, M.; García-Sánchez, A.; Dávila, I.; Isidoro-García, M.; Sanz, C. Genetics and Epigenetics of Atopic Dermatitis: An Updated Systematic Review. Genes 2020, 11, 442. [Google Scholar] [CrossRef] [Green Version]
- Murata, T.; Taguchi, J.; Puri, R.K.; Mohri, H. Sharing of receptor subunits and signal transduction pathway between the IL-4 and IL-13 receptor system. Int. J. Hematol. 1999, 69, 13–20. [Google Scholar] [PubMed]
- Junttila, I.S. Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes. Front. Immunol. 2018, 9, 888. [Google Scholar] [CrossRef]
- Mitamura, Y.; Nunomura, S.; Nanri, Y.; Ogawa, M.; Yoshihara, T.; Masuoka, M.; Tsuji, G.; Nakahara, T.; Hashimoto-Hachiya, A.; Conway, S.J.; et al. The IL-13/periostin/IL-24 pathway causes epidermal barrier dysfunction in allergic skin inflammation. Allergy 2018, 73, 1881–1891. [Google Scholar] [CrossRef]
- Gandhi, N.A.; Pirozzi, G.; Graham, N.M.H. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev. Clin. Immunol. 2017, 13, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.A.; Thaci, D.; Hamilton, J.D.; Graham, N.M.; Bieber, T.; Rocklin, R.; Ming, J.E.; Ren, H.; Kao, R.; Simpson, E.; et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N. Engl. J. Med. 2014, 371, 130–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, J.D.; Suárez-Fariñas, M.; Dhingra, N.; Cardinale, I.; Li, X.; Kostic, A.; Ming, J.E.; Radin, A.R.; Krueger, J.G.; Graham, N.; et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 1293–1300. [Google Scholar] [CrossRef] [Green Version]
- Guttman-Yassky, E.; Bissonnette, R.; Ungar, B.; Suárez-Fariñas, M.; Ardeleanu, M.; Esaki, H.; Suprun, M.; Estrada, Y.; Xu, H.; Peng, X.; et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 155–172. [Google Scholar] [CrossRef] [Green Version]
- Thaçi, D.; Simpson, E.L.; Deleuran, M.; Kataoka, Y.; Chen, Z.; Gadkari, A.; Eckert, L.; Akinlade, B.; Graham, N.; Pirozzi, G.; et al. Efficacy and safety of dupilumab monotherapy in adults with moderate-to-severe atopic dermatitis: A pooled analysis of two phase 3 randomized trials (LIBERTY AD SOLO 1 and LIBERTY AD SOLO 2). J. Dermatol. Sci. 2019, 94, 266–275. [Google Scholar] [CrossRef] [Green Version]
- Blauvelt, A.; de Bruin-Weller, M.; Gooderham, M.; Cather, J.C.; Weisman, J.; Pariser, D.; Simpson, E.L.; Papp, K.A.; Hong, H.C.; Rubel, D.; et al. Long-term management of moderate- to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): A 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017, 389, 2287–2303. [Google Scholar] [CrossRef]
- de Bruin-Weller, M.; Thaçi, D.; Smith, C.H.; Reich, K.; Cork, M.J.; Radin, A.; Zhang, Q.; Akinlade, B.; Gadkari, A.; Eckert, L.; et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: A placebocontrolled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br. J. Dermatol. 2018, 178, 1083–1101. [Google Scholar] [PubMed]
- Halling, A.S.; Loft, N.D.; Silverberg, J.I.; Guttman-Yassky, E.; Thyssen, J.P. Real-world evidence of dupilumab efficacy and risk of adverse events: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2021, 84, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Siegfried, E.C.; Thaçi, D.; Wollenberg, A.; Cork, M.J.; Arkwright, P.D.; Gooderham, M.; Beck, L.A.; Boguniewicz, M.; Sher, L.; et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: A randomized, double-blinded, placebo-controlled phase 3 trial. J. Am. Acad. Dermatol. 2020, 83, 1282–1293. [Google Scholar] [CrossRef]
