Women with Recurrent Pregnancy Loss More Often Have an Older Brother and a Previous Birth of a Boy: Is Male Microchimerism a Risk Factor?
Abstract
1. Introduction
2. Materials and Methods
3. Statistical Analysis
4. Results
4.1. Frequency of an Older Brother and Sister
4.2. Age Difference
4.3. Sex Ratio of Previous Birth
4.4. Combining Sex of Older Siblings and First-Born Child(ren)
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Philipp, T.; Philipp, K.; Reiner, A.; Beer, F.; Kalousek, D.K. Embryoscopic and cytogenetic analysis of 233 missed abortions: Factors involved in the pathogenesis of developmental defects of early failed pregnancies. Hum. Reprod. 2003, 18, 1724–1732. [Google Scholar] [CrossRef]
- Christiansen, O.B.; Kolte, A.M.; Krog, M.C.; Nielsen, H.S.; Egerup, P. Treatment with intravenous immunoglobulin in patients with recurrent pregnancy loss: An update. J. Reprod. Immunol. 2019, 133, 37–42. [Google Scholar] [CrossRef]
- Nielsen, H.S.; Wu, F.; Aghai, Z.; Steffensen, R.; Van Halteren, A.G.; Spierings, E.; Christiansen, O.B.; Miklos, D.; Goulmy, E. H-Y antibody titers are increased in unexplained secondary recurrent miscarriage patients and associated with low male: Female ratio in subsequent live births. Hum. Reprod. 2010, 25, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, O.B. A fresh look at the causes and treatments of recurrent miscarriage, especially its immunological aspects. Hum. Reprod. Update 1996, 2, 271–293. [Google Scholar] [CrossRef] [PubMed]
- Kruse, C.; Steffensen, R.; Varming, K.; Christiansen, O.B. A study of HLA-DR and -DQ alleles in 588 patients and 562 controls confirms that HLA-DRB1*03 associated with recurrent miscarriage. Hum. Reprod. 2004, 19, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- King, K.; Smith, S.; Chapman, M.; Sacks, G. Detailed analysis of peripheral blood natural killer (NK) cells in women with recurrent miscarriage. Hum. Reprod. 2010, 25, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Karami, N.; Boroujerdnia, M.G.; Nikbakht, R.; Khodadadi, A. Enhancement of peripheral blood CD56dim cell and NK cell cytotoxicity in women with recurrent spontaneous abortion or in vitro fertilization failure. J. Reprod. Immunol. 2012, 95, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Kwak-Kim, J.Y.H.; Chung-Bang, H.S.; Ng, S.C.; Ntrivalas, E.I.; Mangubat, C.P.; Beaman, K.D.; Beer, A.E.; Gilman-Sachs, A. Increased T helper 1 cytokine responses by circulating T cells are present in women with recurrent pregnancy losses and in infertile women with multiple implantation failures after IVF. Hum. Reprod. 2003, 18, 767–773. [Google Scholar] [CrossRef]
