Visceral Artery Aneurysms Embolization and Other Interventional Options: State of the Art and New Perspectives
Abstract
:1. Introduction
2. Imaging: Diagnosis and Post Treatment Monitoring
3. Embolization: Techniques and Materials
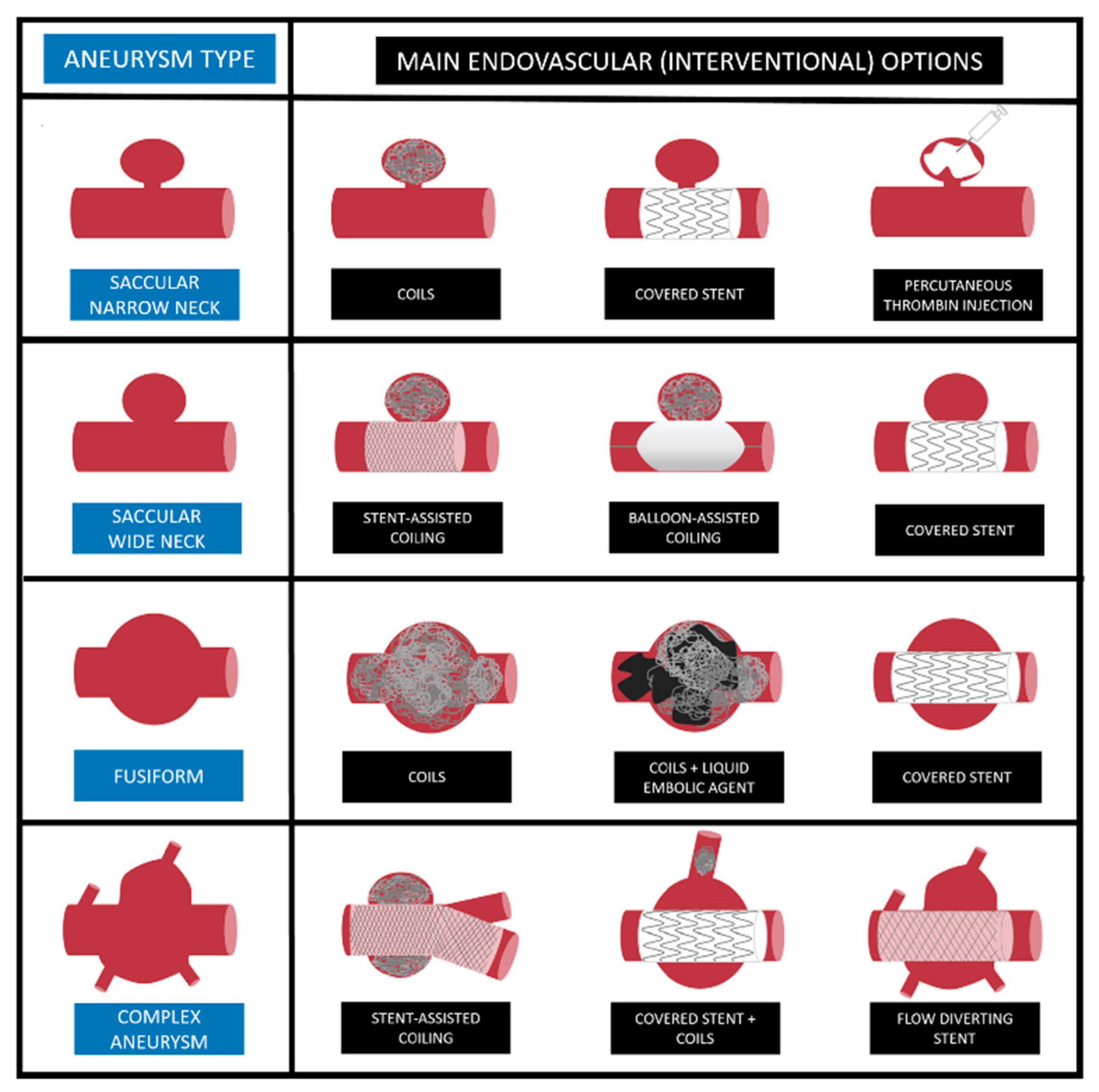
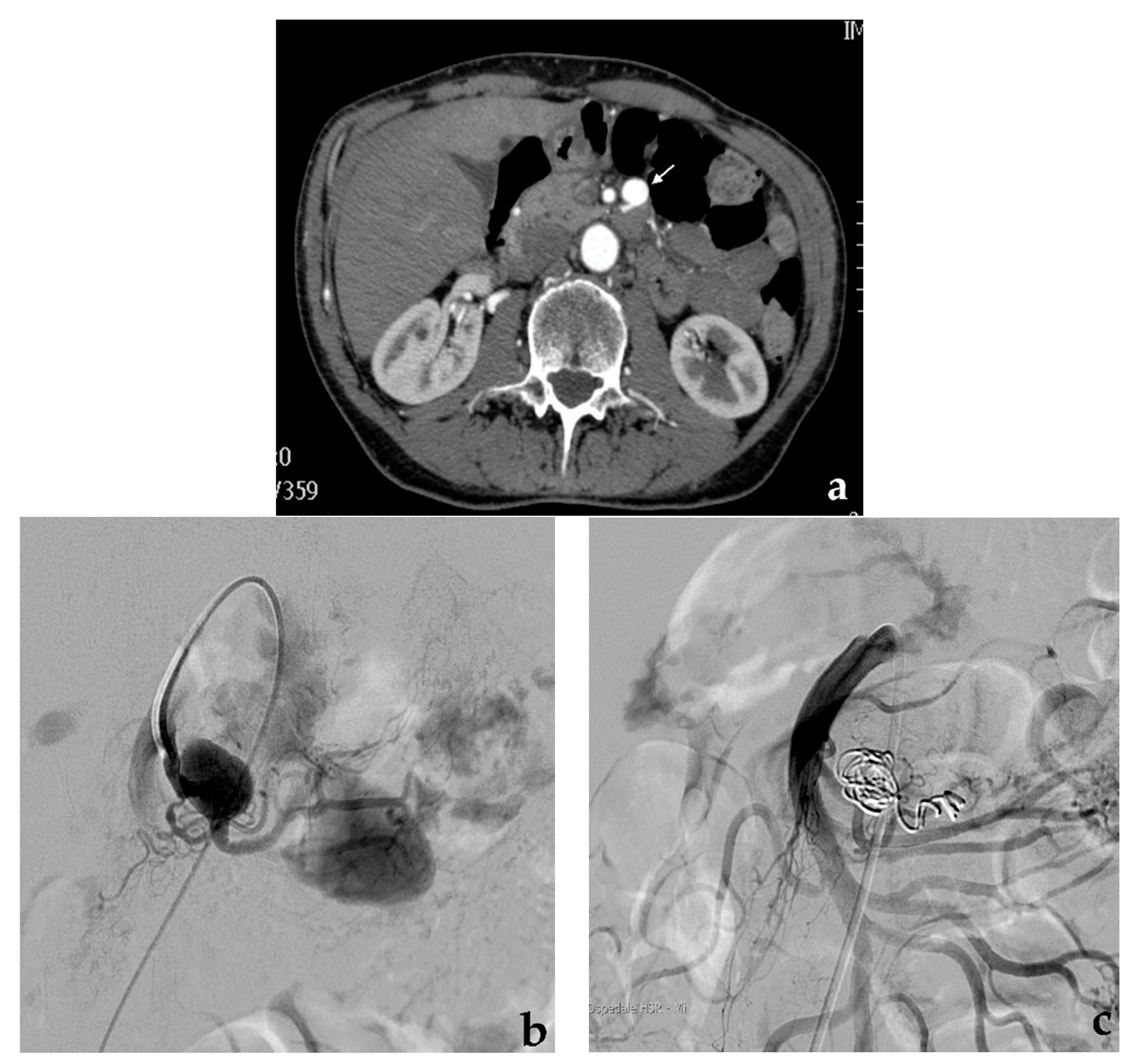
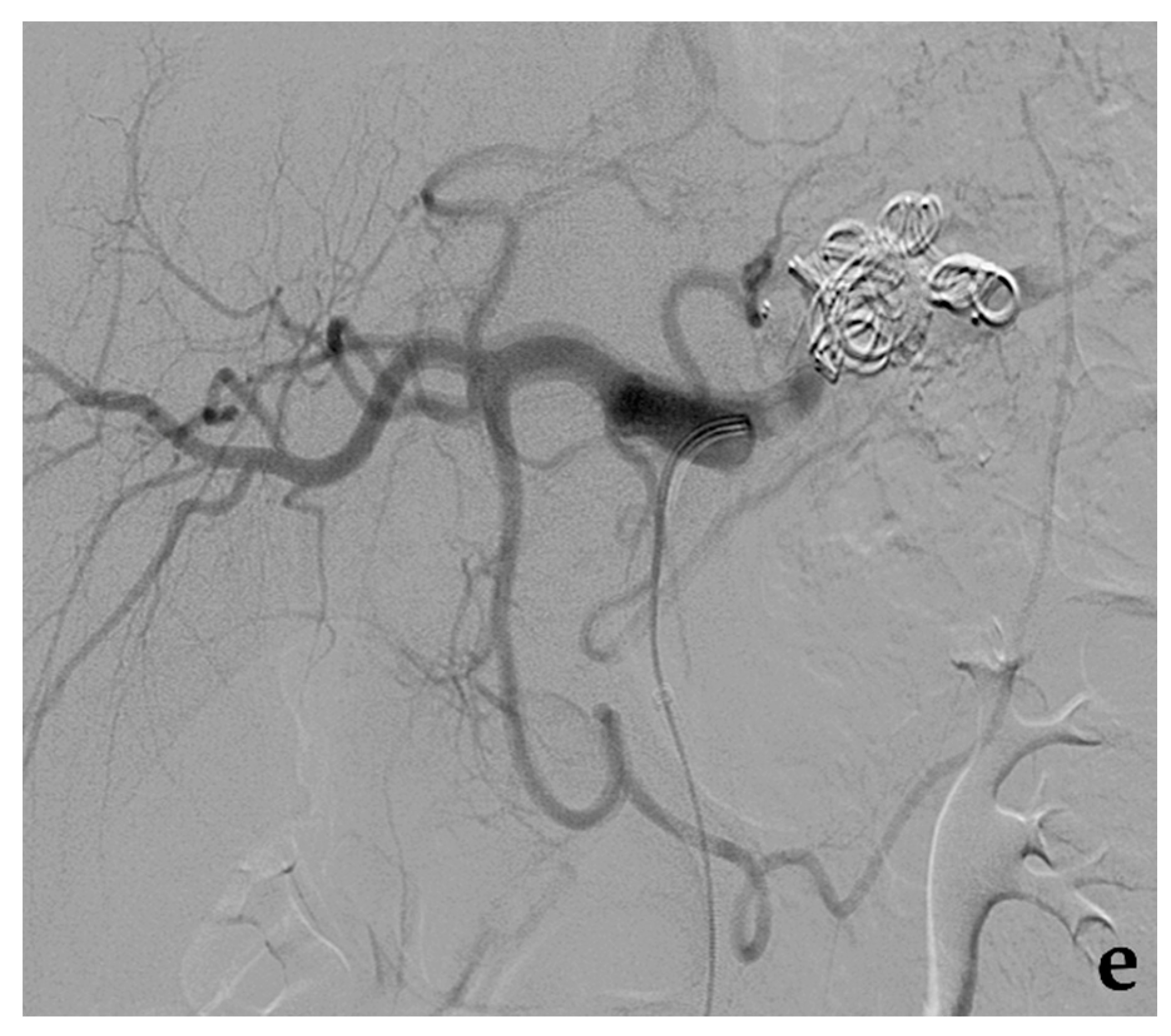
3.1. Coil Embolization
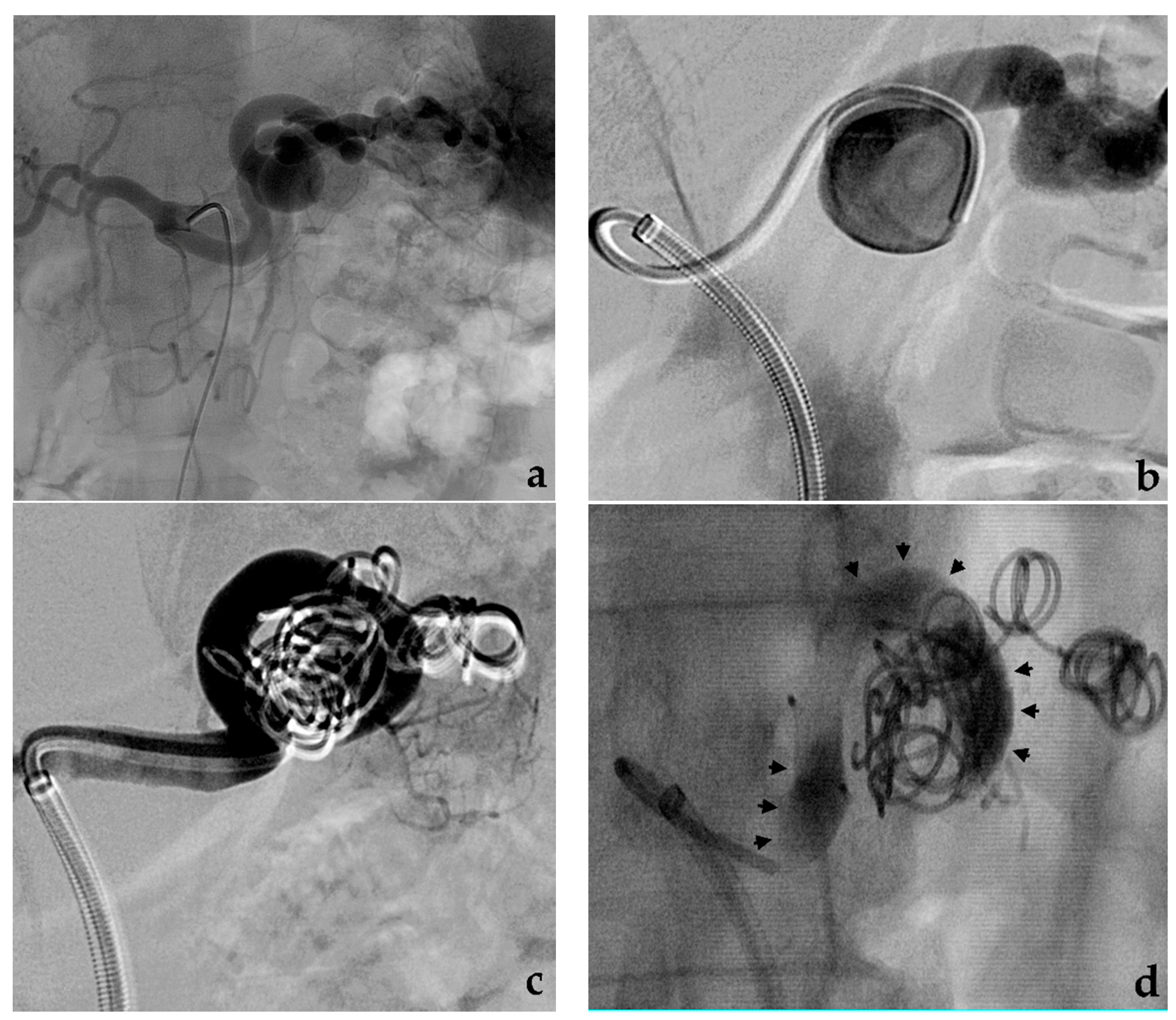
3.2. Stent-Assisted Coil Embolization
3.3. Balloon-Assisted Coil Embolization
3.4. Embolization with Adhesive Liquid Embolic Agents (Glue)
3.5. Embolization with Non-Adhesive Liquid Embolic Agents (Onyx, Squid, Phil)
3.6. Embolization with Plugs, Microplugs
3.7. Embolization with Particles
4. Covered Stent
5. Flow-Diverting Stent
6. Percutaneous (or Endoscopic) Approach
7. Visceral Artery-Related Considerations and Targeted Approaches
7.1. Celiac Trunk
7.2. Splenic Artery
7.3. Hepatic Artery
7.4. Superior Mesenteric Artery
7.5. Gastroduodenal and Pancreaticoduodenal Arteries
7.6. Gastric Artery
7.7. Inferior Mesenteric Artery
7.8. Renal Artery
7.9. Transplanted Arteries
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiesa, R.; Astore, D.; Guzzo, G.; Frigerio, S.; Tshomba, Y.; Castellano, R.; Liberato de Moura, M.R.; Melissano, G. Visceral artery aneurysms. Ann. Vasc. Surg. 2005, 19, 42–48. [Google Scholar] [CrossRef]
- Pitton, M.B.; Dappa, E.; Jungmann, F.; Kloeckner, R.; Schotten, S.; Wirth, G.M.; Mittler, J.; Lang, H.; Mildenberger, P.; Kreitner, K.F.; et al. Visceral artery aneurysms: Incidence, management, and outcome analysis in a tertiary care center over one decade. Eur. Radiol. 2015, 25, 2004–2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakaue, T.; Suzuki, J.; Hamaguchi, M.; Suehiro, C.; Tanino, A.; Nagao, T.; Uetani, T.; Aono, J.; Nakaoka, H.; Kurata, M.; et al. Perivascular adipose tissue angiotensin II type 1 receptor promotes vascular inflammation and aneurysm formation. Hypertension 2017, 70, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.X.; Davila, V.J.; Stone, W.M.; Shamoun, F.E.; Naidu, S.G.; McBane, R.D.; Money, S.R. Natural history and management outcomes of segmental arterial mediolysis. J. Vasc. Surg. 2019, 70, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Parent, B.A.; Cho, S.W.; Buck, D.G.; Nalesnik, M.A.; Gamblin, T.C. Spontaneous rupture of hepatic artery aneurysm associated with polyarteritis nodosa. Am. Surg. 2010, 76, 1416–1419. [Google Scholar] [CrossRef]
- Angeli, E.; Vanzulli, A.; Venturini, M.; Biondi Zoccai, G.; Del Maschio, A. The role of radiology in diagnosis and management of Takayasu’s arteritis. J. Nephrol. 2001, 14, 514–524. [Google Scholar]
- Kordzadeh, A.; Watson, J.; Panayiotopolous, Y.P. Mycotic aneurysm of superior and inferior mesenteric artery. J. Vasc. Surg. 2016, 63, 1638–1646. [Google Scholar] [CrossRef] [Green Version]
- Bolen, M.A.; Brinza, E.; Renapurkar, R.D.; Kim, E.S.H.; Gornik, H.L. Screening CT angiography of the aorta, visceral branch vessels, and pelvic arteries in fibromuscular dysplasia. JACC. Cardiovasc. Imaging 2017, 10, 554–561. [Google Scholar] [CrossRef]
- Venturini, M.; Marra, P.; Colarieti, A.; Agostini, G.; Lanza, C.; Augello, L.; Gusmini, S.; Salvioni, M.; Melissano, G.; Fiorina, P.; et al. Covered stenting and transcatheter embolization of splenic artery aneurysms in diabetic patients: A review of endovascular treatment of visceral artery aneurysms in the current era. Pharm. Res. 2018, 135, 127–135. [Google Scholar] [CrossRef]
- Parfitt, J.; Chalmers, R.T.; Wolfe, J.H. Visceral aneurysms in Ehlers-Danlos syndrome: Case report and review of the literature. J. Vasc. Surg. 2000, 31, 1248–1251. [Google Scholar] [CrossRef]
- Nosher, J.L.; Chung, J.; Brevetti, L.S.; Graham, A.M.; Siegel, R.L. Visceral and renal artery aneurysms: A pictorial essay on endovascular therapy. Radiographics 2006, 26, 1687–1704. [Google Scholar] [CrossRef]
- Wagner, W.H.; Allins, A.D.; Treiman, R.L.; Cohen, J.L.; Foran, R.F.; Levin, P.M.; Cossman, D.V. Ruptured visceral artery aneurysms. Ann. Vasc. Surg. 1997, 11, 342–347. [Google Scholar] [CrossRef]
- Ha, J.F.; Phillips, M.; Faulkner, K. Splenic artery aneurysm rupture in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 146, 133–137. [Google Scholar] [CrossRef]
- Gabrielli, D.; Taglialatela, F.; Mantini, C.; Giammarino, A.; Modestino, F.; Cotroneo, A.R. Endovascular treatment of visceral artery pseudoaneurysms in patients with chronic pancreatitis: Our single-center experience. Ann. Vasc. Surg. 2017, 45, 112–116. [Google Scholar] [CrossRef]
