Is Kummell’s Disease a Misdiagnosed and/or an Underreported Complication of Osteoporotic Vertebral Compression Fractures? A Pattern of the Condition and Available Treatment Modalities
Abstract
:1. Introduction
2. Materials and Methods
- (1)
- study design: randomized control trial, prospective/retrospective cohort studies
- (2)
- population: patients suffering from osteoporosis—vertebral fractures diagnosed with an imaging study
- (3)
- intervention: MIS or conservative treatment
- (4)
- language: articles originally published in English.
- (1)
- authors
- (2)
- year of publication
- (3)
- journal
- (4)
- study design
- (5)
- study type
- (6)
- group abundance
- (7)
- the level of OVCFs
- (8)
- technique of treatment
- (1)
- age
- (2)
- gender
- (3)
- bone mineral density (BMD)
- (4)
- numbers of treated injured levels
- (5)
- technique used as a treatment method
- (6)
- properties of each chosen method
- (7)
- benefits of each chosen method
- (8)
- the results and the efficacy
- (9)
- percentage of complications
- (10)
- adjacent vertebral fracture
- (11)
- kyphosis
- (12)
- pain progression
3. Epidemiology
- (1)
- a middle column injury
- (2)
- a diffuse low-intensity T1-weighted MRI pattern
- (3)
- a fluid-intensity and diffuse low-intensity T2-weighted MRI pattern [21].
4. Pathophysiology
5. Risk Factors
6. Clinical Presentation
Consequences of Delayed Vertebral Compression Fractures
7. Diagnostic Methods
8. Treatment
8.1. Conservative Treatment
Complications of Conservative Treatment
8.2. Surgical Treatment
9. Results
10. Discussion
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hansen, E.J.; Simony, A.; Carreon, L.; Rousing, R.; Tropp, H.T.; Andersen, M.Ø. Vertebroplasty vs. SHAM for Treating Osteoporotic Vertebral Compression Fractures: A Double Blind RCT (VOPE). Integr. J. Orthop. Traumatol. 2019, 2, 1–6. [Google Scholar]
- Zhu, Y.; Cheng, J.; Yin, J.; Zhang, Z.; Liu, C.; Hao, D. Therapeutic effect of kyphoplasty and balloon vertebroplasty on osteoporotic vertebral compression fracture. Medicine 2019, 98, e17810. [Google Scholar] [CrossRef]
- Lou, S.; Shi, X.; Zhang, X.; Lyu, H.; Li, Z.; Wang, Y. Percutaneous vertebroplasty versus non-operative treatment for osteoporotic vertebral compression fractures: A meta-analysis of randomized controlled trials. Osteoporos. Int. 2019, 30, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lin, J.; Li, J.; Yang, Y.; Fei, Q. “Targeted Percutaneous Vertebroplasty” Versus Traditional Percutaneous Vertebroplasty for Osteoporotic Vertebral Compression Fracture. Surg. Innov. 2019, 26, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, N.; Barra, F.; Moraes, L.; Rotta, R.; Casulari, L.A. Percutaneous vertebroplasty: A comparison between the procedure using the traditional and the new side-opening cannula for osteoporotic vertebral fracture. Arq. Neuro-Psiquiatr. 2009, 67, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Bian, J.; Zhang, W.; Zhang, W.; Zhao, C.; Wei, H. Unilateral Versus Bilateral Vertebroplasty for Severe Osteoporotic Vertebral Compression Fractures. J. Spinal Disord. Tech. 2014, 27, E301–E304. [Google Scholar] [CrossRef] [PubMed]
- Noriega, D.C.; Rodrίguez-Monsalve, F.; Ramajo, R.; Sánchez-Lite, I.; Toribio, B.; Ardura, F. Long-term safety and clinical performance of kyphoplasty and SpineJack® procedures in the treatment of osteoporotic vertebral compression fractures: A pilot, monocentric, investigator-initiated study. Osteoporos. Int. 2019, 30, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, J.; Feng, X.; Tao, Y.; Yang, J.; Wang, Y.; Zhang, S.; Cai, J.; Huang, J. A comparison of high viscosity bone cement and low viscosity bone cement vertebroplasty for severe osteoporotic vertebral compression fractures. Clin. Neurol. Neurosurg. 2015, 129, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; McLean, A.L.; Steinberg, A.L.; Ewald, C.; Kalff, R.; Waschke, A. Prospective randomized comparison of early versus newer-generation vertebral access devices for kyphoplasty. Arch. Orthop. Trauma Surg. 2019, 139, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Van Meirhaeghe, J.; Bastian, L.; Boonen, S.; Ranstam, J.; Tillman, J.B.; Wardlaw, D. A Randomized Trial of Balloon Kyphoplasty and Nonsurgical Management for Treating Acute Vertebral Compression Fractures: Vertebral body kyphosis correction and surgical parameters. Spine 2013, 38, 971–983. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Chen, C.; Wang, H.; Wu, Z.; Liu, L. A systematic review of unilateral versus bilateral percutaneous vertebroplasty/percutaneous kyphoplasty for osteoporotic vertebral compression fractures. Acta Orthop. Traumatol. Turc. 2017, 51, 290–297. [Google Scholar] [CrossRef]
- Tang, J.; Guo, W.-C.; Hu, J.-F.; Yu, L. Unilateral and Bilateral Percutaneous Kyphoplasty for Thoracolumbar Osteoporotic Compression Fractures. J. Coll. Physicians Surg. Pak. 2019, 29, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.-K.; Zou, J.-F.; He, X.-L.; Huang, C.-D.; He, C.-J. Bone-filling mesh container versus percutaneous kyphoplasty in treating Kümmell’s disease. Arch. Osteoporos. 2019, 14, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, B.A.; Heller, J.G. Kummel Disease: A Not-So-Rare Complication of Osteoporotic Vertebral Compression Fractures. J. Am. Board Fam. Med. 2009, 22, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Fink, H.A.; Milavetz, D.L.; Palermo, L.; Nevitt, M.C.; Cauley, J.A.; Genant, H.K.; Black, D.M.; Ensrud, K.E.; Fracture Intervention Trial Research Group. What Proportion of Incident Radiographic Vertebral Deformities Is Clinically Diagnosed and Vice Versa? J. Bone Miner. Res. 2005, 20, 1216–1222. [Google Scholar] [CrossRef]