- Simpson, E.L.; Paller, A.S.; Siegfried, E.C.; Boguniewicz, M.; Sher, L.; Gooderham, M.J.; Beck, L.A.; Guttman-Yassky, E.; Pariser, D.; Blauvelt, A.; et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: A phase 3 randomized clinical trial. JAMA Dermatol. 2019, 156, 44–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghamrawi, R.; Bell, K.A.; Balogh, E.A.; Strowd, L.C.; Feldman, S.R. Current and emerging biologics for the treatment of pediatric atopic dermatitis. Expert Opin. Biol. 2020, 20, 1435–1445. [Google Scholar] [CrossRef]
- Deleuran, M.; Thaçi, D.; Beck, L.A.; de Bruin-Weller, M.; Blauvelt, A.; Forman, S.; Bissonnette, R.; Reich, K.; Soong, W.; Hussain, I.; et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J. Am. Acad. Dermatol. 2020, 82, 377–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyle, M.; Cevikbas, F.; Harden, J.L.; Guttman-Yassky, E. Understanding the immune landscape in atopic dermatitis: The era of biologics and emerging therapeutic approaches. Exp. Dermatol. 2019, 28, 756–768. [Google Scholar] [CrossRef] [Green Version]
- Bieber, T. Interleukin-13: Targeting an underestimated cytokine in atopic dermatitis. Allergy 2020, 75, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Bao, K.; Reinhardt, R.L. The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine 2015, 75, 25–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scanlon, S.T.; McKenzie, A.N. The messenger between worlds: The regulation of innate and adaptive type-2 immunity by innate lymphoid cells. Clin. Exp. Allergy 2015, 45, 9–20. [Google Scholar] [CrossRef]
- Hurrell, B.P.; Shafiei Jahani, P.; Akbari, O. Social Networking of Group Two Innate Lymphoid Cells in Allergy and Asthma. Front. Immunol. 2018, 9, 2694. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, L.C.; Rodriguez, E.; Degenhardt, F.; Baurecht, H.; Wehkamp, U.; Volks, N.; Szymczak, S.; Swindell, W.R.; Sarkar, M.K.; Raja, K.; et al. Atopic dermatitis is an IL-13-dominant disease with greater molecular heterogeneity compared to psoriasis. J. Investig. Dermatol. 2019, 139, 1480–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ungar, B.; Garcet, S.; Gonzalez, J.; Dhingra, N.; Correa da Rosa, J.; Shemer, A.; Krueger, J.G.; Suarez-Farinas, M.; Guttman-Yassky, E. An integrated model of atopic dermatitis biomarkers highlights the systemic nature of the disease. J. Investig. Dermatol. 2017, 137, 603–613. [Google Scholar] [CrossRef] [Green Version]
- Ultsch, M.; Bevers, J.; Nakamura, G.; Vandlen, R.; Kelley, R.F.; Wu, L.C.; Eigenbrot, C. Structural basis of signaling blockade by anti-IL-13 antibody Lebrikizumab. J. Mol. Biol. 2013, 425, 1330–1339. [Google Scholar] [CrossRef]
- Simpson, E.L.; Flohr, C.; Eichenfield, L.F.; Bieber, T.; Sofen, H.; Taïeb, A.; Owen, R.; Putnam, W.; Castro, M.; DeBusk, K.; et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: A randomized, placebo-controlled phase II trial (TREBLE). J. Am. Acad. Dermatol. 2018, 78, 863–871. [Google Scholar] [CrossRef] [Green Version]
- Guttman-Yassky, E.; Blauvelt, A.; Eichenfield, L.F.; Paller, A.S.; Armstrong, A.W.; Drew, J.; Gopalan, R.; Simpson, E.L. Efficacy and Safety of Lebrikizumab, a High-Affinity Interleukin 13 Inhibitor, in Adults with Moderate to Severe Atopic Dermatitis: A Phase 2b Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Popovic, B.; Breed, J.; Rees, D.G.; Gardener, M.J.; Vinall, L.M.; Kemp, B.; Spooner, J.; Keen, J.; Minter, R.; Uddin, F.; et al. Structural Characterisation Reveals Mechanism of IL-13-Neutralising Monoclonal Antibody Tralokinumab as Inhibition of Binding to IL-13Ralpha1 and IL 13Ralpha2. J. Mol. Biol. 2017, 429, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Howell, M.D.; Guttman-Yassky, E.; Silverberg, J.I.; Kell, C.; Ranade, K.; Moate, R.; van der Merwe, R. Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J. Allergy Clin. Immunol. 