- Shreve, L.; Jarmakani, M.; Javan, H.; Babin, I.; Nelson, K.; Katrivesis, J.; Lekawa, M.; Kuncir, E.; Fernando, D.; Abi-Jaoudeh, N. Endovascular management of traumatic pseudoaneurysms. CVIR Endovasc. 2020, 3, 88. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Arteaga, A.; Orue-Echebarria, M.I.; Zarain, L.; Lusilla, A.; Martinez, C.M.; Moreno, A.; Cuadrado, M.; Rey, C.; Perez Diaz, M.D.; Leyte, M.G.; et al. Acute bleeding from pseudoaneurysms following liver and pancreatobiliary surgery. Eur. J. Trauma Emerg. Surg. 2017, 43, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, S.; Thome, A.; Reslinger, V.; Atanasiu, C.; Pellerin, O.; Sapoval, M.; Bonnet, S. Compressive hematoma due to pseudoaneurysm of the right hepatic artery: A rare case of obstructive jaundice after single-port cholecystectomy. Surg. Laparosc. Endosc. Percutan. Tech. 2015, 25, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Aldrighetti, L.; Arru, M.; Angeli, E.; Venturini, M.; Salvioni, M.; Ronzoni, M.; Caterini, R.; Ferla, G. Percutaneous vs. surgical placement of hepatic artery indwelling catheters for regional chemotherapy. Hepatogastroenterology 2002, 49, 513–517. [Google Scholar]
- Hemp, J.H.; Sabri, S.S. Endovascular management of visceral arterial aneurysms. Tech. Vasc. Interv. Radiol. 2015, 18, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Regus, S.; Lang, W. Management of true visceral artery aneurysms in 31 cases. J. Visc. Surg. 2016, 153, 347–352. [Google Scholar] [CrossRef]
- Kok, H.K.; Asadi, H.; Sheehan, M.; Given, M.F.; Lee, M.J. Systematic review and single center experience for endovascular management of visceral and renal artery aneurysms. J. Vasc. Interv. Radiol. 2016, 27, 1630–1641. [Google Scholar] [CrossRef]
- Pulli, R.; Dorigo, W.; Troisi, N.; Pratesi, G.; Innocenti, A.A.; Pratesi, C. Surgical treatment of visceral artery aneurysms: A 25-year experience. J. Vasc. Surg. 2008, 48, 334–342. [Google Scholar] [CrossRef] [Green Version]
- Van Petersen, A.; Meerwaldt, R.; Geelkerken, R.; Zeebregts, C. Surgical options for the management of visceral artery aneurysms. J. Cardiovasc. Surg. 2011, 52, 333–343. [Google Scholar]
- Brizzi, V.; Déglise, S.; Dubuisson, V.; Midy, D.; Ducasse, E.; Berard, X. Laparoscopic Resection of a Middle Colic Artery Aneurysm. Ann. Vasc. Surg. 2018, 48, 253.e1–253.e3. [Google Scholar] [CrossRef] [PubMed]
- Marone, E.M.; Peri, A.; Argenti, F.; Pugliese, L.; Rinaldi, L.F.; Pietrabissa, A. Robotic treatment of complex splenic artery aneurysms with deep hilar location: Technical insights and midterm results. Ann. Vasc. Surg. 2020, 68, 50–56. [Google Scholar] [CrossRef]
- Marone, E.M.; Mascia, D.; Kahlberg, A.; Brioschi, C.; Tshomba, Y.; Chiesa, R. Is open repair still the gold standard in visceral artery aneurysm management? Ann. Vasc. Surg. 2011, 25, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Piffaretti, G.; Lomazzi, C.; Carrafiello, G.; Tozzi, M.; Mariscalco, G.; Castelli, P. Visceral artery aneurysms: Management of 48 cases. J. Cardiovasc. Surg. 2011, 52, 557–565. [Google Scholar]
- Chaer, R.A.; Abularrage, C.J.; Coleman, D.M.; Eslami, M.H.; Kashyap, V.S.; Rockman, C.; Murad, M.H. The Society for Vascular Surgery clinical practice guidelines on the management of visceral aneurysms. J. Vasc. Surg. 2020, 72, 3S–39S. [Google Scholar] [CrossRef]
- Barrionuevo, P.; Malas, M.B.; Nejim, B.; Haddad, A.; Morrow, A.; Ponce, O.; Hasan, B.; Seisa, M.; Chaer, R.; Murad, M.H. A systematic review and meta-analysis of the management of visceral artery aneurysms. J. Vasc. Surg. 2020, 72, 40S–45S. [Google Scholar] [CrossRef]
- Kawakubo, K.; Kawakami, H.; Kuwatani, M.; Sakamoto, N. Education and imaging. Hepatobiliary and pancreatic: A splenic artery aneurysm presenting as a calcified pancreatic mass. J. Gastroenterol. Hepatol. 2015, 30, 655. [Google Scholar] [CrossRef]
- Paik, W.H.; Choi, J.H.; Seo, D.W.; Cho, Y.P.; Park, D.H.; Lee, S.S. Clinical usefulness with the combination of color Doppler and contrast-enhanced harmonic EUS for the assessment of visceral vascular diseases. J. Clin. Gastroenterol. 2014, 48, 845–850. [Google Scholar] [CrossRef]
- Saba, L.; Anzidei, M.; Lucatelli, P.; Mallarini, G. The multidetector computed tomography angiography (MDCTA) in the diagnosis of splenic artery aneurysm and pseudoaneurysm. Acta Radiol. 2011, 52, 488–498. [Google Scholar] [CrossRef]
- Jesinger, R.A.; Thoreson, A.A.; Lamba, R. Abdominal and pelvic aneurysms and pseudoaneurysms: Imaging review with clinical, radiologic, and treatment correlation. Radiographics 2013, 33, 71–96. [Google Scholar] [CrossRef]
- Pilleul, F.; Beuf, O. Diagnosis of splanchnic artery aneurysms and pseudoaneurysms, with special reference to contrast enhanced 3D magnetic resonance angiography: A review. Acta Radiol. 2004, 45, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Vosshenrich, R.; Fischer, U. Contrast-enhanced MR angiography of abdominal vessels: Is there still a role for angiography? Eur. Radiol. 2002, 12, 218–230. [Google Scholar] [CrossRef]