- Formica, M.; Zanirato, A.; Cavagnaro, L.; Basso, M.; Divano, S.; Formica, C.; Felli, L. What is the Current Evidence on Vertebral Body Osteonecrosis?: A Systematic Review of the Literature. Asian Spine J. 2018, 12, 586–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanchiku, T.; Imajo, Y.; Suzuki, H.; Yoshida, Y.; Taguchi, T. Usefulness of an Early MRI-based Classification System for Predicting Vertebral Collapse and Pseudoarthrosis After Osteoporotic Vertebral Fractures. J. Spinal Disord. Tech. 2014, 27, E61–E65. [Google Scholar] [CrossRef]
- Tsujio, T.; Nakamura, H.; Terai, H.; Hoshino, M.; Namikawa, T.; Matsumura, A.; Kato, M.; Suzuki, A.; Takayama, K.; Fukushima, W.; et al. Characteristic Radiographic or Magnetic Resonance Images of Fresh Osteoporotic Vertebral Fractures Predicting Potential Risk for Nonunion: A prospective multicenter study. Spine 2011, 36, 1229–1235. [Google Scholar] [CrossRef]
- Takahashi, S.; Hoshino, M.; Takayama, K.; Iseki, K.; Sasaoka, R.; Tsujio, T.; Yasuda, H.; Sasaki, T.; Kanematsu, F.; Kono, H.; et al. Predicting delayed union in osteoporotic vertebral fractures with consecutive magnetic resonance imaging in the acute phase: A multicenter cohort study. Osteoporos. Int. 2016, 27, 3567–3575. [Google Scholar] [CrossRef]
- Hoshino, M.; Tsujio, T.; Terai, H.; Namikawa, T.; Kato, M.; Matsumura, A.; Suzuki, A.; Takayama, K.; Takaoka, K.; Nakamura, H. Impact of Initial Conservative Treatment Interventions on the Outcomes of Patients With Osteoporotic Vertebral Fractures. Spine 2013, 38, E641–E648. [Google Scholar] [CrossRef]
- Inose, H.; Kato, T.; Ichimura, S.; Nakamura, H.; Hoshino, M.; Togawa, D.; Hirano, T.; Tokuhashi, Y.; Ohba, T.; Haro, H.; et al. Risk Factors of Nonunion After Acute Osteoporotic Vertebral Fractures: A Prospective Multicenter Cohort Study. Spine 2020, 45, 895–902. [Google Scholar] [CrossRef]
- Steel, H.H. Kümmell’s disease. Am. J. Surg. 1951, 81, 161–167. [Google Scholar] [CrossRef]
- Stojanovic, J.; Kováč, V. Diagnosis of ischemic vertebral collapse using selective spinal angiography. Rofo 1981, 135, 326–329. [Google Scholar] [CrossRef]
- He, D.; Yu, W.; Chen, Z.; Li, L.; Zhu, K.; Fan, S. Pathogenesis of the intravertebral vacuum of Kümmell’s disease. Exp. Ther. Med. 2016, 12, 879–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, J.; Davis, A. Diagnosis and Management of Vertebral Compression Fractures. Am. Fam. Phys. 2016, 94, 44–50. [Google Scholar]
- Lim, J.; Choi, S.-W.; Youm, J.-Y.; Kwon, H.-J.; Kim, S.-H.; Koh, H.-S. Posttraumatic Delayed Vertebral Collapse: Kummell’s Disease. J. Korean Neurosurg. Soc. 2018, 61, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Liang, C.-Z.; Chen, Q.-X. Kümmell’s Disease, an Uncommon and Complicated Spinal Disorder: A Review. J. Int. Med. Res. 2012, 40, 406–414. [Google Scholar] [CrossRef] [Green Version]
- Swartz, K.; Fee, D. Kümmell’s Disease: A case report and literature review. Spine 2008, 33, E152–E155. [Google Scholar] [CrossRef] [PubMed]
- Matzaroglou, C.; Georgiou, C.S.; Assimakopoulos, K.; Giannakenas, C.; Karageorgos, A.; Saridis, A.; Kafchitsas, K.; Wilke, H.J. Kümmell’s disease: Pathophysiology, diagnosis, treatment and the role of nuclear medicine. Rationale according to our experience. Hell J. Nucl. Med. 2011, 14, 291–299. [Google Scholar] [PubMed]
- Kondo, K.L. Osteoporotic Vertebral Compression Fractures and Vertebral Augmentation. Semin. Interv. Radiol. 2008, 25, 412–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leidig, G.; Minne, H.W.; Sauer, P.; Wüster, C.; Wüster, J.; Lojen, M.; Raue, F.; Ziegler, R. A study of complaints and their relation to vertebral destruction in patients with osteoporosis. Bone Miner. 1990, 8, 217–229. [Google Scholar] [CrossRef]
- O’Neill, T.W.; Felsenberg, D.; Varlow, J.; Cooper, C.; Kanis, J.A.; Silman, A.J. The prevalence of vertebral deformities in European men and women: The european vertebral osteoporosis study. J. Bone Miner. Res. 1996, 11, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Drożdżowska, B. Osteoporotic fractures. Endokrynol. Pol. 2009, 60, 4998–5502. [Google Scholar]
- Klotzbuecher, C.M.; Ross, P.D.; Landsman, P.B.; Abbott, T.A.; Berger, M. Patients with Prior Fractures Have an Increased Risk of Future Fractures: A Summary of the Literature and Statistical Synthesis. J. Bone Miner. Res. 2000, 15, 721–739. [Google Scholar] [CrossRef] [Green Version]
- Franek, E.; Wichrowska, H.; Gozdowski, D.; Puzianowska-Kuźnicka, M. WHO fracture risk calculator (FRAX) in the assessment of obese patients with osteoporosis. Endokrynol. Pol. 2009, 60, 82–87. [Google Scholar]
- Muratore, M.; Ferrera, A.; Masse, A.; Bistolfi, A. Osteoporotic vertebral fractures: Predictive factors for conservative treatment failure. A systematic review. Eur. Spine J. 2018, 27, 2565–2576. [Google Scholar] [CrossRef]
- Hatgis, J.; Palea, O.; Ghormi, Y.; Granville, M.; Berti, A.; Jacobson, R.E.; Ghomri, Y. Radiologic Evaluation of Chronic Vertebral Compression Fractures and Role of Vertebral Augmentation. Cureus 2018, 10, e3208. [Google Scholar] [CrossRef] [Green Version]
- Vaccaro, A.; Kandziora, F.; Fehlings, M.; Shanmughanathan, R. Rationale for Fracture Classification: A0, A1, A2, A3, A4, B1, B2, B3, C. Available online: https://surgeryreference.aofoundation.org/spine/trauma/thoracolumbar/further-reading/rationale-for-fracture-classification-a0-a1-a2-a3-a4-b1-b2-b3-c (accessed on 2 August 2020).