- Bianchi, D.W.; Zickwolf, G.K.; Weil, G.J.; Sylvester, S.; Demaria, M.A. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc. Natl. Acad. Sci. USA 1996, 93, 705–708. [Google Scholar] [CrossRef]
- Verdijk, R.M.; Kloosterman, A.; Pool, J.; Van De Keur, M.; Naipal, A.M.I.H.; Van Halteren, A.G.S.; Brand, A.; Mutis, T.; Goulmy, E. Pregnancy induces minor histocompatibility antigen-specific cytotoxic T cells: Implications for stem cell transplantation and immunotherapy. Blood 2004, 103, 1961–1964. [Google Scholar] [CrossRef]
- Gammill, H.S.; Nelson, J.L. Naturally acquired microchimerism. Int. J. Dev. Biol. 2010, 54, 531–543. [Google Scholar] [CrossRef]
- Lissauer, D.; Piper, K.; Goodyear, O.; Kilby, M.D.; Moss, P.A.H. Fetal-Specific CD8 + Cytotoxic T Cell Responses Develop during Normal Human Pregnancy and Exhibit Broad Functional Capacity. J. Immunol. 2012, 189, 1072–1080. [Google Scholar] [CrossRef]
- Gammill, H.S.; Stephenson, M.D.; Aydelotte, T.M.; Nelson, J.L. Microchimerism in recurrent miscarriage. Cell. Mol. Immunol. 2014, 11, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Lambert, N.C.; Guthrie, K.A.; Porter, A.J.; Loubiere, L.S.; Madeleine, M.M.; Stevens, A.M.; Hermes, H.M.; Nelson, J.L. Male microchimerism in women without sons: Quantitative assessment and correlation with pregnancy history. Am. J. Med. 2005, 118, 899–906. [Google Scholar] [CrossRef]
- Klonisch, T.; Drouin, R. Fetal-maternal exchange of multipotent stem/progenitor cells: Microchimerism in diagnosis and disease. Trends Mol. Med. 2009, 15, 510–518. [Google Scholar] [CrossRef]
- Jørggensen, K.T.; Pedersen, B.V.; Nielsen, N.M.; Jacobsen, S.; Frisch, M. Childbirths and risk of female predominant and other autoimmune diseases in a population-based Danish cohort. J. Autoimmun. 2012, 38, J81–J87. [Google Scholar] [CrossRef]
- Kekow, M.; Barleben, M.; Drynda, S.; Jakubiczka, S.; Kekow, J.; Brune, T. Long-term persistence and effects of fetal microchimerisms on disease onset and status in a cohort of women with rheumatoid arthritis and systemic lupus erythematosus. BMC Musculoskelet. Disord. 2013, 14, 325. [Google Scholar] [CrossRef]
- Loren, A.W.; Bunin, G.R.; Boudreau, C.; Champlin, R.E.; Cnaan, A.; Horowitz, M.M.; Loberiza, F.R.; Porter, D.L. Impact of Donor and Recipient Sex and Parity on Outcomes of HLA-Identical Sibling Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2006, 12, 758–769. [Google Scholar] [CrossRef]
- The ESHRE Guideline Group; Atik, R.B.; Christiansen, O.B.; Elson, J.; Kolte, A.M.; Lewis, S.; Middeldorp, S.; Nelen, W.; Peramo, B.; Quenby, S.; et al. ESHRE guideline: Recurrent pregnancy loss. Hum. Reprod. Open 2018, 10, 1–12. [Google Scholar] [CrossRef]