- Kawai, T.; Shimohira, M.; Suzuki, K.; Ohta, K.; Kurosaka, K.; Hashizume, T.; Nishikawa, H.; Muto, M.; Arai, N.; Kan, H.; et al. Time-resolved magnetic resonance angiography as a follow-up method for visceral artery aneurysms treated with coil-embolisation. Pol. J. Radiol. 2018, 83, e137–e142. [Google Scholar] [CrossRef]
- Koganemaru, M.; Abe, T.; Nonoshita, M.; Iwamoto, R.; Kusumoto, M.; Kuhara, A.; Kugiyama, T. Follow-up of true visceral artery aneurysm after coil embolization by three-dimensional contrast-enhanced MR angiography. Diagn. Interv. Radiol. 2014, 20, 129–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfister, K.; Kasprzak, P.M.; Jung, E.M.; Müller-Wille, R.; Wohlgemuth, W.; Kopp, R.; Schierling, W. Contrast-enhanced ultrasound to evaluate organ microvascularization after operative versus endovascular treatment of visceral artery aneurysms. Clin. Hemorheol. Microcirc. 2016, 64, 689–698. [Google Scholar] [CrossRef]
- Sousa, J.; Costa, D.; Manshila, A. Visceral artery aneurysms: Review on indications and current treatment strategies. Int. Angiol. 2019, 38, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Dudeck, O.; Bulla, K.; Wieners, G.; Ruehl, R.; Ulrich, G.; Holger, A.; Ricke, J.; Pech, M. Embolization of the gastroduodenal artery before selective internal radiotherapy: A prospectively randomized trial comparing standard pushable coils with fibered interlock detachable coils. Cardiovasc. Interv. Radiol. 2011, 34, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Ekeh, A.P.; Khalaf, S.; Ilyas, S.; Kauffman, S.; Walusimbi, M.; McCarthy, M.C. Complications arising from splenic artery embolization: A review of an 11-year experience. Am. J. Surg. 2013, 205, 250–254. [Google Scholar] [CrossRef]
- Jana, M.; Gamanagatti, S.; Mukund, A.; Paul, S.; Gupta, P.; Garg, P.; Chattopadhyay, T.K.; Sahni, P. Endovascular management in abdominal visceral arterial aneurysms: A pictorial essay. World. J. Radiol. 2011, 28, 182–187. [Google Scholar] [CrossRef]
- Kato, K.; Kawashima, K.; Suzuki, T.; Hamano, M.; Yoshida, S.; Yoshioka, K. Embolization of medium-sized vessels with the penumbra occlusion device: Evaluation of anchoring function. CVIR Endovasc. 2020, 3, 24. [Google Scholar] [CrossRef]
- Koganemaru, M.; Abe, T.; Nonoshita, M.; Iwamoto, R.; Kusumoto, M.; Kuhara, A.; Kugiyama, T. Management of visceral artery embolization using 0.010-inch detachable microcoils. Diagn. Interv. Radiol. 2014, 20, 345–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, F.; Dunn, J.; Rundback, J.; Pellerito, J.; Galmer, A. Visceral artery aneurysms: Diagnosis, surveillance, and treatment. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 97. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, O.; Nakasone, Y.; Tamura, Y.; Yamashita, Y. Endovascular management of visceral artery pseudoaneurysms: Transcatheter embolization using the isolation technique. Cardiovasc. Interv. Radiol. 2010, 33, 1128–1134. [Google Scholar] [CrossRef]
- Yasumoto, T.; Osuga, K.; Yamamoto, H.; Ono, Y.; Masada, M.; Mikami, K.; Kanamori, D.; Nakamura, M.; Tanaka, K.; Nakazawa, T.; et al. Long-term outcomes of coil packing for visceral aneurysms: Correlation between packing density and incidence of coil compaction or recanalization. J. Vasc. Interv. Radiol. 2013, 24, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Venturini, M.; Marra, P.; Colombo, M.; Alparone, M.; Agostini, G.; Bertoglio, L.; Sallemi, C.; Salvioni, M.; Gusmini, S.; Balzano, G.; et al. Endovascular treatment of visceral artery aneurysms and pseudoaneurysms in 100 Patients: Covered stenting vs transcatheter embolization. J. Endovasc. Ther. 2017, 24, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Etezadi, V.; Gandhi, R.T.; Benenati, J.F.; Rochon, P.; Gordon, M.; Benenati, M.; Alehashemi, S.; Katzen, B.T.; Geisbüsch, P. Endovascular treatment of visceral and renal artery aneurysms. J. Vasc. Interv. Radiol. 2011, 22, 1246–1253. [Google Scholar] [CrossRef]
- Wei, X.; Sun, Y.; Wu, Y.; Li, Z.; Zhu, J.; Zhao, Z.; Feng, R.; Jing, Z. Management of wide-based renal artery aneurysms using non covered stent-assisted coil embolization. J. Vasc. Surg. 2017, 66, 850–857. [Google Scholar] [CrossRef] [Green Version]
- Secco, G.; Chevallier, O.; Falvo, N.; Guillen, K.; Comby, P.O.; Mousson, C.; Majbri, N.; Midulla, M.; Loffroy, R. Packing technique with or without remodeling for endovascular coil embolization of renal artery aneurysms: Safety, efficacy and mid-term outcomes. J. Clin. Med. 2021, 10, 326. [Google Scholar] [CrossRef]