2019, 143, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Wollenberg, A.; Blauvelt, A.; Guttman-Yassky, E.; Worm, M.; Lynde, C.; Lacour, J.P.; Spelman, L.; Katoh, N.; Saeki, H.; Poulin, Y.; et al. Tralokinumab for moderate-to-severe atopic dermatitis: Results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br. J. Dermatol. 2021, 184, 437–449. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Toth, D.; Bieber, T.; Alexis, A.F.; Elewski, B.E.; Pink, A.E.; Hijnen, D.; Jensen, T.N.; Bang, B.; Olsen, C.K.; et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: Results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br. J. Dermatol. 2021, 184, 450–463. [Google Scholar] [CrossRef]
- Gadina, M.; Le, M.T.; Schwartz, D.M.; Silvennoinen, O.; Nakayamada, S.; Yamaoka, K.; O’Shea, J.J. Janus kinases to jakinibs: From basic insights to clinical practice. Rheumatology 2019, 58 (Suppl. 1), i4–i16. [Google Scholar] [CrossRef]
- Howell, M.D.; Fitzsimons, C.; Smith, P.A. JAK/STAT inhibitors and other small molecules cytokine antagonists for the treatment of allergic disease. Ann. Allergy Asthma Immunol. 2018, 120, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Stark, G.R.; Cheon, H.; Wang, Y. Responses to cytokine and interferons that depend upon JAKs and STATs. Cold Spring Harb. Perspect. Biol. 2018, 10, a028555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esaki, H.; Ewald, D.A.; Ungar, B.; Rozenblit, M.; Zheng, X.; Xu, H.; Estrada, Y.D.; Peng, X.; Mitsui, H.; Litman, T.; et al. Identification of novel immune and barier genes in atopic dermatitis by means of laser capture microdissection. J. Allergy Clin. Immunol. 2015, 135, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Suarez-Farinas, M.; Ungar, B.; Correa da Rossa, J.; Ewald, D.A.; Rozenblit, M.; Gonzalez, J.; Xu, H.; Zheng, X.; Peng, X.; Estrada, Y.D.; et al. RNA sequencing atopic dermatitis transcriptome profiling provides insihgts into novel disease mechanisms with potential therapeutic implication. J. Allergy Clin. Immunol. 2015, 135, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Fukada, T.; Nishida, K.; Nakayama, M.; Matsuda, M.; Miura, I.; Dainichi, T.; Fukuda, S.; Kabashima, K.; Nakaoka, S.; et al. Hyperactiovation of JAK1 tyrosine kinase induces stepwise, progressive pruritic dermatitis. J. Clin. Invsetig. 2016, 126, 2064–2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunduz, O. JAK/STAT pathway modulation: Does it work in dermatology? Dermatology 2019, 2, e12903. [Google Scholar]
- Howell, M.D.; Kuo, F.I.; Smith, P.A. Targeting the Janus Kinase Family in Autoimmune Skin Diseases. Front. Immunol. 2019, 10, 2342. [Google Scholar] [CrossRef]
- Szalus, K.; Trzeciak, M.; Nowicki, R.J. JAK-STAT Inhibitors in Atopic Dermatitis from Pathogenesis to Clinical Trials Results. Microorganisms 2020, 8, 1743. [Google Scholar] [CrossRef]
- He, H.; Guttman-Yassky, E. JAK Inhibitors for Atopic Dermatitis: An Update. Am. J. Clin. Dermatol. 2018, 20, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Heron, C.E.; Ghamrawi, R.I.; Strowd, L.C.; Feldman, S.R. Emerging Role of Janus Kinase Inhibitors for the Treatment of Atopic Dermatitis. Immunotargets 2020, 9, 255–272. [Google Scholar] [CrossRef]
- Tanimoto, Y.; Shinozaki, Y.; Yamamoto, Y.; Katsuda, E.; Taniai-Riya, K.; Toyoda, K.; Kakimoto, K.; Kimoto, Y.; Amano, W.; Konishi, N.; et al. A novel JAK inhibitor JTE-052 reduces skin inflammation and ameliorates chronic dermatitis in rodent models: Comparison with conventional therapeutic agents. Exp. Dermatol. 2018, 27, 22–29. [Google Scholar] [CrossRef]