- Robertson, P. Thoracolumbar Injury Classification and Severity Score (TLICS)|Radiology Reference Article. Available online: https://radiopaedia.org/articles/thoracolumbar-injury-classification-and-severity-score-tlics-1 (accessed on 2 August 2020).
- Hadjipavlou, A.G.; Katonis, P.G.; Tzermiadianos, M.N.; Tsoukas, G.M.; Sapkas, G. Principles of management of osteometabolic disorders affecting the aging spine. Eur. Spine J. 2003, 12, S113–S131. [Google Scholar] [CrossRef] [Green Version]
- Li, J.B.; Na, S.B.; Gong, W.Q.; Lyu, Z.S.; Liu, L.; Zhang, S.K. Progress on clinical treatment of Kümmell’s disease. Zhongguo Gu Shang 2020, 33, 81–86. (In Chinese) [Google Scholar] [CrossRef]
- Wiggins, M.C.; Sehizadeh, M.; Pilgram, T.K.; Gilula, L.A. Importance of Intravertebral Fracture Clefts in Vertebroplasty Outcome. Am. J. Roentgenol. 2007, 188, 634–640. [Google Scholar] [CrossRef]
- Jang, J.-S.; Kim, D.-Y.; Lee, S.-H. Efficacy of Percutaneous Vertebroplasty in the Treatment of Intravertebral Pseudarthrosis Associated With Noninfected Avascular Necrosis of the Vertebral Body. Spine 2003, 28, 1588–1592. [Google Scholar] [CrossRef]
- Stallenberg, B.; Madani, A.; Burny, F.; Gevenois, P.A. The Vacuum Phenomenon: A CT sign of nonunited fracture. Am. J. Roentgenol. 2001, 176, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Pilitsis, J.G. Vertebral Compression Fractures, American Association of Neurological Surgeons Vertebral Compression Fractures—Symptoms, Complications, Diagnosis and Treatments. Available online: https://www.aans.org/Patients/Neurosurgical-Conditions-and-Treatments/Vertebral-Compression-Fractures (accessed on 2 August 2020).
- Skovrlj, B.; Gilligan, J.; Cutler, H.S.; Qureshi, S.A. Minimally invasive procedures on the lumbar spine. World J. Clin. Cases 2015, 3, 1–9. [Google Scholar] [CrossRef]
- Pierce, T.P.; Jauregui, J.J.; Elmallah, R.K.; Lavernia, C.J.; Mont, M.A.; Nace, J. A current review of core decompression in the treatment of osteonecrosis of the femoral head. Curr. Rev. Musculoskelet. Med. 2015, 8, 228–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaccaro, A.; Kandziora, F.; Fehlings, M.; Shanmughanathan, R. Nonoperative Treatment/Bracing for A0 Minor, Nonstructural Fractures. Available online: https://surgeryreference.aofoundation.org/spine/trauma/thoracolumbar/a0/nonoperative-treatment-bracing (accessed on 2 August 2020).
- Parry, S.M.; Puthucheary, Z.A. The impact of extended bed rest on the musculoskeletal system in the critical care environment. Extreme Physiol. Med. 2015, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saurabh Dang, M. 2020. [When To Consider A Back Brace. [Online] Spine-Health. Available online: https://www.spine-health.com/treatment/alternative-care/when-consider-back-brace (accessed on 28 December 2020).
- Mazanec, D.J.; Podichetty, V.K.; Mompoint, A.; Potnis, A. Vertebral compression fractures: Manage aggressively to prevent sequelae. Clevel. Clin. J. Med. 2003, 70, 147–156. [Google Scholar] [CrossRef]
- Boszczyk, B.M.; Bierschneider, M.; Schmid, K.; Grillhösl, A.; Robert, B.; Jaksche, H. Microsurgical interlaminary vertebro- and kyphoplasty for severe osteoporotic fractures. J. Neurosurg. Spine 2004, 100, 32–37. [Google Scholar] [CrossRef]