- Ma, T.; He, Y.; Zhong, W.; Luo, G.; Li, Q.; Wang, Z.; Zhang, H.; Wu, Z.; Qiu, C. Mid-term results of coil embolization alone or stent-assisted coil embolization for renal artery aneurysms. Ann. Vasc. Surg. 2021. [Google Scholar] [CrossRef]
- Mihalea, C.; Caroff, J.; Rouchaud, A.; Pescariu, S.; Moret, J.; Spelle, L. Treatment of wide-neck bifurcation aneurysm using “WEB device waffle cone technique”. World. Neurosurg. 2018, 113, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Bracale, U.M.; Narese, D.; Ficarelli, I.; De Laurentis, M.; Spalla, F.; Dinoto, E.; Vitale, G.; Solari, D.; Bajardi, G.; Pecoraro, F. Stent-assisted detachable coil embolization of wide-necked renal artery aneurysms. Diagn. Interv. Radiol. 2017, 23, 77–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modestino, F.; Cappelli, A.; Mosconi, C.; Peta, G.; Bruno, A.; Vara, G.; De Benedictis, C.; Golfieri, R. Balloon-assisted coil embolization of a wide-necked aneurysm of the inferior pancraticoduodenal artery. CVIR Endovasc. 2020, 3, 62. [Google Scholar] [CrossRef] [PubMed]
- Murray, T.E.; Brennan, P.; Maingard, J.T.; Chandra, R.V.; Little, D.M.; Brooks, D.M.; Kok, H.K.; Asadi, H.; Lee, M.J. Treatment of visceral artery aneurysms using novel neurointerventional devices and techniques. J. Vasc. Interv. Radiol. 2019, 30, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Onal, Y.; Samanci, C.; Cicek, E.D. Double-lumen balloons, are they only useful in neurointerventions? Preliminary outcomes of double-lumen balloon-assisted embolization of visceral artery aneurysms. Vasc. Endovasc. Surg. 2020, 54, 214–219. [Google Scholar] [CrossRef]
- Gorsi, U.; Chaluvashetty, S.; Kaira, N.; Kang, M.; Bathia, V.; Lai, A.; Singhal, M.; Vyas, S.; Ahuja, C.K.; Kumar, A.; et al. Percutaneous glue embolization as a primary treatment for visceral pseudoaneurysms. Minim. Invasive Ther. Allied Technol. 2020, 29, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Madhusudhan, K.S.; Gamanagatti, S.; Garg, P.; Pal, S.; Sahni, P.; Gupta, A.K. Endovascular embolization of visceral artery pseudoaneurysms using modified injection technique with N-Butyl Cyanoacrylate glue. J. Vasc. Interv. Radiol. 2015, 26, 1718–1725. [Google Scholar] [CrossRef]
- Bailey, M.A.; McPherson, S.J.; Troxler, M.A.; Peach, A.H.; Patel, J.V.; Scott, D.J. Ischemic skin ulceration complicating glue embolization of type II endoleak after endovascular aneurysm repair. J. Vasc. Interv. Radiol. 2011, 22, 163–167. [Google Scholar] [CrossRef]
- Ashour, R.; Ali Aziz-Sultan, M. Onyx HD-500 for embolization of cerebral aneurysms. Neurol. Res. 2014, 36, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Bratby, M.J.; Lehmann, E.D.; Bottomley, J.; Kessel, D.O.; Nicholson, A.A.; McPherson, S.J.; Morgan, R.A.; Belli, A.M. Endovascular embolization of visceral artery aneurysms with ethylene-vinyl alcohol (Onyx): A case series. Cardiovasc. Interv. Radiol. 2006, 29, 1125–1128. [Google Scholar] [CrossRef]
- Gautier, S.; Chevallier, O.; Mastier, C.; d’Athis, P.; Falvo, N.; Pilleul, F.; Midulla, M.; Rat, P.; Facy, O.; Loffroy, R. Portal vein embolization with ethylene-vinyl alcohol copolymer for contralateral lobe hypertrophy before liver resection: Safety, feasibility and initial experience. Quant. Imaging. Med. Surg. 2021, 11, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Mozes, G.D.; Pather, K.; Oderich, G.S.; Mirza, A.; Colglazier, J.J.; Shuja, F.; Mendes, B.C.; Kalra, M.; Bjarnason, H.; Bower, T.C.; et al. Outcomes of Onyx embolization of type II endoleaks after endovascular repair of abdominal aortic aneurysms. Ann. Vasc. Surg. 2020, 67, 223–231. [Google Scholar] [CrossRef]
- Venturini, M.; Lanza, C.; Marra, P.; Colarieti, A.; Panzeri, M.; Augello, L.; Gusmini, S.; Salvioni, M.; De Cobelli, F.; Del Maschio, A. Transcatheter embolization with Squid, combined with other embolic agents or alone, in different abdominal diseases: A single-center experience in 30 patients. CVIR Endovasc. 2019, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Venturini, M.; Augello, L.; Lanza, C.; Curti, M.; Coppola, A.; Piacentino, F.; De Cobelli, F. Emergency tips recanalisation and gastroesophageal varices embolisation with an ethylene vinyl alcohol copolymer agent (Squid) and detachable coils. Eur. Radiol. Exp. 2020, 4, 67. [Google Scholar] [CrossRef]
- Lucatelli, P.; Corona, M.; Teodoli, L.; Nardis, P.; Cannavale, A.; Rocco, B.; Trobiani, C.; Cipollari, S.; Zilahi de Gyurgyokai, S.; Bezzi, M.; et al. Use of Phil embolic agent for bleeding in non-neurological interventions. J. Clin. Med. 2021, 10, 701. [Google Scholar] [CrossRef]
