The Evaluation of Efficacy and Safety of A Radiofrequency Hydro-Injector Device for the Skin around the Eye Area
Abstract
:1. Introduction
2. Materials and Methods
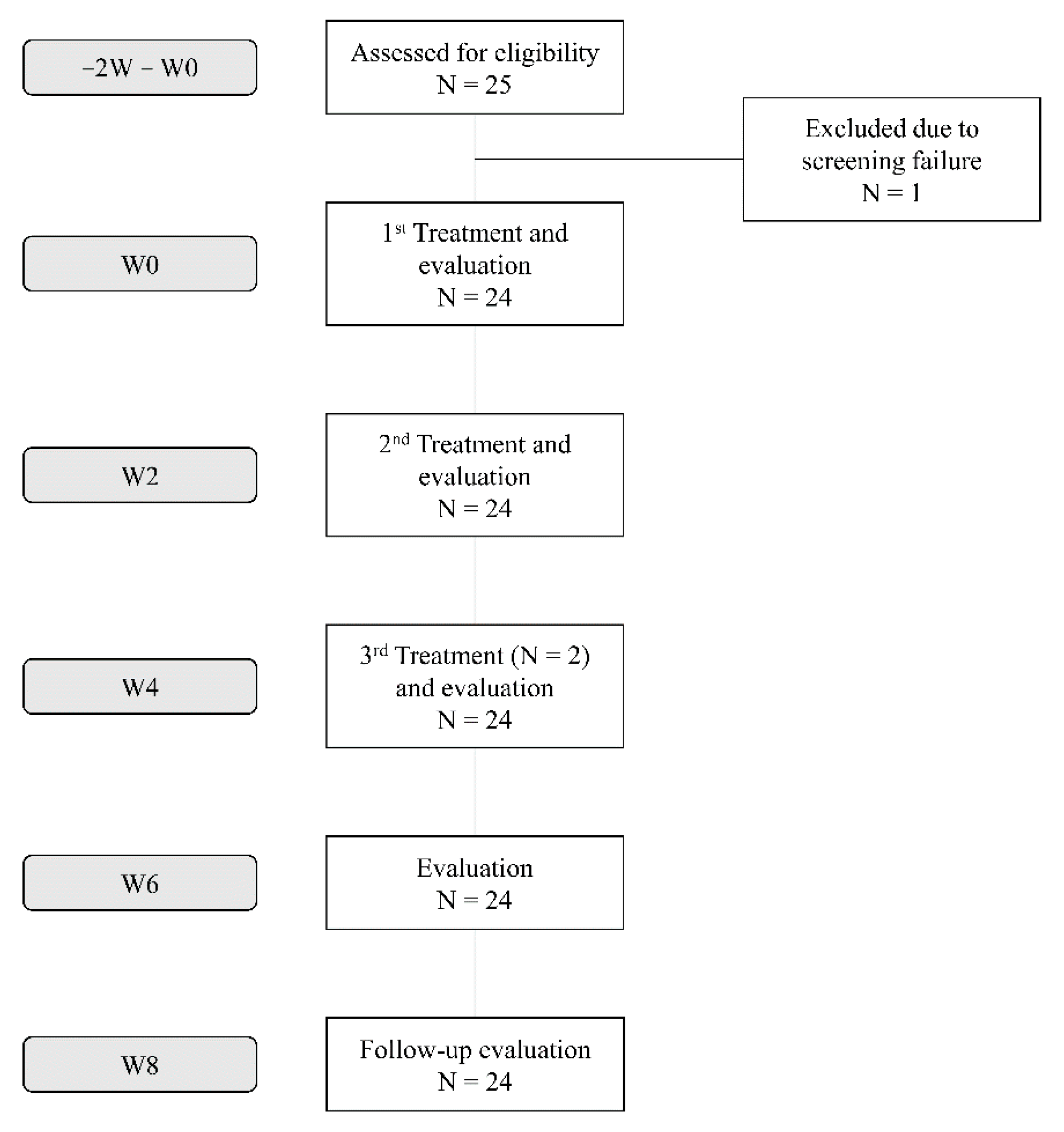
2.1. Study Population
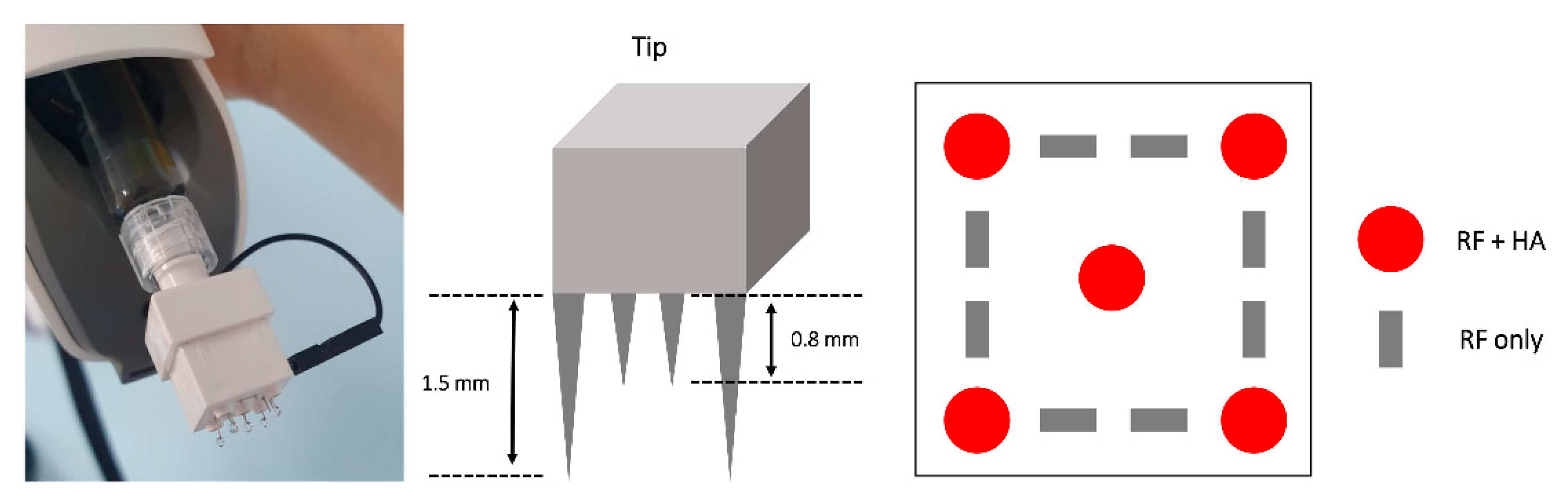
2.2. Materials
2.3. Treatment Procedure
2.4. Efficacy Outcome Measures
2.5. Statistical Analyses
3. Results
3.1. Subjects and Efficacy Outcomes
3.2. Skin Hydration and Elasticity
3.3. Wrinkles, Roughness, and Pore Volume
3.4. Safety Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sumino, H.; Ichikawa, S.; Abe, M.; Endo, Y.; Ishikawa, O.; Kurabayashi, M. Effects of aging, menopause, and hormone replacement therapy on forearm skin elasticity in women. J. Am. Geriatr. Soc. 2004, 52, 945–949. [Google Scholar] [CrossRef]
- Carruthers, J.; Carruthers, A. Shrinking upper and lower eyelid skin with a novel radiofrequency tip. Dermatol. Surg. 2007, 33, 802–809. [Google Scholar] [CrossRef]
- Elsaie, M.L.; Choudhary, S.; Leiva, A.; Nouri, K. Nonablative radiofrequency for skin rejuvenation. Dermatol. Surg. 2010, 36, 577–589. [Google Scholar] [CrossRef]
- Zelickson, B.D.; Kist, D.; Bernstein, E.; Brown, D.B.; Ksenzenko, S.; Burns, J.; Kilmer, S.; Mehregan, D.; Pope, K. Histological and ultrastructural evaluation of the effects of a radiofrequency-based nonablative dermal remodeling device: A pilot study. Arch. Dermatol. 2004, 140, 204–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hantash, B.M.; Renton, B.; Berkowitz, R.L.; Stridde, B.C.; Newman, J. Pilot clinical study of a novel minimally invasive bipolar microneedle radiofrequency device. Lasers Surg. Med. 2009, 41, 87–95. [Google Scholar] [CrossRef]
- Philipp-Dormston, W.G.; Bergfeld, D.; Sommer, B.M.; Sattler, G.; Cotofana, S.; Snozzi, P.; Wollina, U.; Hoffmann, K.P.J.; Salavastru, C.; Fritz, K. Consensus statement on prevention and management of adverse effects following rejuvenation procedures with hyaluronic acid-based fillers. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Gerth, D.J. Structural and volumetric changes in the aging face. Facial Plast. Surg. 2015, 31, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Garza, L.A.; Kang, S.; Varani, J.; Orringer, J.S.; Fisher, G.J.; Voorhees, J.J. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch. Dermatol. 2007, 143, 155–163. [Google Scholar] [CrossRef]
- Seok, J.; Hong, J.Y.; Choi, S.Y.; Park, K.Y.; Kim, B.J. A potential relationship between skin hydration and stamp-type microneedle intradermal hyaluronic acid injection in middle-aged male face. J. Cosmet. Dermatol. 2016, 15, 578–582. [Google Scholar] [CrossRef]
- Kane, M.A.; Blitzer, A.; Brandt, F.S.; Glogau, R.G.; Monheit, G.D.; Narins, R.S.; Paty, J.A.; Waugh, J.M. Development and validation of a new clinically-meaningful rating scale for measuring lateral canthal line severity. Aesthet Surg. J. 2012, 32, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Kim, S.; Lee, H.; Moon, S.; Chang, I. Correlation between a Cutometer and quantitative evaluation using Moire topography in age-related skin elasticity. Ski. Res. Technol. 2007, 13, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Zaman, S.U.; Khan, B.A.; Amir, M.N.; Ebrahimzadeh, M.A. Calendula extract: Effects on mechanical parameters of human skin. Acta Pol. Pharm. 2011, 68, 693–701. [Google Scholar]
- Kerscher, M.; Bayrhammer, J.; Reuther, T. Rejuvenating influence of a stabilized hyaluronic acid-based gel of nonanimal origin on facial skin aging. Dermatol. Surg. 2008, 34, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Sykes, J.M. Hyaluronic acid fillers: History and overview. Facial Plast. Surg. 2011, 27, 523–528. [Google Scholar] [CrossRef] [PubMed]
- el-Domyati, M.; el-Ammawi, T.S.; Medhat, W.; Moawad, O.; Brennan, D.; Mahoney, M.G.; Uitto, J. Radiofrequency facial rejuvenation: Evidence-based effect. J. Am. Acad. Dermatol. 2011, 64, 524–535. [Google Scholar] [CrossRef]