- Cosman, F.; De Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R.; National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.H.; Jay, B. Vertebroplasty. Semin. Interv. Radiol. 2013, 30, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Noriega, D.; Maestretti, G.; Renaud, C.; Francaviglia, N.; Ould-Slimane, M.; Queinnec, S.; Ekkerlein, H.; Hassel, F.; Gumpert, R.; Sabatier, P.; et al. Clinical Performance and Safety of 108 SpineJack Implantations: 1-Year Results of a Prospective Multicentre Single-Arm Registry Study. BioMed Res. Int. 2015, 2015, 173872. [Google Scholar] [CrossRef] [Green Version]
- McGraw, J.K.; Cardella, J.; Barr, J.D.; Mathis, J.M.; Sanchez, O.; Schwartzberg, M.S.; Swan, T.L.; Sacks, D. Society of Interventional Radiology quality improvement guidelines for percutaneous vertebroplasty. J. Vasc. Interv. Radiol. 2003, 14 Pt2, 311–315. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Liang, D.; Jiang, X.; Ye, L.; Yao, Z. Comparison of effectiveness between percutaneous vertebroplasty and percutaneous kyphoplasty for treatment of osteoporotic vertebral compression fracture with intravertebral vacuum cleft. Zhongguo Xiu Fu chong Jian Wai Ke Za Zhi 2016, 30, 1104–1110. (In Chinese) [Google Scholar] [CrossRef]
- Wei, H.; Dong, C.; Zhu, Y.; Ma, H. Analysis of two minimally invasive procedures for osteoporotic vertebral compression fractures with intravertebral cleft: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2020, 15, 401. [Google Scholar] [CrossRef] [PubMed]
- Krüger, A.; Oberkircher, L.; Figiel, J.; Floßdorf, F.; Bolzinger, F.; Noriega, D.C.; Ruchholtz, S. Height restoration of osteoporotic vertebral compression fractures using different intravertebral reduction devices: A cadaveric study. Spine J. 2015, 15, 1092–1098. [Google Scholar] [CrossRef]
- Laredo, J.-D. Expert’s comment concerning Grand Rounds case entitled “Kümmell’s disease: Delayed post-traumatic osteonecrosis of the vertebral body” (by Ma, R., Chow, R., Shen, F.H.). Eur. Spine J. 2010, 19, 1071–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.S.; Kim, H.S.; Ju, C.I.; Kim, S.W. Delayed Bone Cement Displacement Following Balloon Kyphoplasty. J. Korean Neurosurg. Soc. 2008, 43, 212–214. [Google Scholar] [CrossRef]
- Kuhn, K.; Höntzsch, D. Augmentation mit PMMA-Zement [Augmentation with PMMA cement]. Der Unfallchirurg 2015, 118, 737–748. (In German) [Google Scholar] [CrossRef]
- Zhu, J.; Yang, S.; Cai, K.; Wang, S.; Qiu, Z.; Huang, J.; Jiang, G.; Wang, X.; Fang, X. Bioactive poly (methyl methacrylate) bone cement for the treatment of osteoporotic vertebral compression fractures. Theranostics 2020, 10, 6544–6560. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhai, Q.; Hu, M.; Cao, C.; Wang, J.; Yang, H.; Li, B. Bone cements for percutaneous vertebroplasty and balloon kyphoplasty: Current status and future developments. J. Orthop. Transl. 2015, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Costa, F.; Ortolina, A.; Galbusera, F.; Cardia, A.; Sala, G.; Ronchi, F.; Uccelli, C.; Grosso, R.; Fornari, M. Pedicle screw cement augmentation. A mechanical pullout study on different cement augmentation techniques. Med Eng. Phys. 2016, 38, 181–186. [Google Scholar] [CrossRef]
- Sawakami, K.; Yamazaki, A.; Ishikawa, S.; Ito, T.; Watanabe, K.; Endo, N. Polymethylmethacrylate Augmentation of Pedicle Screws Increases the Initial Fixation in Osteoporotic Spine Patients. J. Spinal Disord. Tech. 2012, 25, E28–E35. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, H.S.; Lee, S.K.; Kim, S.W. Bone Cement-Augmented Percutaneous Short Segment Fixation: An Effective Treatment for Kummell’s Disease? J. Korean Neurosurg. Soc. 2015, 58, 54–59. [Google Scholar] [CrossRef]