- Venturini, M.; Marra, P.; Augello, L.; Colarieti, A.; Guazzarotti, G.; Palumbo, D.; Lanza, C.; Melissano, G.; Chiesa, R.; De Cobelli, F. Elective embolization of splenic artery aneurysms with an ethylene vinyl alcohol copolymer agent (Squid) and detachable coils. J. Vasc. Interv. Radiol. 2020, 31, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Virgilio, E.; Laurino, F.; Orgera, G.; Menè, P.; Pirozzi, N.; Ziparo, V.; Cavallini, M. Giant hepatic artery aneurysm associated with immunoglobulin G4-related disease successfully treated using a liquid embolic agent. Korean J. Radiol. 2015, 16, 953–954. [Google Scholar] [CrossRef] [Green Version]
- Simoncini, F.; Mastrorilli, D.; Mezzetto, L.; Angrisano, A.; Scorsone, L.; Bergamaschi, G.; Veraldi, G.F. Management of distal aneurysm of the superior mesenteric artery by percutaneous ultrasound-guided Onyx injection: A case report. Vascular 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Venturini, M.; Della Corte, A.; Lanza, C.; Fontana, F.; Chiesa, R.; De Cobelli, F. Embolization of 2 coexisting intraparenchymal renal artery aneurysms with an ethylene vinyl alcohol copolymer agent (Squid) and coils. Cardiovasc. Interv. Radiol. 2020, 43, 942–944. [Google Scholar] [CrossRef] [PubMed]
- Kolber, M.K.; Shukla, P.A.; Kumar, A.; Silberzweig, J.E. Ethylene Vinyl Alcohol Copolymer (Onyx) embolization for acute hemorrhage: A systematic review of peripheral applications. J. Vasc. Interv. Radiol. 2015, 26, 809–815. [Google Scholar] [CrossRef]
- Lozupone, E.; Bracco, S.; Trombatore, P.; Milonia, L.; D’Argento, F.; Alexandre, A.; Valente, I.; Semeraro, V.; Cioni, S.; Pedicelli, A. Endovascular treatment of cerebral dural arteriovenous fistulas with Squid 12. Interv. Neuroradiol. 2020, 26, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Piacentino, F.; Fontana, F.; Curti, M.; Coppola, A.; Venturini, M. Bronchial artery embolization (BAE) with an ethylene vinyl alcohol copolymer agent (Squid) and polyvinyl alcohol particles (PVA) for treatment of hemoptysis. Diagn. Interv. Radiol. 2021, in press. [Google Scholar]
- Prashar, A.; Butt, S.; Shaida, N. Introducing PHIL (precipitating hydrophobic injectable liquid)—A new embolic agent for the body interventional radiologist. Diagn. Interv. Radiol. 2020, 26, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Lopera, J.E. The Amplatzer vascular plug: Review of evolution and current applications. Semin. Interv. Radiol. 2015, 32, 356–369. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Li, H.; Tam, M.D.; Zhou, D.; Wang, D.X.; Spain, J. The amplatzer vascular plug: A review of the device and its clinical applications. Cardiovasc. Interv. Radiol. 2012, 35, 725–740. [Google Scholar] [CrossRef]
- Balderi, A.; Antonietti, A.; Ferro, L.; Peano, E.; Pedrazzini, F.; Fonio, P.; Grosso, M. Endovascular treatment of visceral artery aneurysms and pseudoaneurysms: Our experience. Rad. Med. 2012, 117, 815–830. [Google Scholar] [CrossRef]
- Ocal, O.; Streitparth, T.; Seidensticker, M.; Streitparth, F. Embolization of a giant hepatic venous aneurysm by complementary use of coils, vascular plug, and thrombin. J. Vasc. Interv. Radiol. 2020, 31, 1958–1960. [Google Scholar] [CrossRef]
- Bailey, C.R.; Arun, A.; Towsley, M.; Choi, W.K.; Betz, J.F.; MacKenzie, S.; Areda, M.A.; Duvvuri, M.; Mitchell, S.; Weiss, C.R. MVP™ micro vascular plug systems for the treatment of pulmonary arteriovenous malformations. Cardiovasc. Interv. Radiol. 2019, 42, 389–395. [Google Scholar] [CrossRef]
- Sildiroglu, O.; Arslan, B.; Turba, U.C. Endovascular management of fusiform renal artery aneurysm in a patient with refractory hypertension using hydrocoils and embospheres. Clin. Imaging. 2012, 36, 409–412. [Google Scholar] [CrossRef]
- He, Y.X.; Li, G.; Liu, Y.; Tang, H.; Chong, Z.Y.; Wu, X.J.; Jin, X.; Zhang, S.Y.; Wang, M. Endovascular treatment of visceral aneurysms and pseudoaneurysms. J. Biol. Regul. Homeost. Agents 2021, 35, 131–140. [Google Scholar] [PubMed]
- Laganà, D.; Carrafiello, G.; Mangini, M.; Dionigi, G.; Caronno, R.; Castelli, P.; Fugazzola, C. Multimodal approach to endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Eur. J. Radiol. 2006, 59, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Rebonato, A.; Greco, L.; Citone, M.; David, V. Endovascular exclusion of visceral artery aneurysms with stent-grafts: Technique and long term follow-up. Cardiovasc. Interv. Radiol. 2008, 31, 36–42. [Google Scholar] [CrossRef]
- Herzog, T.; Suelberg, D.; Belyaev, O.; Uhl, W.; Seemann, M.; Seelig, M.H. Treatment of acute delayed visceral hemorrhage after pancreatic surgery from hepatic arteries with covered stents. J. Gastrointest. Surg. 2011, 15, 496–502. [Google Scholar] [CrossRef]
- Künzle, S.; Glenck, M.; Puippe, G.; Schadde, E.; Mayer, D.; Pfammatter, T. Stent-graft repairs of visceral and renal artery aneurysms are effective and result in long-term patency. J. Vasc. Interv. Radiol. 2013, 24, 989–996. [Google Scholar] [CrossRef]