- Fukuyama, T.; Ehling, S.; Cook, E.; Baumer, W. Topically administered Janus kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory reponses in a model of allergic dermatitis. J. Pharm. Exp. 2015, 354, 394–405. [Google Scholar] [CrossRef] [Green Version]
- Jin, W.; Huang, W.; Chen, L.; Jin, M.; Wang, Q.; Gao, Z.; Jin, Z. Topical application of JAK1/JAK2 inhibitor momelotinib exhibits significant anti-inflammatory responses in DNCB-induced atopic dermatitis model mice. Int. J. Mol. Sci. 2018, 19, 3973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amano, W.; Nakajima, S.; Kunugi, H.; Numata, Y.; Kitoh, A.; Egawa, G.; Dainichi, T.; Honda, T.; Otsuka, A.; Kimoto, Y.; et al. The Janus kinase inhibitor JTE-052 improves skin barrier function through suppressing signal transducer and activator of transcription 3 signaling. J. Allergy Clin. Immunol. 2015, 136, 667–677.e7. [Google Scholar] [CrossRef] [Green Version]
- Clarysse, K.; Pfaff, C.; Marquardt, Y.; Huth, L.; Kortekaas, K.; Kluwig, D.; Lüscher, B.; Gutermuth, J.; Baron, J. JAK1/3 inhibition preserves epidermal morphology in full-thickness 3D skin models of atopic dermatitis and psoriasis. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Chen, X.F.; Li, Q.; Zhang, S.T. CYT387, a novel JAK2 inhibitor, suppresses IL-13-induced epidermal barrier dysfunction via miR-143 targeting IL-13Rα1 and STAT3. Biochem. Genet. 2020, 59, 531–546. [Google Scholar] [CrossRef]
- Tanimoto, A.; Ogawa, Y.; Oki, C.; Kimoto, Y.; Nozawa, K.; Amano, W.; Noji, S.; Shiozaki, M.; Matsuo, A.; Shinozaki, Y.; et al. Pharmacological properties of JTE-052: A novel potent JAK inhibitor that suppresses various inflammatory responses in vitro and in vivo. Inflamm. Res. 2015, 64, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Kaino, H.; Nagata, T. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J. Am. Acad. Dermatol. 2020, 82, 823–831. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Murata, R.; Kaino, H.; Nagata, T. Long-term safety and efficacy of delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with atopic dermatitis. J. Dermatol. 2020, 47, 114–120. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, H.; Nemoto, O.; Igarashi, A.; Saeki, H.; Oda, M.; Kabashima, K.; Nagata, T. Phase 2 clinical study of delgocitinib ointment in pediatric patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 144, 1575–1583. [Google Scholar] [CrossRef]
- Bissonnette, R.; Papp, K.A.; Poulin, Y.; Gooderham, M.; Raman, M.; Mallbris, L.; Wang, C.; Purohit, V.; Mamolo, C.; Papacharalambous, J.; et al. Topical tofacitinib for atopic dermatitis: A phase IIa randomized trial. Br. J. Dermatol. 2016, 175, 902–911. [Google Scholar] [CrossRef]
- Kim, B.S.; Sun, K.; Papp, K.; Venturanza, M.; Nasir, A.; Kuligowski, M.E. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: Results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study. J. Am. Acad. Dermatol. 2020, 82, 1305–1313. [Google Scholar] [CrossRef] [Green Version]
- Papp, K.; Szepietowski, J.C.; Kircik, L.; Toth, D.; Kuligowski, M.E.; Venturanza, M.E.; Sun, K.; Simpson, E.L. Efficacy and Safety of Ruxolitinib Cream for the Treatment of Atopic Dermatitis: Results from Two Phase 3, Randomized, Double-Blind Studies. SKIN J. Cutan. Med. 2020, 4, s95. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Anderson, K.R.; Tollefson, M.M. New and emerging therapies for pediatric atopic dermatitis. Paediatr. Drugs 2019, 21, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.; Lacour, J.; Spelman, L.; Galimberti, R.; Eichenfield, L.; Bissonnette, R.; King, B.; Thyssen, J.; Silverberg, J.; Bieber, T.; et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: Results from two randomized monotherapy phase III trials. Br. J. Dermatol. 2020, 183, 242–255. [Google Scholar] [CrossRef]
