The Impact of Chronic Heart Failure on Retinal Vessel Density Assessed by Optical Coherence Tomography Angiography in Children with Dilated Cardiomyopathy
Abstract
:1. Introduction
2. Material and Methods
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathew, T.; Williams, L.; Navaratnam, G.; Rana, B.; Wheeler, R.; Collins, K.; Harkness, A.; Jones, R.; Knight, D.; O’Gallagher, K.; et al. Diagnosis and assessment of dilated cardiomyopathy: A guideline protocol from the British Society of Echocardiography. Echo Res. Pract. 2017, 4, G1–G13. [Google Scholar] [CrossRef] [Green Version]
- Japp, A.G.; Gulati, A.; Cook, S.A.; Cowie, M.R.; Prasad, S.K. The Diagnosis and Evaluation of Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 67, 2996–3010. [Google Scholar] [CrossRef]
- Puggia, I.; Merlo, M.; Barbati, G.; Rowland, T.J.; Stolfo, D.; Gigli, M.; Ramani, F.; Di Lenarda, A.; Mestroni, L.; Sinagra, G. Natural History of Dilated Cardiomyopathy in Children. J. Am. Heart Assoc. 2016, 5, e003450. [Google Scholar] [CrossRef] [Green Version]
- Fadl, S.; Wåhlander, H.; Fall, K.; Cao, Y.; Sunnegårdh, J. The highest mortality rates in childhood dilated cardiomyopathy occur during the first year after diagnosis. Acta Paediatr. 2018, 107, 672–677. [Google Scholar] [CrossRef] [Green Version]
- Burke, M.A.; Cook, S.A.; Seidman, J.G.; Seidman, C.E. Clinical and Mechanistic Insights into the Genetics of Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 68, 2871–2886. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Cochran, T.R.; Briston, D.A.; Brown, S.R.; Sambatakos, P.J.; Miller, T.L.; Carrillo, A.A.; Corcia, L.; Sanchez, J.E.; Diamond, M.B.; et al. Pediatric cardiomyopathies: Causes, epidemiology, clinical course, preventive strategies and therapies. Future Cardiol. 2013, 9, 817–848. [Google Scholar] [CrossRef] [Green Version]
- Jeewa, A.; Denfield, S.W. Clinical Characteristics and Treatment of Cardiomyopathies in Children. Curr. Cardiol. Rev. 2016, 12, 85–98. [Google Scholar] [CrossRef]
- Vikhorev, P.G.; Vikhoreva, N.N. Cardiomyopathies and Related Changes in Contractility of Human Heart Muscle. Int. J. Mol. Sci. 2018, 19, 2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osborne, N.N.; Casson, R.J.; Wood, J.P.; Chidlow, G.; Graham, M.; Melena, J. Retinal ischemia: Mechanisms of damage and potential therapeutic strategies. Prog. Retin. Eye Res. 2004, 23, 91–147. [Google Scholar] [CrossRef]
- Wangsa-Wirawan, N.D. Retinal Oxygen Fundamental and Clinical. Arch. Ophthalmol. 2003, 121, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Flammer, J.; Konieczka, K.; Bruno, R.M.; Virdis, A.; Flammer, A.J.; Taddei, S. The eye and the heart. Eur. Heart J. 2013, 34, 1270–1278. [Google Scholar] [CrossRef]
- Carpineto, P.; Mastropasqua, R.; Marchini, G.; Toto, L.; Di Nicola, M.; Di Antonio, L. Reproducibility and repeatability of foveal avascular zone measurements in healthy subjects by optical coherence tomography angiography. Br. J. Ophthalmol. 2016, 100, 671–676. [Google Scholar] [CrossRef]
- Dubis, A.M.; Hansen, B.R.; Cooper, R.F.; Beringer, J.; Dubra, A.; Carroll, J. Relationship between the foveal avascular zone and foveal pit morphology. Investig. Opthalmol. Vis. Sci. 2012, 53, 1628–1636. [Google Scholar] [CrossRef]
- McClintic, B.R.; McClintic, J.I.; Bisognano, J.D.; Block, R.C. The Relationship between retinal microvascular abnormalities and coronary heart disease: A review. Am. J. Med. 2010, 123, 374.e1–374.e7. [Google Scholar] [CrossRef] [Green Version]