- Venturini, M.; Marra, P.; Colombo, M.; Panzeri, M.; Gusmini, S.; Sallemi, C.; Salvioni, M.; Lanza, C.; Agostini, G.; Balzano, G.; et al. Endovascular repair of 40 visceral artery aneurysms and pseudoaneurysms with the Viabahn Stent-Graft: Technical aspects, clinical outcome and mid-term patency. Cardiovasc. Interv. Radiol. 2018, 41, 385–397. [Google Scholar] [CrossRef]
- Venturini, M.; Angeli, E.; Salvioni, M.; De Cobelli, F.; Trentin, C.; Carlucci, M.; Staudacher, C.; Del Maschio, A. Hemorrhage from a right hepatic artery pseudoaneurysm: Endovascular treatment with a coronary stent-graft. J. Endovasc. Ther. 2002, 9, 221–224. [Google Scholar] [CrossRef] [PubMed]
- McGraw, J.K.; Patzik, S.B.; Gale, S.S.; Dodd, J.T.; Boorstein, J.M. Autogenous vein-covered stent for the endovascular management of a superior mesenteric artery pseudoaneurysm. J. Vasc. Interv. Radiol. 1998, 9, 779–782. [Google Scholar] [CrossRef]
- Tipaldi, M.A.; Pisano, A.; Krokidis, M.; Laurino, F.; Ginanni Corradini, L.; Lucatelli, P.; Venturini, M.; Laghi, A.; Rossi, M. Extravascular migration of thrombosed covered stents after endovascular exclusion of splenic or hepatic artery aneurysms and pseudoaneurysms: An underestimated phenomenon. J. Vasc. Interv. Radiol. 2021, 32, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Cagnazzo, F.; di Carlo, D.T.; Cappucci, M.; Lefevre, P.-H.; Costalat, V.; Perrini, P. Acutely ruptured intracranial aneurysms treated with flow-diverter stents: A systematic review and meta-analysis. AJNR Am. J. Neuroradiol. 2018, 39, 1669–1675. [Google Scholar] [CrossRef]
- Colombi, D.; Bodini, F.C.; Bossalini, M.; Rossi, B.; Michieletti, E. Extracranial visceral artery aneurysms/pseudoaneurysms repaired with flow diverter device developed for cerebral aneurysms: Preliminary results. Ann. Vasc. Surg. 2018, 53, 272.e1–272.e9. [Google Scholar] [CrossRef] [PubMed]
- Rabuffi, P.; Bruni, A.; Antonuccio, E.G.M.; Ambrogi, C.; Vagnarelli, S. Treatment of visceral artery aneurysms and pseudoaneurysms with the use of cerebral flow diverting stents: Initial experience. CVIR Endovasc. 2020, 3, 48. [Google Scholar] [CrossRef]
- Ruffino, M.A.; Rabbia, C.; Italian Cardiatis Registry Investigators Group. Endovascular repair of peripheral and visceral aneurysms with the Cardiatis multilayer flow modulator: One-year results from the Italian Multicenter Registry. J. Endovasc. Ther. 2012, 19, 599–610. [Google Scholar] [CrossRef]
- Balderi, A.; Antonietti, A.; Pedrazzini, F.; Sortino, D.; Vinay, C.; Grosso, M. Treatment of visceral aneurysm using multilayer stent: Two-year follow-up results in five consecutive patients. Cardiovasc. Interv. Radiol. 2013, 36, 1256–1261. [Google Scholar] [CrossRef]
- Ferrero, E.; Ferri, M.; Carbonatto, P.; Viazzo, A.; Calvo, A.; Nessi, F. Disconnection of multilayer stents 2 years after treatment of a hepatic artery aneurysm. J. Endovasc. Ther. 2013, 20, 393–397. [Google Scholar] [CrossRef]
- Shlomovitz, E.; Jaskolka, J.D.; Tan, K.T. Use of a flow-diverting uncovered stent for the treatment of a superior mesenteric artery aneurysm. J. Vasc. Interv. Radiol. 2011, 22, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Eldem, G.; Erdoğan, E.; Peynircioğlu, B.; Arat, A.; Balkancı, F. Endovascular treatment of true renal artery aneurysms: A single center experience. Diagn. Interv. Radiol. 2019, 25, 62–70. [Google Scholar] [CrossRef]
- Seshadhri, S.; Janiga, G.; Beuing, O.; Skalej, M.; Thévenin, D. Impact of stents and flow diverters on hemodynamics in idealized aneurysm models. J. Biomech. Eng. 2011, 133, 071005. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, P.; Ganslandt, O.; Bäzner, H.; Henkes, H.; Pérez, M.A. The fate of side branches covered by flow diverters-results from 140 patients. World Neurosurg. 2017, 103, 789–798. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiang, D.; Lu, Q.; Wu, M.; Cui, J.A. systematic review and meta-analysis of the performance of flow-diverting stents in the treatment of peripheral and visceral artery aneurysms. Catheter. Cardiovasc. Interv. 2021, 97, 461–469. [Google Scholar] [CrossRef]
- Piffaretti, G.; Tozzi, M.; Carrafiello, G.; Caronno, R.; Laganà, D.; Recaldini, C.; Castelli, P. A case of gastroduodenal artery aneurysm in a HIV-positive patient treated by combined percutaneous thrombin injection and endovascular coil embolization. J. Cardiovasc. Surg. 2008, 49, 659–661. [Google Scholar]
- Anderloni, A.; Auriemma, F.; Samarasena, J. Visceral artery pseudoaneurysm: Is EUS-guided treatment ready for prime time? Endosc. Int. Open 2020, 8, E413–E414. [Google Scholar] [PubMed] [Green Version]