- Timmins, P. Lilly and Incyte Announce Top-Line Results from Phase 3 Study (BREEZE-AD4) of Oral Selective JAK Inhibitor Baricitinib in Combination with Topical Corticosteroids in Patients with Moderate to Severe Atopic Dermatitis Not Controlled with Cyclosporine; Press Release; Eli Lilly and Company: Indianapolis, IN, USA, 2020. [Google Scholar]
- Guttman-Yassky, E.; Thaçi, D.; Pangan, A.L.; Hong, H.C.; Papp, K.A.; Reich, K.; Beck, L.A.; Mohamed, M.F.; Othman, A.A.; Anderson, J.K.; et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebocontrolled trial. J. Allergy Clin. Immunol. 2020, 145, 877–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gooderham, M.J.; Forman, S.B.; Bissonnette, R.; Beebe, J.S.; Zhang, W.; Banfield, C.; Zhu, L.; Papacharalambous, J.; Vincent, M.S.; Peeva, E. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: A phase 2 randomized clinical trial. JAMA Dermatol. 2019, 155, 1371. [Google Scholar] [CrossRef] [Green Version]
- Simpson, E.L.; Sinclair, R.; Forman, S.; Wollenberg, A.; Aschoff, R.; Cork, M.; Bieber, T.; Thyssen, J.P.; Yosipovitch, G.; Flohr, C.; et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet 2020, 396, 255–266. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Simpson, E.L.; Thyssen, J.P.; Gooderham, M.; Chan, G.; Feeney, C.; Biswas, P.; Valdez, H.; DiBonaventura, M.; Nduaka, C.; et al. Efficacy and Safety of Abrocitinib in Patients with Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 863–873. [Google Scholar] [CrossRef]
- Bissonnette, R.; Maari, C.; Forman, S.; Bhatia, N.; Lee, M.; Fowler, J.; Tyring, S.; Pariser, D.; Sofen, H.; Dhawan, S.; et al. The oral Janus kinase/spleen tyrosine kinase inhibitor ASN002 demonstrates efficacy and improves associated systemic inflammation in patients with moderate-to-severe atopic dermatitis: Results from a randomized double-blind placebo-controlled study. Br. J. Dermatol. 2019, 181, 733–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, J.; Choi, Y.; Simpson, E.L. Therapeutic New Era for Atopic Dermatitis: Part 2. Small Molecules. Ann. Dermatol. 2021, 33, 101–107. [Google Scholar] [CrossRef]
- Czarnowicki, T.; Esaki, H.; Gonzalez, J.; Malajian, D.; Shemer, A.; Noda, S.; Talasila, S.; Berry, A.; Gray, J.; Becker, L.; et al. Early pediatric atopic dermatitis shows only a cutaneous lymphocyte antigen (CLA)() TH2/TH1 cell imbalance, whereas adults acquire CLA() TH22/TC22 cell subsets. J. Allergy Clin. Immunol. 2015, 136, 941.e51. [Google Scholar] [CrossRef] [Green Version]
- Jones, B.C.; Logsdon, N.J.; Walter, M.R. Structure of IL-22 bound to its high-affinity IL-22R1 chain. Structure 2008, 16, 1333–1344. [Google Scholar] [CrossRef] [Green Version]
- Jin, M.; Yoon, J. From Bench to Clinic: The Potential of Therapeutic Targeting of the IL-22 Signaling Pathway in Atopic Dermatitis. Immune Netw. 2018, 18, e42. [Google Scholar] [CrossRef] [PubMed]
- Renert-Yuval, Y.; Guttman-Yassky, E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann. Allergy Asthma Immunol. 2020, 124, 28–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guttman-Yassky, E.; Brunner, P.M.; Neumann, A.U.; Khattri, S.; Pavel, A.B.; Malik, K.; Singer, G.K.; Baum, D.; Gilleaudeau, P.; Sullivan-Whalen, M.; et al. Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: A randomized, double-blind, phase 2a trial. J. Am. Acad. Dermatol. 2018, 78, 872–881.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunner, P.M.; Pavel, A.B.; Khattri, S.; Leonard, A.; Malik, K.; Rose, S.; Jim On, S.; Vekaria, A.S.; Traidl-Hoffmann, C.; Singer, G.K.; et al. Baseline IL-22 expression in patients with atopic dermatitis stratifies tissue responses to fezakinumab. J. Allergy Clin. Immunol. 2019, 143, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Nograles, K.E.; Zaba, L.C.; Guttman-Yassky, E.; Fuentes-Duculan, J.; Suárez-Fariñas, M.; Cardinale, I.; Khatcherian, A.; Gonzalez, J.; Pierson, K.C.; White, T.R.; et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br. J. Dermatol. 2008, 159, 1092–1102. [Google Scholar] [CrossRef] [Green Version]