- Prinz, V.; Bayerl, S.; Renz, N.; Trampuz, A.; Czabanka, M.; Woitzik, J.; Vajkoczy, P.; Finger, T. High frequency of low-virulent microorganisms detected by sonication of pedicle screws: A potential cause for implant failure. J. Neurosurg. Spine 2019, 31, 424–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitner, L.; Malaj, I.; Sadoghi, P.; Amerstorfer, F.; Glehr, M.; Vander, K.; Leithner, A.; Radl, R. Pedicle screw loosening is correlated to chronic subclinical deep implant infection: A retrospective database analysis. Eur. Spine J. 2018, 27, 2529–2535. [Google Scholar] [CrossRef] [PubMed]
- McKoy, B.; Yuehuei, H. An injectable cementing screw for fixation in osteoporotic bone. J. Biomed. Mater. Res. 2000, 53, 216–222. [Google Scholar] [CrossRef]
- Cho, Y. Posterior Vertebrectomy and Circumferential Fusion for the Treatment of Advanced Thoracolumbar Kümmell Disease with Neurologic Deficit. Asian Spine J. 2017, 11, 634–640. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-S.; Ge, C.-Y.; Feng, H.; Zhang, H.-P.; Niu, X.-B.; Shi, S.-Y.; Zhu, Z.-Q.; Hao, D.-J. Bone Cement-Augmented Short-Segment Pedicle Screw Fixation for Kümmell Disease with Spinal Canal Stenosis. Med. Sci. Monit. 2018, 24, 928–935. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.-C.; Guo, H.; Guo, D.-Q.; Luo, P.-J.; Li, Y.-X.; Mo, G.-Y.; Ma, Y.-H.; Peng, J.-C.; Liang, D.; Zhang, S.-C. Effect and potential risks of using multilevel cement-augmented pedicle screw fixation in osteoporotic spine with lumbar degenerative disease. BMC Musculoskelet. Disord. 2020, 21, 1–12. [Google Scholar] [CrossRef]
- Goetzen, M.; Windolf, M.; Schmoelz, W. Augmented screws in angular stable plating of the proximal humerus: What to do when revision is needed? Clin. Biomech. 2014, 29, 1023–1026. [Google Scholar] [CrossRef]
- Blattert, T.R.; Glasmacher, S.; Riesner, H.-J.; Josten, C. Revision characteristics of cement-augmented, cannulated fenestrated pedicle screws in the osteoporotic vertebral body: A biomechanical in vitro investigation. Technical note. J. Neurosurg. Spine 2009, 11, 23–27. [Google Scholar] [CrossRef]
- Bullmann, V.; Schmoelz, W.; Richter, M.; Grathwohl, C.; Schulte, T.L. Revision of Cannulated and Perforated Cement-Augmented Pedicle Screws: A biomechanical study in human cadavers. Spine 2010, 35, E932–E939. [Google Scholar] [CrossRef]
- Choy, W.J.; Walsh, W.R.; Phan, K.; Mobbs, R.J. Technical Note: Pedicle Cement Augmentation with Proximal Screw Toggle and Loosening. Orthop. Surg. 2019, 11, 510–515. [Google Scholar] [CrossRef]
- Sudo, H.; Ito, M.; Kaneda, K.; Abumi, K.; Kotani, Y.; Nagahama, K.; Minami, A.; Iwasaki, N. Anterior decompression and strut graft versus posterior decompression and pedicle screw fixation with vertebroplasty for osteoporotic thoracolumbar vertebral collapse with neurologic deficits. Spine J. 2013, 13, 1726–1732. [Google Scholar] [CrossRef]
- Winder, M.J.; Gilhooly, P.M. Accuracy of minimally invasive percutaneous thoracolumbar pedicle screws using 2D fluoroscopy: A retrospective review through 3D CT analysis. J. Spine Surg. 2017, 3, 193–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, P.G.M.; Cadman, J.; Tomka, J.; Dabirrahmani, D.; Appleyard, R.; Kam, A. Pullout force of minimally invasive surgical and open pedicle screws-a biomechanical cadaveric study. J. Spine Surg. 2020, 6, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Schmidt, F.A.; Kirnaz, S.; Wipplinger, C.; Schwartz, T.H.; Härtl, R. MIS approaches in the cervical spine. J. Spine Surg. 2019, 5, S74–S83. [Google Scholar] [CrossRef]