- Hashimoto, Y.; Ohno, I.; Takahashi, H.; Sasaki, M.; Imaoka, H.; Watanabe, K.; Umemoto, K.; Kimura, G.; Mitsunaga, S.; Ikeda, M. EUS-guided n-butyl-2-cyanoacrylate injection therapy for ruptured isolated left gastric artery pseudoaneurysm. Endosc. Ultrasound 2019, 8, 58–59. [Google Scholar] [CrossRef]
- Zhang, W.; Fu, Y.F.; Wei, P.L.; Bei, E.; Li, D.C.; Xu, J. Endovascular repair of celiac artery aneurysm with the use of stent grafts. J. Vasc. Interv. Radiol. 2016, 27, 14–18. [Google Scholar] [CrossRef]
- Coskun, B.; Rozanes, I.; Erkan, M.; Ates, S.; Akpek, S. Giant celiac artery aneurysm treated with a flow-diverting multilayer stent: Early rupture as a fatal complication. J. Vasc. Interv. Radiol. 2017, 28, 468–470. [Google Scholar] [CrossRef]
- Gorsi, U.; Agarwal, V.; Nair, V.; Kang, M.; Kalra, N.; Sreedhara, B.C.; Gupta, R.; Rana, S.S.; Dutta, U.; Sandhu, M.S. Endovascular and percutaneous transabdominal embolisation of pseudoaneurysms in pancreatitis: An experience from a tertiary-care referral centre. Clin. Radiol. 2021, 76, 314.e17–314.e23. [Google Scholar] [CrossRef]
- Yamamoto, S.; Hirota, S.; Maeda, H.; Achiwa, S.; Arai, K.; Kobayashi, K.; Nakao, N. Transcatheter coil embolization of splenic artery aneurysm. Cardiovasc. Interv. Radiol. 2008, 31, 527–534. [Google Scholar] [CrossRef]
- Wojtaszek, M.; Lamparski, K.; Wnuk, E.; Ostrowski, T.; Maciąg, R.; Rix, T.; Maj, E.; Milczarek, K.; Korzeniowski, K.; Rowiński, O. Selective occlusion of splenic artery aneurysms with the coil packing technique: The impact of packing density on aneurysm reperfusion correlated between contrast-enhanced MR angiography and digital subtraction angiography. Radiol. Med. 2019, 124, 450–459. [Google Scholar] [CrossRef]
- Yoon, T.; Kwon, T.; Kwon, H.; Han, Y.; Cho, Y. Transcatheter arterial embolization of splenic artery aneurysms: A single-center experience. Vasc. Spec. Int. 2014, 30, 120–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasaki, R.; Miyamoto, N.; Oki, H.; Okada, T.; Yamaguchi, M.; Gomi, T.; Higashino, T.; Washio, T.; Maruta, T.; Sugimura, K.; et al. Flow-preserved coil embolization using a side-holed indwelling catheter for common hepatic artery pseudoaneurysm: Report of three cases. Surg. Today 2015, 45, 772–776. [Google Scholar] [CrossRef]
- Erben, Y.; De Martino, R.R.; Bjarnason, H.; Duncan, A.A.; Kalra, M.; Oderich, G.S.; Bower, T.C.; Gloviczki, P. Operative management of hepatic artery aneurysms. J. Vasc. Surg. 2015, 62, 610–615. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; Lee, J.; Duncan, J.R.; Picus, D.D.; Darcy, M.D.; Sauk, S. Endovascular treatment of superior mesenteric artery pseudoaneurysms using covered stents in six patients. AJR Am. J. Roentgenol. 2014, 203, 432–438. [Google Scholar] [CrossRef]
- Ozaki, T.; Kimura, M.; Yoshimura, N.; Hori, Y.; Takano, T.; Kamura, T.; Yamamoto, S.; Sasai, K. Endovascular treatment of spontaneous isolated dissecting aneurysm of the superior mesenteric artery using stent-assisted coil embolization. Cardiovasc. Interv. Radiol. 2006, 29, 435–437. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, M.S.; Han, H.Y.; Lee, H.M. Endovascular management of ruptured middle colic artery aneurysm and review of the literature. Ann. Vasc. Surg. 2019, 59, 310.e13–310.e16. [Google Scholar] [CrossRef] [PubMed]
- Gandini, R.; Abrignani, S.; Perrone, O.; Lauretti, D.L.; Merolla, S.; Scaggiante, J.; Vasili, E.; Floris, R.; Cioni, R. Retrograde Endovascular Stenting of Preocclusive Celiac Artery Stenosis with Loop Technique Associated with Pancreaticoduodenal Artery Aneurysm Embolization. J. Vasc. Interv. Radiol. 2017, 28, 1607–1609. [Google Scholar] [CrossRef]
- Sadek, M.; Rockman, C.B.; Berland, T.L.; Maldonado, T.S.; Jacobowitz, G.R.; Adelman, M.A.; Mussa, F.F. Coil embolization of a gastroduodenal artery pseudoaneurysm secondary to cholangitis: Technical aspects and review of the literature. Vasc. Endovasc. Surg. 2012, 46, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Somani, P.; Sunkara, T.; Prajapati, R.; Talele, R. Endoscopic ultrasound-guided coil embolization and thrombin injection of a bleeding gastroduodenal artery pseudoaneurysm. Endoscopy 2019, 51, E36–E37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bains, L.; Kori, R.; Sharma, R.; Kaur, D. Replaced right hepatic artery pseudoaneurysm managed with coil embolisation. BMJ Case Rep. 2019, 12, e227921. [Google Scholar] [CrossRef]
- Nagarajan, K.; Sunilkumar, D.; Ramakrishnaiah, V.P.N.; Amuthabarathi, M. Left gastric pseudoaneurysm in a case of chronic pancreatitis: A case report with review of literature. Vasc. Endovasc. Surg. 2021, 55, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Vargas, H.A.; Cousins, C.; Higgins, J.N.; See, T.C. Left gastric artery aneurysm: Successful embolization with ethylene vinyl alcohol copolymer (Onyx). Cardiovasc. Interv. Radiol. 2008, 31, 418–421. [Google Scholar] [CrossRef]