- Nielsen, H.S.; Steffensen, R.; Varming, K.; van Halteren, A.G.S.; Spierings, E.; Ryder, L.P.; Goulmy, E.; Christiansen, O.J. Association of HY-restricting HLA class II alleles with pregnancy outcome in patients with recurrent miscarriage subsequent to a firstborn boy. Hum. Mol. Genet. 2009, 18, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, O.B.; Pedersen, B.; Nielsen, H.S.; Andersen, A.M.N. Impact of the sex of first child on the prognosis in secondary recurrent miscarriage. Hum. Reprod. 2004, 19, 2946–2951. [Google Scholar] [CrossRef]
- Nielsen, H.S.; Andersen, A.M.N.; Kolte, A.M.; Christiansen, O.B. A firstborn boy is suggestive of a strong prognostic factor in secondary recurrent miscarriage: A confirmatory study. Fertil. Steril. 2008, 89, 907–911. [Google Scholar] [CrossRef]
- Egerup, P.; Lindschou, J.; Gluud, C.; Christiansen, O.B. The effects of intravenous immunoglobulins in women with recurrent miscarriages: A systematic review of randomised trials with meta-analyses and trial sequential analyses including individual patient data. PLoS ONE 2015, 10, e0141588. [Google Scholar] [CrossRef]
- Wang, S.W.; Zhong, S.Y.; Lou, L.J.; Hu, Z.F.; Sun, H.Y.; Zhu, H.Y. The effect of intravenous immunoglobulin passive immunotherapy on unexplained recurrent spontaneous abortion: A meta-analysis. Reprod. Biomed. Online 2016, 33, 720–736. [Google Scholar] [CrossRef]
- Winkelhorst, D.; Oepkes, D.; Lopriore, E. Fetal and neonatal alloimmune thrombocytopenia: Evidence based antenatal and postnatal management strategies. Expert Rev. Hematol. 2017, 10, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Kolte, A.M.; Steffensen, R.; Christiansen, O.B.; Nielsen, H.S. Maternal HY-restricting HLA class II alleles are associated with poor long-term outcome in recurrent pregnancy loss after a boy. Am. J. Reprod. Immunol. 2016, 76, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Loubière, L.S.; Lambert, N.C.; Flinn, L.J.; Erickson, T.D.; Yan, Z.; Guthrie, K.A.; Vickers, K.T.; Nelson, J.L. Maternal microchimerism in healthy adults in lymphocytes, monocyte/macrophages and NK cells. Lab. Investig. 2006, 86, 1185–1192. [Google Scholar] [CrossRef]
- Khosrotehrani, K.; Johnson, K.L.; Lau, J.; Dupuy, A.; Cha, D.H.; Bianchi, D.W. The Influence of Fetal Loss on the Presence of Fetal Cell Microchimerism: A Systematic Review. Arthritis Rheum. 2003, 48, 3237–3241. [Google Scholar] [CrossRef] [PubMed]
- Mold, J.E.; Michaëlsson, J.; Burt, T.D.; Muench, M.O.; Beckerman, K.P.; Busch, M.P.; Lee, T.H.; Nixon, D.F.; McCune, J.M. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 2008, 322, 1562–1565. [Google Scholar] [CrossRef]