- McCall, T.; Cole, C.; Dailey, A. Vertebroplasty and kyphoplasty: A comparative review of efficacy and adverse events. Curr. Rev. Musculoskelet. Med. 2008, 1, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornelis, F.H.; Joly, Q.; Nouri-Neuville, M.; Ben-Ammar, M.; Kastler, B.; Kastler, A.; Amoretti, N.; Hauger, O. Innovative Spine Implants for Improved Augmentation and Stability in Neoplastic Vertebral Compression Fracture. Medicina 2019, 55, 426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamze, B.; Odri, G.; Funck-Brentano, T.; Orcel, P.; Laredo, J.-D.; Bousson, V. Percutaneous Vertebral Augmentation Techniques in Osteoporotic and Traumatic Fractures. Semin. Interv. Radiol. 2018, 35, 309–323. [Google Scholar] [CrossRef]
- Taylor, R.S.; Taylor, R.J.; Fritzell, P. Balloon Kyphoplasty and Vertebroplasty for Vertebral Compression Fractures: A comparative systematic review of efficacy and safety. Spine 2006, 31, 2747–2755. [Google Scholar] [CrossRef]
- Hulme, P.A.; Krebs, J.; Ferguson, S.J.; Berlemann, U. Vertebroplasty and Kyphoplasty: A Systematic Review of 69 Clinical Studies. Spine 2006, 31, 1983–2001. [Google Scholar] [CrossRef] [PubMed]
- Papanastassiou, I.D.; Phillips, F.M.; Van Meirhaeghe, J.; Berenson, J.R.; Andersson, G.B.J.; Chung, G.; Small, B.J.; Aghayev, K.; Vrionis, F.D. Comparing effects of kyphoplasty, vertebroplasty, and non-surgical management in a systematic review of randomized and non-randomized controlled studies. Eur. Spine J. 2012, 21, 1826–1843. [Google Scholar] [CrossRef] [Green Version]
- Cortet, B.; Cotten, A.; Boutry, N.; Flipo, R.M.; Duquesnoy, B.; Chastanet, P.; Delcambre, B. Percutaneous vertebroplasty in the treatment of osteoporotic vertebral compression fractures: An open prospective study. J. Rheumatol. 1999, 26, 2222–2228. [Google Scholar]
- Wang, B.; Zhao, C.-P.; Song, L.-X.; Zhu, L. Balloon kyphoplasty versus percutaneous vertebroplasty for osteoporotic vertebral compression fracture: A meta-analysis and systematic review. J. Orthop. Surg. Res. 2018, 13, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.-Z.; Bei, M.-J.; Shu, D.-P.; Sun, C.-J.; Chen, J.-B.; Xiao, Y.-P. Comparison of the clinical outcomes of percutaneous vertebroplasty vs. kyphoplasty for the treatment of osteoporotic Kümmell’s disease: A prospective cohort study. BMC Musculoskelet. Disord. 2020, 21, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamska, O.; Modzelewski, K.; Stolarczyk, A.; Kseniuk, J. Delayed posttraumatic vertebral body collapse “Kummell disease”. Med. Case Rep. Study Protoc. 2021, 2, e0092. [Google Scholar] [CrossRef]
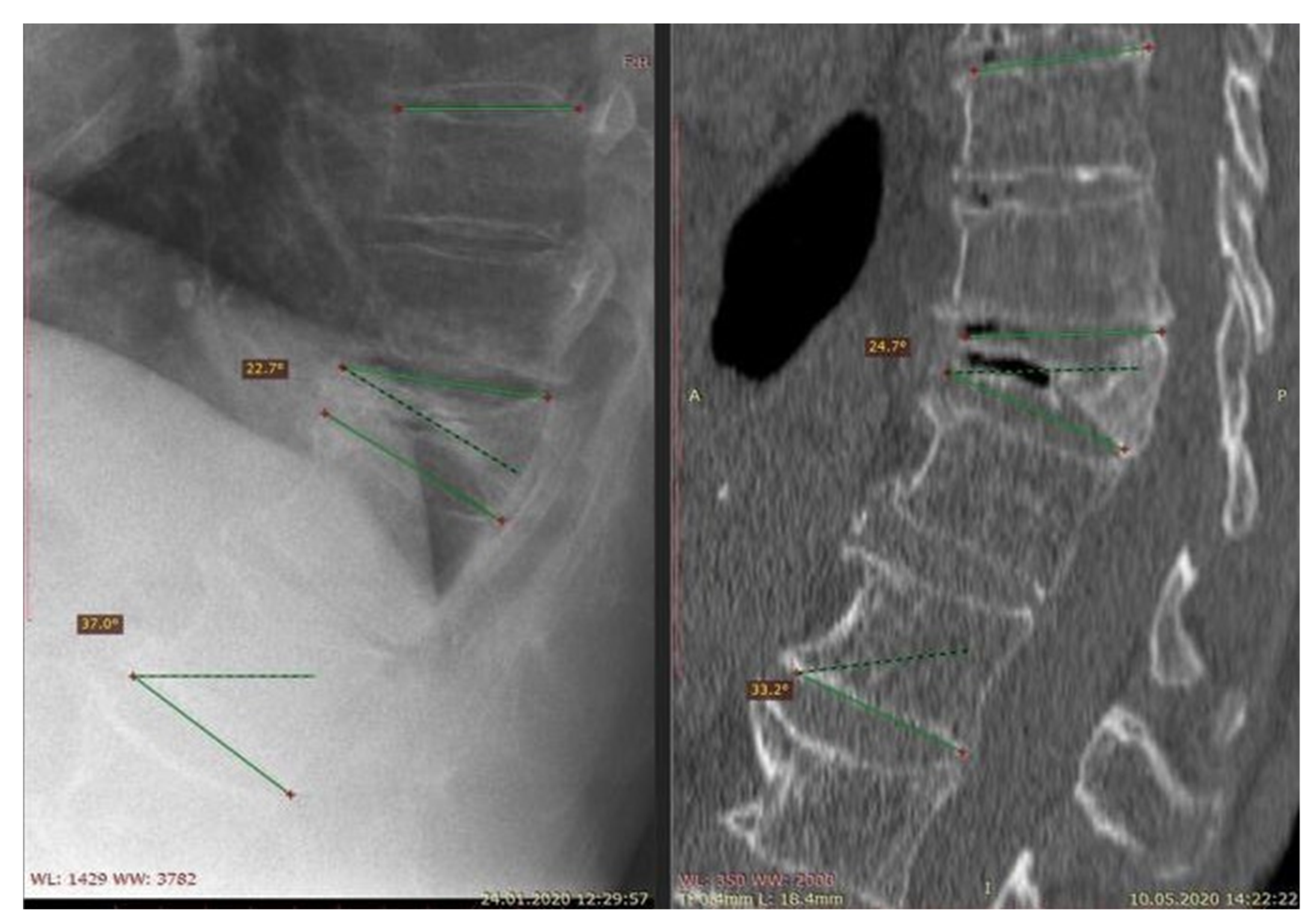
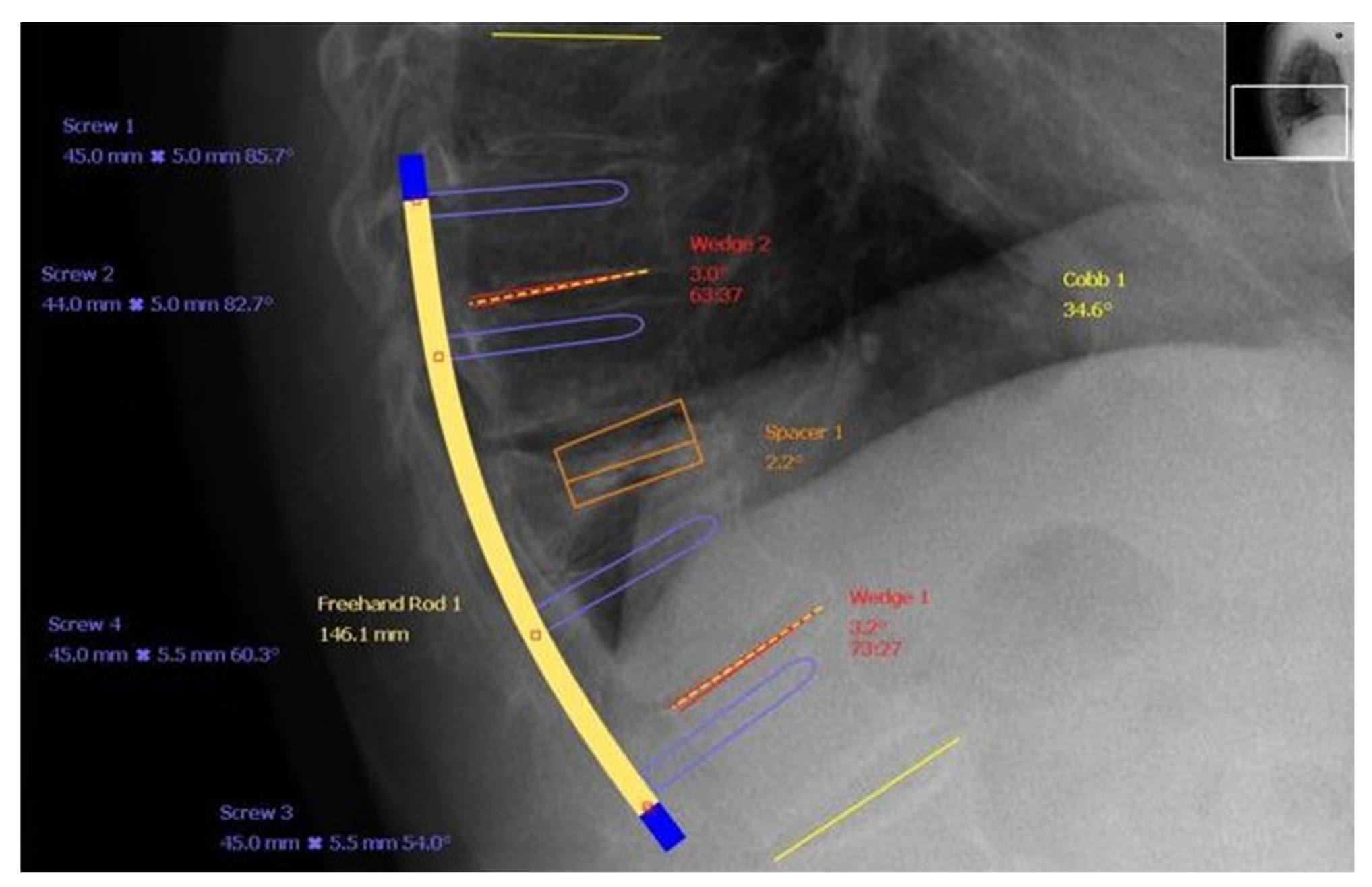
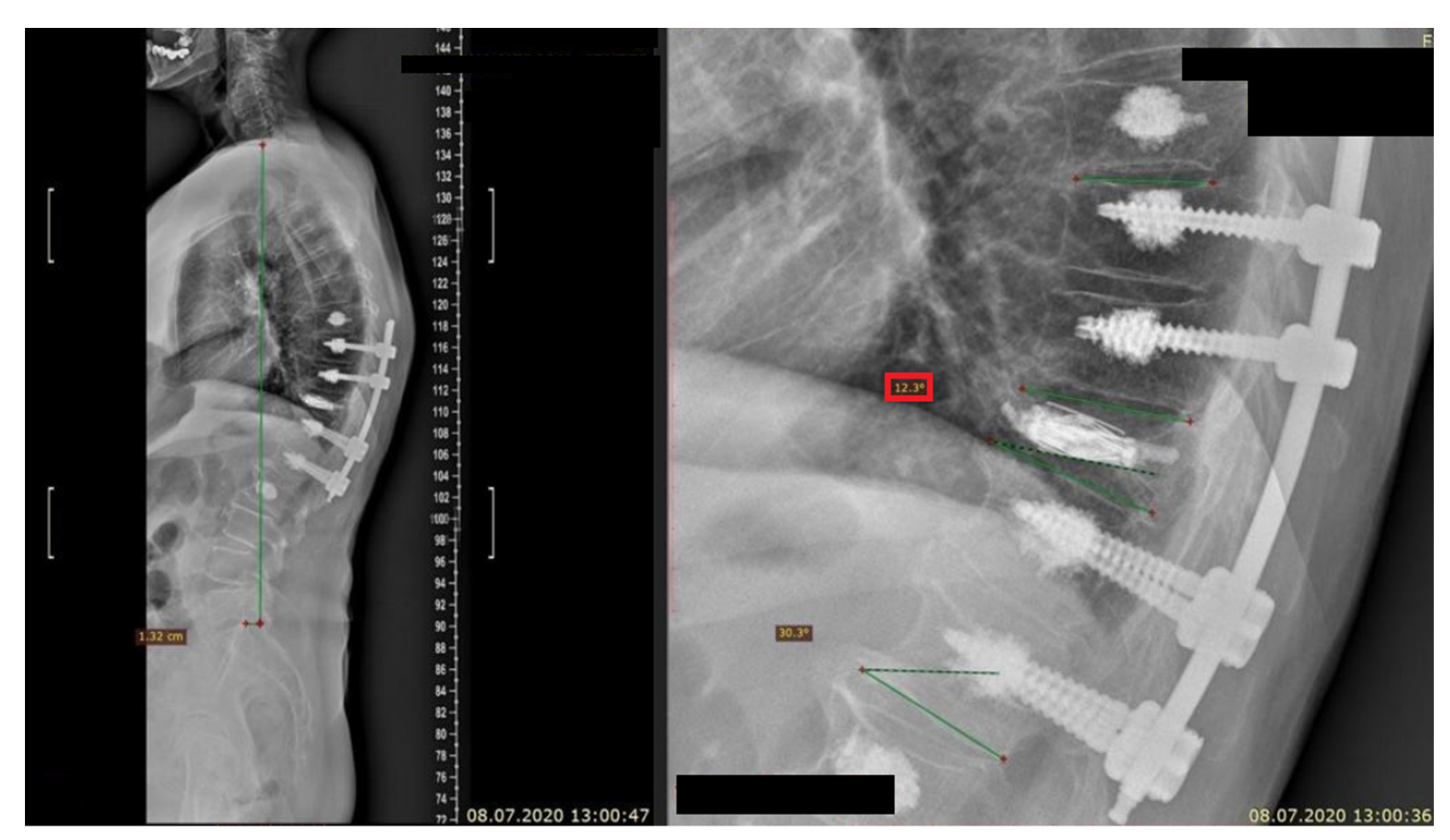
| [Reference Number] Authors | Date of Publication | Journal | Study Design | Clinical/Non-Clinical | Group Abundance | Level of Fracture Appearance | Technique of Treatment | Source | Accessed Date |
|---|---|---|---|---|---|---|---|---|---|
| [1] Hansen E.J. | September 2019 | Integrative Journal of Orthopaedics and Traumatology | RCT | clinical | 46 | T5-L5 | vertebroplasty vs. placebo | https://www.researchgate.net/publication/336149174_Vertebroplasty_vs_SHAM_for_Treating_Osteoporotic_Vertebral_Compression_Fractures_A_Double_Blind_RCT | 21 January 2021 |