- Kim, J.E.; Chang, S.; Won, C.H.; Kim, C.H.; Park, K.H.; Choi, J.H.; Lee, M.W. Combination treatment using bipolar radiofrequency-based intense pulsed light, infrared light and diode laser enhanced clinical effectiveness and histological dermal remodeling in Asian photoaged skin. Dermatol. Surg. 2012, 38, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Akita, H.; Sasaki, R.; Yokoyama, Y.; Negishi, K.; Matsunaga, K. The clinical experience and efficacy of bipolar radiofrequency with fractional photothermolysis for aged Asian skin. Exp. Dermatol. 2014, 23, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, K.Y.; Choi, S.Y.; Koh, H.J.; Park, S.Y.; Park, W.S.; Bae, I.H.; Kim, B.J. The efficacy, longevity, and safety of combined radiofrequency treatment and hyaluronic Acid filler for skin rejuvenation. Ann. Dermatol. 2014, 26, 447–456. [Google Scholar] [CrossRef]
- England, L.J.; Tan, M.H.; Shumaker, P.R.; Egbert, B.M.; Pittelko, K.; Orentreich, D.; Pope, K. Effects of monopolar radiofrequency treatment over soft-tissue fillers in an animal model. Lasers Surg. Med. 2005, 37, 356–365. [Google Scholar] [CrossRef]
- Goldman, M.P.; Alster, T.S.; Weiss, R. A randomized trial to determine the influence of laser therapy, monopolar radiofrequency treatment, and intense pulsed light therapy administered immediately after hyaluronic acid gel implantation. Dermatol. Surg. 2007, 33, 535–542. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lee, Y.H.; Kim, H.; Koh, H.J.; Park, S.Y.; Park, W.S.; Bae, I.H.; Park, K.Y.; Kim, B.J. A combination trial of intradermal radiofrequency and hyaluronic acid filler for the treatment of nasolabial fold wrinkles: A pilot study. J. Cosmet. Laser Ther. 2014, 16, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, A.; Rotsztejn, H. Nonablative radiofrequency treatment for the skin in the eye area: Clinical and cutometrical analysis. J. Cosmet. Dermatol. 2016, 15, 427–433. [Google Scholar] [CrossRef]