- Patton, N.; Aslam, T.; MacGillivray, T.; Pattie, A.; Deary, I.J.; Dhillon, B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: A rationale based on homology between cerebral and retinal microvasculatures. J. Anat. 2005, 206, 319–348. [Google Scholar] [CrossRef] [PubMed]
- Kromer, R.; Tigges, E.; Rashed, N.; Pein, I.; Klemm, M.; Blankenberg, S. Association between optical coherence tomography based retinal microvasculature characteristics and myocardial infarction in young men. Sci. Rep. 2018, 8, 5615. [Google Scholar] [CrossRef] [PubMed]
- Amini, Z.; Miri, M.; Rabbani, H.; Kafieh, R. A Comprehensive Study of Retinal Vessel Classification Methods in Fundus Images. J. Med. Signals Sens. 2017, 7, 59–70. [Google Scholar] [CrossRef]
- Ffytche, T.J.; Shilling, J.S.; Chisholm, I.H.; Federman, J.L. Indications for fluorescein angiography in disease of the ocular fundus: A review. J. R. Soc. Med. 1980, 73, 362–365. [Google Scholar] [CrossRef] [Green Version]
- Le Rouic, J.-F.; Peronnet, P.; Barrucand, A.; Delaunay, S.; Dupouy, S.; Badat, I.; Becquet, F. Indications d’angiographie à la fluorescéine et d’OCT-angiographie en consultation de rétine médicale: Comparaison entre 2015 et 2018. J. Fr. Ophtalmol. 2020, 43, 397–403. [Google Scholar] [CrossRef]
- Tsang, S.H.; Sharma, T. Fluorescein Angiography. Chem. Biol. Pteridines Folates 2018, 1085, 7–10. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Kashani, A.H.; Chen, C.-L.; Gahm, J.K.; Zheng, F.; Richter, G.M.; Rosenfeld, P.J.; Shi, Y.; Wang, R.K. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Prog. Retin. Eye Res. 2017, 60, 66–100. [Google Scholar] [CrossRef]
- Tan, A.C.S.; Tan, G.S.; Denniston, A.K.; Keane, P.A.; Ang, M.; Milea, D.; Chakravarthy, U.; Cheung, C.M.G. An overview of the clinical applications of optical coherence tomography angiography. Eye 2018, 32, 262–286. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Jia, Y.; Gao, S.S.; Lumbroso, B.; Rispoli, M. Optical Coherence Tomography Angiography Using the Optovue Device. Integr. Intraoperative Ocul. Coherence Tomogr. Pediatr. Ocul. Surg. 2016, 56, 6–12. [Google Scholar] [CrossRef]
- Ji, Y.S.; Alagorie, A.R.; Byon, I.; Sadda, S.R. Impact of Scan Tilt on Quantitative Assessments Using Optical Coherence Tomography Angiography. Transl. Vis. Sci. Technol. 2020, 9, 46. [Google Scholar] [CrossRef]
- Prati, F.; Ramazzotti, V.; Gatto, L.; Albertucci, M. Optical coherence tomography. ESC CardioMed 2018, 631–636. [Google Scholar] [CrossRef]
- Rakusiewicz, K.; Kanigowska, K.; Hautz, W.; Ziółkowska, L. Investigating Ganglion Cell Complex Thickness in Children with Chronic Heart Failure due to Dilated Cardiomyopathy. J. Clin. Med. 2020, 9, 2882. [Google Scholar] [CrossRef]
- McNally, E.M.; Mestroni, L. Dilated Cardiomyopathy: Genetic Determinants and Mechanisms. Circ. Res. 2017, 121, 731–748. [Google Scholar] [CrossRef]
- Inanc, M.; Tekin, K.; Kiziltoprak, H.; Ozalkak, S.; Doguizi, S.; Aycan, Z. Changes in Retinal Microcirculation Precede the Clinical Onset of Diabetic Retinopathy in Children with Type 1 Diabetes Mellitus. Am. J. Ophthalmol. 2019, 207, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Xu, Y.; Zeng, X.; Yang, N.; Jiang, M.; Zhang, X.; Yang, J.; He, T.; Xing, Y. Use of optical coherence tomography angiography for assessment of microvascular changes in the macula and optic nerve head in hypertensive patients without hypertensive retinopathy. Microvasc. Res. 2020, 129, 103969. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, M.F.; Can, M.E.; Kazancı, E.G. Effects of iron deficiency anemia on peripapillary and macular vessel density determined using optical coherence tomography angiography on children. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.K.H.; Xie, J.; Wang, J.J. Retinal arteriolar diameter and the prevalence and incidence of hypertension: A systematic review and meta-analysis of their association. Curr. Hypertens. Rep. 2012, 14, 144–151. [Google Scholar] [CrossRef]