- Guettier, C.; Sebagh, M.; Buard, J.; Feneux, D.; Ortin-Serrano, M.; Gigou, M.; Tricottet, V.; Reynès, M.; Samuel, D.; Féray, C. Male cell microchimerism in normal and diseased female livers from fetal life to adulthood. Hepatology 2005, 42, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Bucher, C.; Stern, M.; Buser, A.; Heim, D.; Tsakiris, D.; Droll, A.; Meyer-, S.; Tichelli, A.; Passweg, J.; Gratwohl, A. Role of primacy of birth in HLA-identical sibling transplantation. Blood 2007, 110, 468–469. [Google Scholar] [CrossRef] [PubMed]
- Dobbelstein, C.; Ahn, K.W.; Haagenson, M.; Hale, G.A.; Van Rood, J.J.; Miklos, D.; Waller, E.K.; Spellman, S.R.; Fernandez-Vina, M.; Ganser, A.; et al. Birth order and transplantation outcome in hla-identical sibling stem cell transplantation: An analysis on behalf of the center for international blood and marrow transplantation. Biol. Blood Marrow Transplant. 2013, 19, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Cockrill, T.; del Junco, D.J.; Arnett, F.C.; Assassi, S.; Tan, F.K.; McNearney, T.; Fischbach, M.; Perry, M.; Mayes, M.D. Separate influences of birth order and gravidity/parity on the development of systemic sclerosis. Arthritis Care Res. 2010, 62, 418–424. [Google Scholar] [CrossRef] [PubMed]

| All RPL Patients (n = 383) | pRPL (n = 201) | sRPL (n = 182) | |
|---|---|---|---|
| No older siblings, n (%) | 181 (47.3) | 104 (51.7) | 77 (42.3) |
| Older siblings of both sexes, n (%) | 40 (10.4) | 16 (8.0) | 24 (13.2) |
| Only older brother(s), n (%) | 97 (25.3) a | 45 (22.4) | 52 (28.6) b |
| Only older sister(s), n (%) | 65 (17.0) a | 36 (17.9) | 29 (15.9) b |
| At least one older brother, n (%) | 137 (35.7) | 61 (30.4) | 76 (41.8) |
| At least one older sister, n (%) | 105 (27.4) | 52 (25.9) | 53 (29.1) |
| Only younger brother(s), n (%) | 76 (19.8) | 42 (21.0) | 34 (18.7) |
| Only younger sister(s), n (%) | 88 (23.0) | 48 (24.0) | 39 (21.4) |
| Younger siblings of both sexes, n (%) | 49 (12.8) | 27 (13.5) | 22 (12.1) |
| No younger siblings, n (%) | 170 (44.4) | 83 (41.3) | 87 (47.8) |
| At least one younger brother, n (%) | 125 (32.6) | 69 (34.3) | 56 (30.8) |
| At least one younger sister, n (%) | 137 (35.8) | 76 (37.8) | 61 (33.5) |
| Demographic | Only Older Brother(s) (n = 97) | Only Older Sister(s) (n = 65) | Older Siblings of Both Sexes (n = 40) | No Older Siblings (n = 181) |
|---|---|---|---|---|
| Age, years Mean (SD) | 33.3 (5.6) | 32.6 (5.2) | 32.4 (5.8) | 32.8 (5.1) |
| No. of consecutive losses, Median (range) | 4 (3;12) | 4 (3;13) | 4 (3;10) | 4 (3;12) |