- Hantash, B.M.; Ubeid, A.A.; Chang, H.; Kafi, R.; Renton, B. Bipolar fractional radiofrequency treatment induces neoelastogenesis and neocollagenesis. Lasers Surg. Med. 2009, 41, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tammi, R.; Pasonen-Seppanen, S.; Kolehmainen, E.; Tammi, M. Hyaluronan synthase induction and hyaluronan accumulation in mouse epidermis following skin injury. J. Investig. Dermatol. 2005, 124, 898–905. [Google Scholar] [CrossRef]
- Aust, M.C.; Fernandes, D.; Kolokythas, P.; Kaplan, H.M.; Vogt, P.M. Percutaneous collagen induction therapy: An alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr. Surg. 2008, 121, 1421–1429. [Google Scholar] [CrossRef] [Green Version]
- Jeon, I.K.; Chang, S.E.; Park, G.H.; Roh, M.R. Comparison of microneedle fractional radiofrequency therapy with intradermal botulinum toxin a injection for periorbital rejuvenation. Dermatology 2013, 227, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, H.T.; Lee, Y.J.; Paik, S.H.; Moon, Y.S.; Lee, W.J.; Chang, S.E.; Lee, M.W.; Choi, J.H.; Jung, J.M.; et al. Comparison of the effects of polynucleotide and hyaluronic acid fillers on periocular rejuvenation: A randomized, double-blind, split-face trial. J. Dermatolog. Treat. 2020, 1–7. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, Y.-K.; Jung, C.-J.; Lee, M.-Y.; Moon, I.-J.; Won, C.-H. The Evaluation of Efficacy and Safety of A Radiofrequency Hydro-Injector Device for the Skin around the Eye Area. J. Clin. Med. 2021, 10, 2582. https://doi.org/10.3390/jcm10122582
Lim Y-K, Jung C-J, Lee M-Y, Moon I-J, Won C-H. The Evaluation of Efficacy and Safety of A Radiofrequency Hydro-Injector Device for the Skin around the Eye Area. Journal of Clinical Medicine. 2021; 10(12):2582. https://doi.org/10.3390/jcm10122582
Chicago/Turabian StyleLim, Young-Kyoung, Chang-Jin Jung, Mi-Young Lee, Ik-Jun Moon, and Chong-Hyun Won. 2021. "The Evaluation of Efficacy and Safety of A Radiofrequency Hydro-Injector Device for the Skin around the Eye Area" Journal of Clinical Medicine 10, no. 12: 2582. https://doi.org/10.3390/jcm10122582