| [2] Zhu Y. et al. | November 2019 | Medicine | RCT | clinical | 1077 | NR | vertebroplasty vs. kyphoplasty | https://journals.lww.com/md-journal/FullText/2019/11080/Therapeutic_effect_of_kyphoplasty_and_balloon.28.aspx | 21 January 2021 |
| [3] Lou S. et al. | December 2019 | Osteoporosis international | RCT | clinical | 1624 | T5-L5 | vertebroplasty vs. conservative treatment/SHAM | https://pubmed.ncbi.nlm.nih.gov/31375875/ | 21 January 2021 |
| [4] Xu J. et al. | October 2019 | Surgical innovation | RCT | clinical | 42 | T10-L4 | targeted percutaneous vertebroplasty vs. traditional percutaneous vertebroplasty | https://pubmed.ncbi.nlm.nih.gov/31167616/ | 21 January 2021 |
| [5] Figueiredo N. et al. | June 2009 | Arquivos de neuro-psiquiatria | RCT | clinical | 47 | T4-L5 | frontal vs. side- opening cannula vertebroplasty | https://pubmed.ncbi.nlm.nih.gov/19623429/ | 21 January 2021 |
| [6] Chen C. et al. | December 2014 | Journal of spinal disorders and techniques | RCT | clinical | 39 | NR | unilateral vs. bilateral vertebroplasty | https://pubmed.ncbi.nlm.nih.gov/24901876/ | 21 January 2021 |
| [7] Noriega DC. et al. | March 2019 | Osteoporosis International | RCT | clinical | 30 | T7-L3 | kyphoplasty vs. SpineJack | https://pubmed.ncbi.nlm.nih.gov/30488273/ | 21 January 2021 |
| [8] Zhang L. et al. | February 2015 | Clinical neurology and neurosurgery | RCT | clinical | 32 | NR | high viscosity vs. low viscosity cement vertebroplasty | https://pubmed.ncbi.nlm.nih.gov/25524481/ | 21 January 2021 |
| [9] Schwarz F. et al. | November 2019 | Archives of orthopaedic and trauma surgery | RCT | clinical | 65 | L1-L4 | early versus newer generation vertebral devices access for kyphoplasty | https://pubmed.ncbi.nlm.nih.gov/31278508/ | 21 January 2021 |
| [10] Van Meirhaeghe J. et al. | May 2013 | Spine | RCT | clinical | 300 | NR | kyphoplasty vs. nonsurgical methods | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3678891/ | 21 January 2021 |
| [11] Yang S. et al. | July 2017 | Acta orthopaedica et traumatologica turcica | SR based on RCTs | clinical | 850 | NR | unilateral vs. bilateral vertebroplasty/kyphoplasty | https://pubmed.ncbi.nlm.nih.gov/28647158/ | 21 January 2021 |
| [12] Tang J. et al. | October 2019 | Journal of the College of Physicians and Surgeons | SR based on RCTs | clinical | 178 | T11-L2 | unilateral vs. bilateral balloon kyphoplasty | https://pubmed.ncbi.nlm.nih.gov/31564267/ | 21 January 2021 |
| [13] Duan Z. K. et al. | November 2019 | Archives of osteoporosis | RCT | clinical | 40 | T11-L3 | Bone-filling mesh container vs. kyphoplasty | https://pubmed.ncbi.nlm.nih.gov/31741066/ | 21 January 2021 |
| Reference | Mean Age (year) | Gender (M/F) | BMD T-Score | No. Levels Treated | VAS at Baseline | VAS at the Follow Up | Weighted Mean Difference (95% Confidence Interval) |
|---|---|---|---|---|---|---|---|
| [1] SHAM/ PVP | 69 71 | 2/22 4/18 | −2.2 −2.7 | 28/27 | 5.30 4.06 | 1.6 1.6 | NR |
| [2] BKP/ PVP | 70 72 | 117/419 119/422 | NR | NR | NR | NR | −0.19 (−0.39, 0.01) |
| [3] PVP/ CG | 73 82 | 602/212 680/130 | NR | NR | 7.5 8.8 | NR | NR |
| [4] targeted PVP/ PVP | 68.5 | 3/18 2/19 | NR | NR | 7.38 2.48 | NR | NR |
| [5] SOC/ FOC | NR | NR | NR | 22/25 | 8.04 7.92 | 1.05 1.36 | NR |
| [6] UPVP/ BPVP | 69.5 69 | NR | −3.18/ −3.32 | 23/21 | 7.99 7.66 | 2.82 2.61 | NR |
| [7] BKP/ SJ | 68 68 | 13/2 11/4 | NR | 17/16 | 8.43 8.05 | 2.5 1.44 | NR |
| [8] HV PVP/ LV PVP | 75.5/75.8 | 2/12 3/15 | NR | 17/22 | 8.4 8.6 | 2.2 1.9 | NR |