| BMI, kg/m2 Mean (SD) | 26.1 (5.4) | 26.5 (6.5) | 26.2 (5.8) | 26.2 (5.7) |
| Smoking, % | 13.4 | 18.5 | 5.0 | 9.4 |
| All RPL | pRPL | sRPL | |
|---|---|---|---|
| Age difference to the youngest older brother (year) Median (P10:P90) | 3.5 (1.5:7.0) | 3.0 (1.5:7.0) | 4.5 (2.0:7.0) |
| Age difference to the youngest older sister (year) Median (P10:P90) | 3.5 (2.0:7.0) | 3.5 (2.0:5.0) | 3.5 (1.5:7.0) |
| Only Older Brother(s) | Only Older Sister(s) | Both Older Brother(s) and Sister(s) | No Older Siblings | At Least One Older Brother | All sRPL | |
|---|---|---|---|---|---|---|
| Sex ratio of births >22 weeks a | 1.33 | 2.50 | 2.00 | 1.23 | 1.48 | 1.51 b |
| Minimum one boy, n (%) c | 34 (65.4) | 21 (72.4) | 17 (70.8) | 47 (61.0) | 51 (67.1) | 119 (65.4) |
| Minimum one girl, n (%) c | 28 (53.9) | 9 (31.0) | 10 (41.7) | 40 (52.0) | 38 (50.0) | 87 (47.8) |
| Observed | Expected * | |||
|---|---|---|---|---|
| Frequency | Percentage (%) | Frequency | Percentage (%) | |
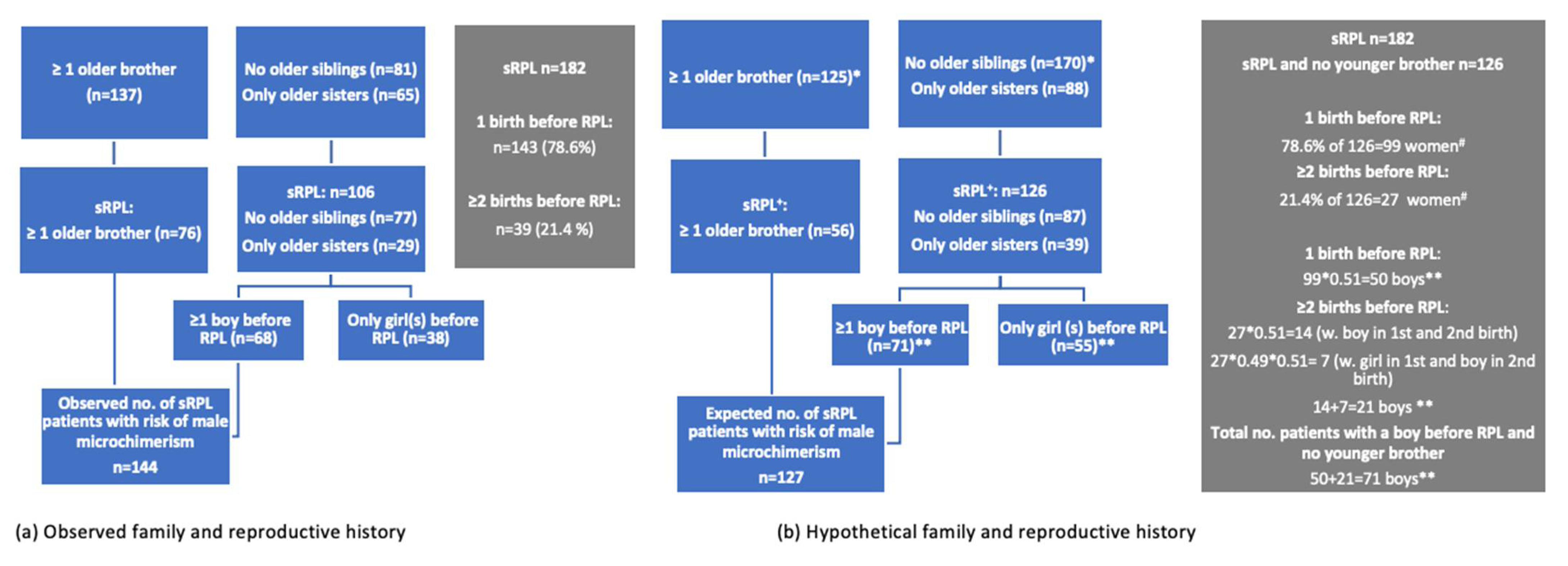
| Older brother and/or a prior boy | 144 | 79.1 | 127 | 69.8 |
| No older brother and no prior boy | 38 | 20.9 | 55 | 30.2 |
| Observed | Expected * | |||
|---|---|---|---|---|
| Frequency | Percentage (%) | Frequency | Percentage (%) | |
| Older brother and/or a prior boy | 205 | 53.5 | 189 | 49.0 |
| No older brother and no prior boy | 178 | 46.5 | 194 | 51.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nørgaard-Pedersen, C.; Kesmodel, U.S.; Christiansen, O.B. Women with Recurrent Pregnancy Loss More Often Have an Older Brother and a Previous Birth of a Boy: Is Male Microchimerism a Risk Factor? J. Clin. Med. 2021, 10, 2613. https://doi.org/10.3390/jcm10122613
Nørgaard-Pedersen C, Kesmodel US, Christiansen OB. Women with Recurrent Pregnancy Loss More Often Have an Older Brother and a Previous Birth of a Boy: Is Male Microchimerism a Risk Factor? Journal of Clinical Medicine. 2021; 10(12):2613. https://doi.org/10.3390/jcm10122613
Chicago/Turabian StyleNørgaard-Pedersen, Caroline, Ulrik Schiøler Kesmodel, and Ole B. Christiansen. 2021. "Women with Recurrent Pregnancy Loss More Often Have an Older Brother and a Previous Birth of a Boy: Is Male Microchimerism a Risk Factor?" Journal of Clinical Medicine 10, no. 12: 2613. https://doi.org/10.3390/jcm10122613
APA StyleNørgaard-Pedersen, C., Kesmodel, U. S., & Christiansen, O. B. (2021). Women with Recurrent Pregnancy Loss More Often Have an Older Brother and a Previous Birth of a Boy: Is Male Microchimerism a Risk Factor? Journal of Clinical Medicine, 10(12), 2613. https://doi.org/10.3390/jcm10122613