- Dhingra, N.; Guttman-Yassky, E. A possible role for IL-17A in establishing Th2 inflammation in murine models of atopic dermatitis. J. Investig. Dermatol. 2014, 134, 2071–2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krueger, J.G.; Brunner, P.M. Interleukin-17 alters the biology of many cell types involved in the genesis of psoriasis, systemic inflammation and associated comorbidities. Exp. Dermatol. 2018, 27, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Chiricozzi, A.; Nograles, K.E.; Johnson-Huang, L.M.; Fuentes-Duculan, J.; Cardinale, I.; Bonifacio, K.M.; Gulati, N.; Mitsui, H.; Guttman-Yassky, E.; Suárez-Fariñas, M.; et al. IL-17 induces an expanded range of downstream genes in reconstituted human epidermis model. PLoS ONE 2014, 9, e90284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutowska-Owsiak, D.; Schaupp, A.L.; Salimi, M.; Selvakumar, T.A.; McPherson, T.; Taylor, S.; Ogg, G.S. IL-17 downregulates filaggrin and affects keratinocyte expression of genes associated with cellular adhesion. Exp. Dermatol. 2012, 21, 104–110. [Google Scholar] [CrossRef]
- Ungar, B.; Pavel, A.B.; Li, R.; Kimmel, G.; Nia, J.; Hashim, P.; Kim, H.J.; Chima, M.; Vekaria, A.S.; Estrada, Y.; et al. Phase 2 randomized, double-blind study of IL-17 targeting with secukinumab in atopic dermatitis. J. Allergy Clin. Immunol. 2021, 147, 394–397. [Google Scholar] [CrossRef]
- Mitamura, Y.; Nunomura, S.; Furue, M.; Izuhara, K. IL-24: A new player in the pathogenesis of pro-inflammatory and allergic skin diseases. Allergol. Int. 2020, 69, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.H.; Kuo, Y.C.; Tsai, M.H.; Ho, C.C.; Tsai, H.T.; Hsu, C.Y.; Chen, Y.C.; Lin, P. Interleukin-24 as a target cytokine of environmental aryl hydrocarbon receptor agonist exposure in the lung. Toxicol. Appl. Pharm. 2017, 324, 1–11. [Google Scholar] [CrossRef]
- Liu, G.; Asanoma, K.; Takao, T.; Tsukimori, K.; Uchi, H.; Furue, M.; Kato, K.; Wake, N. Aryl hydrocarbon receptor SNP -130 C/T associates with dioxins susceptibility through regulating its receptor activity and downstream effectors including interleukin 24. Toxicol. Lett. 2015, 232, 384–392. [Google Scholar] [CrossRef]
- Vu, Y.H.; Hashimoto-Hachiya, A.; Takemura, M.; Yumine, A.; Mitamura, Y.; Nakahara, T.; Furue, M.; Tsuji, G. IL-24 Negatively Regulates Keratinocyte Differentiation Induced by Tapinarof, an Aryl Hydrocarbon Receptor Modulator: Implication in the Treatment of Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 9412. [Google Scholar] [CrossRef]
- Otsuka, A.; Doi, H.; Egawa, G.; Maekawa, A.; Fujita, T.; Nakamizo, S.; Nakashima, C.; Nakajima, S.; Watanabe, T.; Miyachi, Y.; et al. Possible new therapeutic strategy to regulate atopic dermatitis through upregulating filaggrin expression. J. Allergy Clin. Immunol. 2014, 133, 139–146. [Google Scholar] [CrossRef]