| [9] PKP VAD/RI/ST | 67/74/74 | 9/21//15/17//10/19 | −3.98/−3.54/−3.70 | 30/32/29 | NR | NR | NR |
| [10] PKP/ CG | 72.2 74.1 | 34/115 34/117 | Normal: 28/20 Osteopenic:54/57 Osteoporosis: 53/51 | 188 151 | 6.79 6.93 | 2.7 4.35 | NR |
| [11] UVP/BVP UKP/BKP | 67.9 | 308/416 + 126 not differentiated | NR | ca 906 | NR/3.11 NR/3.17 | NR/2.16 NR/1.28 | NR |
| [12] UBKP/ BBKP | 72.3 73.9 | 26/57 32/63 | NR | 83 95 | 7.9 7.8 | 2.7 2.6 | NR |
| [13]BFMC/ BKP | <60 <60 | 9/11 8/12 | <−3.0 | 20 20 | 7.5 7 | 1.5 1 | NR |
| Reference | Duration of Follow Up Period | Oswestry Disability Index | Height in the Middle of Injured Vertebrae | Cobb Angle | Mean Operation Time (min) | Re-Fracture of Adjacent Vertebral Bodies | Mean Cement Volume (mL) | Cement Leakage |
|---|---|---|---|---|---|---|---|---|
| [3] PVP/ CG | <36 months | NR | NR | NR | NR | 16.43% 5.83% | NR | NR |
| [4] Targeted PVP/ PVP | NR | 73.11/34.71 79.73/48.28 | NR | NR | 20.05 25.43 | NR | 4 ml | 4.76% 42.9% |
| [5] SOC/ FOC | 6 months | NR | NR | NR | NR | NR | 5.5 6.3 | 27% 68% |
| [6] UPVP/ BPVP | NR | 42.82/18.43 39.42/22.37 | NR | NR | 31.12 52.34 | NR | 3.17 4.36 | 45% 78.9% |
| [7] SJ/ BKP | 36 months | 65.4 59.9 | 86%/81% 82%/79% | −3.2° ± 4.3°/ −2.5° ± 4.4° | 23 32 | 6.67% 6.67% | 4.9 5.1 | 6.67% 0 |
| [8] HV PVP/ LL PVP | 24.5 months | 73.9/29.8 75.5/32.8 | 29.7%/45.6% 32.8%/50.7% | 20.8/14.8 19.3/14.9 | 41.8 44.8 | 29.4% 68.2% | 3.4 3.5 | 35.7% 83% |
| [9] PKP VAD/RI/ST | NR | NR | NR | NR | 31/28/29 | NR | 5.5/6/6 | NR |
| [10] BKP/ CG | 24 months | NR | 8.2%/6% 0%/−2% | 3.4°/3.1° 0°/0.8° | 65 | 7.38% 4.63% | 4.8 | NR |
| [11] UVP/BVP UKP/BKP | <54 months | NR | −0.10, 95% CI, −0.42 to 0.23; SMD = 0.10, 95% CI, −0.35 to 0.55 SMD = −0.13, 95% CI, −0.43 to 0.17 | SMD = −0.05, 95% CI, −0.28 to 0.18 | NR | NR | NR | NR |
| [12] UBKP/BBKP | 6 months | 87.3/86.4 23.5/22.9 | 15.3/15.7 23.6/24.3 | 34.3/33.8 23.4/22.6 | 29.8 31.5 | 6.02% 7.37% | 3.1 3.5 | NR |
| [13] BFMC/ KP | 6 months | 75.45/11.75 75.5/12.75 | NR | 23.16°/16.79° | 43.8 43.3 | 20% 25% | NR | 5% 40% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamska, O.; Modzelewski, K.; Stolarczyk, A.; Kseniuk, J. Is Kummell’s Disease a Misdiagnosed and/or an Underreported Complication of Osteoporotic Vertebral Compression Fractures? A Pattern of the Condition and Available Treatment Modalities. J. Clin. Med. 2021, 10, 2584. https://doi.org/10.3390/jcm10122584
Adamska O, Modzelewski K, Stolarczyk A, Kseniuk J. Is Kummell’s Disease a Misdiagnosed and/or an Underreported Complication of Osteoporotic Vertebral Compression Fractures? A Pattern of the Condition and Available Treatment Modalities. Journal of Clinical Medicine. 2021; 10(12):2584. https://doi.org/10.3390/jcm10122584
Chicago/Turabian StyleAdamska, Olga, Krzysztof Modzelewski, Artur Stolarczyk, and Jurij Kseniuk. 2021. "Is Kummell’s Disease a Misdiagnosed and/or an Underreported Complication of Osteoporotic Vertebral Compression Fractures? A Pattern of the Condition and Available Treatment Modalities" Journal of Clinical Medicine 10, no. 12: 2584. https://doi.org/10.3390/jcm10122584
APA StyleAdamska, O., Modzelewski, K., Stolarczyk, A., & Kseniuk, J. (2021). Is Kummell’s Disease a Misdiagnosed and/or an Underreported Complication of Osteoporotic Vertebral Compression Fractures? A Pattern of the Condition and Available Treatment Modalities. Journal of Clinical Medicine, 10(12), 2584. https://doi.org/10.3390/jcm10122584