- Nagarajan, K.; Elango, S.; Ishita, L.; Shanmugam, D. Traumatic pseudoaneurysm of the inferior mesenteric artery branch: A rare cause of lower GI bleeding and treatment with selective coil embolization. Cardiovasc. Interv. Radiol. 2016, 39, 1358–1360. [Google Scholar] [CrossRef]
- Hansraj, N.; Hamdi, A.; Wise, E.S.; Di Chiacchio, L.; Sarkar, R.; Toursavadkohi, S. Open and endovascular management of inferior mesenteric artery aneurysms: A report of two cases. Ann. Vasc. Surg. 2017, 43, 316.e9–316.e14. [Google Scholar] [CrossRef]
- Tang, H.; Tang, X.; Fu, W.; Luo, J.; Shi, Z.; Wang, L.; Liu, F.; Guo, D. Coil embolization of renal artery bifurcation and branch aneurysms with flow preservation. J. Vasc. Surg. 2018, 68, 451–458. [Google Scholar] [CrossRef]
- Abath, C.; Andrade, G.; Cavalcanti, D.; Brito, N.; Marques, R. Complex renal artery aneurysms: Liquids or coils? Tech. Vasc. Interv. Radiol. 2007, 10, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Mounayer, C.; Aymard, A.; Saint-Maurice, J.P.; Chapot, R.; Merland, J.J.; Houdart, E. Balloon-assisted coil embolization for large-necked renal artery aneurysms. Cardiovasc. Interv. Radiol. 2000, 23, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Onal, Y.; Acunas, B.; Samanci, C.; Ugurlucan, M.; Umutlu, M.R.; Oztas, D.M.; Alpagut, U. Preliminary results of stent-assisted coiling of wide-necked visceral artery aneurysms via self-expandable neurointerventional stents. J. Vasc. Interv. Radiol. 2019, 30, 49–53. [Google Scholar] [CrossRef]
- Maingard, J.; Lamanna, A.; Kok, H.K.; Ranatunga, D.; Ravi, R.; Chandra, R.V.; Lee, M.J.; Brooks, D.M.; Asadi, H. Endovascular treatment of visceral artery and renal aneurysms (VRAA) using a constant mesh density flow diverting stent. CVIR Endovasc. 2019, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Y.; Jing, Z.; Feng, R. Endovascular treatment for a true aneurysm of the transplant renal artery using noncovered stent-assisted coil embolization. Ann. Vasc. Surg. 2018, 51, 325.e5–325.e8. [Google Scholar] [CrossRef] [PubMed]
- Bassi, R.; Niewczas, M.A.; Biancone, L.; Bussolino, S.; Merugumala, S.; Tezza, S.; D’Addio, F.; Ben Nasr, M.; Valderrama-Vasquez, A.; Usuelli, V.; et al. Metabolomic profiling in individuals with a failing kidney allograft. PLoS ONE 2017, 12, e0169077. [Google Scholar] [CrossRef]
- Zhang, J.; Khalifeh, A.; Santini-Dominguez, R.; Barth, R.N.; Bruno, D.; Desikan, S.; Gupta, A.; Toursavadkohi, S. Endovascular reconstruction of the hepatic arterial system for the management of mycotic pseudoaneurysm in a liver transplant patient. Ann. Vasc. Surg. 2019, 61, 473.e7–473.e11. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Vicaretti, M.; Lee, T.; Amaratunga, R.; Cocco, N.; Laurence, J.; Rogers, N.; Wong, G.; Webster, A.; Hawthorne, W.; et al. Endovascular management of mycotic pseudoaneurysm after pancreas transplantation: Case report and literature review. Transplant. Proc. 2020, 52, 660–666. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venturini, M.; Piacentino, F.; Coppola, A.; Bettoni, V.; Macchi, E.; De Marchi, G.; Curti, M.; Ossola, C.; Marra, P.; Palmisano, A.; et al. Visceral Artery Aneurysms Embolization and Other Interventional Options: State of the Art and New Perspectives. J. Clin. Med. 2021, 10, 2520. https://doi.org/10.3390/jcm10112520
Venturini M, Piacentino F, Coppola A, Bettoni V, Macchi E, De Marchi G, Curti M, Ossola C, Marra P, Palmisano A, et al. Visceral Artery Aneurysms Embolization and Other Interventional Options: State of the Art and New Perspectives. Journal of Clinical Medicine. 2021; 10(11):2520. https://doi.org/10.3390/jcm10112520
Chicago/Turabian StyleVenturini, Massimo, Filippo Piacentino, Andrea Coppola, Valeria Bettoni, Edoardo Macchi, Giuseppe De Marchi, Marco Curti, Christian Ossola, Paolo Marra, Anna Palmisano, and et al. 2021. "Visceral Artery Aneurysms Embolization and Other Interventional Options: State of the Art and New Perspectives" Journal of Clinical Medicine 10, no. 11: 2520. https://doi.org/10.3390/jcm10112520
APA StyleVenturini, M., Piacentino, F., Coppola, A., Bettoni, V., Macchi, E., De Marchi, G., Curti, M., Ossola, C., Marra, P., Palmisano, A., Cappelli, A., Basile, A., Golfieri, R., Cobelli, F. D., Piffaretti, G., Tozzi, M., Carcano, G., & Fontana, F. (2021). Visceral Artery Aneurysms Embolization and Other Interventional Options: State of the Art and New Perspectives. Journal of Clinical Medicine, 10(11), 2520. https://doi.org/10.3390/jcm10112520