- Hatano, Y.; Man, M.Q.; Uchida, Y.; Crumrine, D.; Mauro, T.M.; Feingold, K.R.; Elias, P.M.; Holleran, W.M. Murine atopic dermatitis responds to peroxisome proliferator-activated receptors alpha and beta/delta (but not gamma) and liver X receptor activators. J. Allergy Clin. Immunol. 2010, 125, 160–169.e1-5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Gurevich, I.; Aneskievich, B.J. Organotypic modeling of human keratinocyte response to peroxisome proliferators. Cells Tissues Organs 2012, 196, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Wallmeyer, L.; Lehnen, D.; Eger, N.; Sochorová, M.; Opálka, L.; Kováčik, A.; Vávrová, K.; Hedtrich, S. Stimulation of PPARα normalizes the skin lipid ratio and improves the skin barrier of normal and filaggrin deficient reconstructed skin. J. Dermatol. Sci. 2015, 80, 102–110. [Google Scholar] [CrossRef]
- Jia, T.; Qiao, W.; Yao, Q.; Wu, W.; Kaku, K. Treatment with Docosahexaenoic Acid Improves Epidermal Keratinocyte Differentiation and Ameliorates Inflammation in Human Keratinocytes and Reconstructed Human Epidermis Models. Molecules 2019, 24, 3156. [Google Scholar] [CrossRef] [Green Version]
- Czarnowicki, T.; Dohlman, A.B.; Malik, K.; Antonini, D.; Bissonnette, R.; Chan, T.C.; Zhou, L.; Wen, H.C.; Estrada, Y.; Xu, H.; et al. Effect of short-term liver X receptor activation on epidermal barrier features in mild to moderate atopic dermatitis: A randomized controlled trial. Ann. Allergy Asthma Immunol. 2018, 120, 631–640.e11. [Google Scholar] [CrossRef] [PubMed]
- Schmuth, M.; Jiang, Y.J.; Dubrac, S.; Elias, P.M.; Feingold, K.R. Thematic review series: Skin lipids. Peroxisome proliferator-activated receptors and liver X receptors in epidermal biology. J. Lipid Res. 2008, 49, 499–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ming, M.; Zhao, B.; Shea, C.R.; Shah, P.; Qiang, L.; White, S.R.; Sims, D.M.; He, Y.Y. Loss of sirtuin 1 (SIRT1) disrupts skin barrier integrity and sensitizes mice to epicutaneous allergen challenge. J. Allergy Clin. Immunol. 2015, 135, 936–945.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielach-Bazyluk, A.; Zbroch, E.; Mysliwiec, H.; Rydzewska-Rosolowska, A.; Kakareko, K.; Flisiak, I.; Hryszko, T. Sirtuin 1 and Skin: Implications in Intrinsic and Extrinsic Aging–A Systematic Review. Cells 2021, 10, 813. [Google Scholar] [CrossRef]
- Jin, T.; Park, K.Y.; Seo, S.J. Adiponectin Upregulates Filaggrin Expression via SIRT1-Mediated Signaling in Human Normal Keratinocytes. Ann. Dermatol. 2017, 29, 407–413. [Google Scholar] [CrossRef]
- Dainichi, T.; Nakano, Y.; Wakae, K.; Otsuka, M.; Muramatsu, M.; Kabashima, K. APOBEC3 regulates keratinocyte differentiation and expression of Notch3. Exp. Dermatol. 2019, 28, 1341–1347. [Google Scholar] [CrossRef]
- Czarnowicki, T.; Malajian, D.; Khattri, S.; Correa da Rosa, J.; Dutt, R.; Finney, R.; Dhingra, N.; Xiangyu, P.; Xu, H.; Estrada, Y.D.; et al. Petrolatum: Barrier repair and antimicrobial responses underlying this “inert” moisturizer. J. Allergy Clin. Immunol. 2016, 137, 1091–1102.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grether-Beck, S.; Felsner, I.; Brenden, H.; Kohne, Z.; Majora, M.; Marini, A.; Jaenicke, T.; Rodriguez-Martin, M.; Trullas, C.; Hupe, M.; et al. Urea uptake enhances barrier function and antimicrobial defense in humans by regulating epidermal gene expression. J. Investig. Dermatol. 2012, 132, 1561–1572. [Google Scholar] [CrossRef] [Green Version]
- Páyer, E.; Szabó-Papp, J.; Ambrus, L.; Szöllősi, A.G.; Andrási, M.; Dikstein, S.; Kemény, L.; Juhász, I.; Szegedi, A.; Bíró, T.; et al. Beyond the physico-chemical barrier: Glycerol and xylitol markedly yet differentially alter gene expression profiles and modify signalling pathways in human epidermal keratinocytes. Exp. Dermatol. 2018, 27, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Sun, R.; Hupe, M.; Kim, P.L.; Park, K.; Crumrine, D.; Lin, T.K.; Santiago, J.L.; Mauro, T.M.; Elias, P.M.; et al. Topical apigenin improves epidermal permeability barrier homoeostasis in normal murine skin by divergent mechanisms. Exp. Dermatol. 2013, 22, 210–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che, D.N.; Cho, B.O.; Shin, J.Y.; Kang, H.J.; Kim, J.; Choi, J.; Jang, S.I. Anti-atopic dermatitis effects of hydrolyzed celery extract in mice. J. Food Biochem. 2020, 44, e13198. [Google Scholar] [CrossRef]
- Che, D.N.; Cho, B.O.; Shin, J.Y.; Kang, H.J.; Kim, J.S.; Oh, H.; Kim, Y.S.; Jang, S.I. Apigenin Inhibits IL-31 Cytokine in Human Mast Cell and Mouse Skin Tissues. Molecules 2019, 24, 1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, M.; Tsang, M.S.; He, R.; Lam, C.W.; Quan, Z.B.; Wong, C.K. The Active Compounds and Therapeutic Mechanisms of Pentaherbs Formula for Oral and Topical Treatment of Atopic Dermatitis Based on Network Pharmacology. Plants 2020, 9, 1166. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.T.T.; Fang, M.; Hwang, E.; Kim, Y.; Park, B.; Seo, S.A.; Do, N.Q.; Nguyen, Q.; Yi, T.H. Inhibitory Effects of Urtica thunbergiana Ethanol Extract on Atopic Dermatitis-Induced NC/Nga Mice. Antioxidants 2020, 9, 197. [Google Scholar] [CrossRef] [Green Version]
| Targets |
|---|
| Gene-based approach: “Read-through” drugs |
| Direct replacement of FLG |
| Indirect replacement therapy: Topical application of FLG metabolites: PCA, UCA L-histidine |
| Inhibiton of cytokine-mediated FLG downregulation: IL-4/IL-13 inhibitors IL-13 inhibitors JAK inhibitors IL-22 inhibitors IL-17 inhibitors IL-24 inhibitors |
| Enhancement FLG expression: AHR agonists JTC-801 Peroxisome proliferator-activated receptors (PPARs) agonists Liver X receptor (LXR) agonists Sirtuin 1 (SIRT1) Apolipoprotein B mRNA editing enzyme complex (APOBEC3) Petrolatum, urea, glycerol Herbal medicines: apigenin, quercetin, luteolin, ursolic acid, rosmarinic acid |
| Pathway | Mechanisms Regulating FLG Expression | Effect on FLG Expression | Mechanisms of Targeted Therapy |
|---|---|---|---|
| IL-4/IL13 | Upregulation of JAK/STAT signalling Downregulation of OVOL1 signalling Upregulation of periostin-IL-24 axis | Downregulation | Anti-IL4Rα mAb |
| IL-13 | Upregulation of JAK/STAT signalling Downregulation of OVOL1 signalling Upregulation of periostin-IL24 axis | Downregulation | Anti-IL-13 mAb |
| Janus kinase (JAK) | Upregulation of STAT phosphorylation | Downregulation | JAK inhibitors |
| IL-22 | Upregulation of JAK/STAT3 signalling Upregulation of IL-24 | Downregulation | Anti-IL-22 mAb |
| IL-17A | Upregulation of C/CAAT-enhancer-binding proteins, particularly C/EBPB | Downregulation | Anti-IL-17A mAb |
| IL-24 | Upregulation of JAK/STAT3 signalling | Downregulation | JAK inhibitors |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębińska, A. New Treatments for Atopic Dermatitis Targeting Skin Barrier Repair via the Regulation of FLG Expression. J. Clin. Med. 2021, 10, 2506. https://doi.org/10.3390/jcm10112506
Dębińska A. New Treatments for Atopic Dermatitis Targeting Skin Barrier Repair via the Regulation of FLG Expression. Journal of Clinical Medicine. 2021; 10(11):2506. https://doi.org/10.3390/jcm10112506
Chicago/Turabian StyleDębińska, Anna. 2021. "New Treatments for Atopic Dermatitis Targeting Skin Barrier Repair via the Regulation of FLG Expression" Journal of Clinical Medicine 10, no. 11: 2506. https://doi.org/10.3390/jcm10112506
APA StyleDębińska, A. (2021). New Treatments for Atopic Dermatitis Targeting Skin Barrier Repair via the Regulation of FLG Expression. Journal of Clinical Medicine, 10(11), 2506. https://doi.org/10.3390/jcm10112506