- Wang, J.J.; Liew, G.; Klein, R.; Rochtchina, E.; Knudtson, M.D.; Klein, B.E.; Wong, T.Y.; Burlutsky, G.; Mitchell, P. Retinal vessel diameter and cardiovascular mortality: Pooled data analysis from two older populations. Eur. Heart J. 2007, 28, 1984–1992. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, J.; Zhang, Y.; Qian, Y.W.; Zhang, J.F.; Wang, Z.L. Retinal and choroidal vascular changes in coronary heart disease: An optical coherence tomography angiography study. Biomed. Opt. Express 2019, 10, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- Witt, N.; Wong, T.Y.; Hughes, A.D.; Chaturvedi, N.; Klein, B.E.; Evans, R.; McNamara, M.; Thom, S.A.M.; Klein, R. Abnormalities of retinal microvascular structure and risk of mortality from ischemic heart disease and stroke. Hypertension 2006, 47, 975–981. [Google Scholar] [CrossRef] [Green Version]
- Tikellis, G.; Wong, T.Y.; Arnett, D.K.; Skelton, T.N.; Taylor, H.W.; Klein, R.; Couper, D.J.; Sharrett, A.R. Retinal Arteriolar Narrowing and Left Ventricular Hypertrophy in African Americans. The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Hypertens. 2008, 21, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Cheung, N.; Bluemke, D.A.; Klein, R.; Sharrett, A.R.; Islam, F.A.; Cotch, M.F.; Klein, B.E.K.; Criqui, M.H.; Wong, T.Y. Retinal arteriolar narrowing and left ventricular remodeling: The multi-ethnic study of atherosclerosis. J. Am. Coll. Cardiol. 2007, 50, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.Y.; Islam, F.M.A.; Klein, R.; Klein, B.E.K.; Cotch, M.F.; Castro, C.; Sharrett, A.R.; Shahar, E. Retinal vascular caliber, cardiovascular risk factors, and inflammation: The multi-ethnic study of atherosclerosis (MESA). Investig. Opthalmol. Vis. Sci. 2006, 47, 2341–2350. [Google Scholar] [CrossRef] [PubMed]
- McGeechan, K. Meta-analysis: Retinal vessel caliber and risk for coronary heart disease. Ann. Intern. Med. 2009, 151, 404–413. [Google Scholar] [CrossRef]
- Wong, T.Y.; Kamineni, A.; Klein, R.; Sharrett, A.R.; Klein, B.E.; Siscovick, D.S.; Cushman, M.; Duncan, B.B. Quantitative Retinal Venular Caliber and Risk of Cardiovascular Disease in Older Persons: The Cardiovascular Health Study. Arch. Intern. Med. 2006, 166, 2388–2394. [Google Scholar] [CrossRef] [Green Version]
- Cheung, N.; Wong, T.Y. Microvascular changes in the retina as a risk marker for cardiovascular disease. Curr. Cardiovasc. Risk Rep. 2008, 3, 51–58. [Google Scholar] [CrossRef]
- Phan, K.; Mitchell, P.; Liew, G.; Plant, A.J.; Wang, S.B.; Au, C.; Chiha, J.; Kovoor, P.; Thiagalingam, A.; Burlutsky, G.; et al. Association between retinal arteriolar and venule calibre with prevalent heart failure: A cross-sectional study. PLoS ONE 2015, 10, e0144850. [Google Scholar] [CrossRef] [Green Version]
- Nägele, M.P.; Barthelmes, J.; Ludovici, V.; Cantatore, S.; Von Eckardstein, A.; Enseleit, F.; Lüscher, T.F.; Ruschitzka, F.; Sudano, I.; Flammer, A.J. Retinal microvascular dysfunction in heart failure. Eur. Heart J. 2017, 39, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Arnould, L.; Guenancia, C.; Gabrielle, P.-H.; Pitois, S.; Baudin, F.; Pommier, T.; Zeller, M.; Bron, A.; Creuzot-Garcher, C.; Cottin, Y. Influence of cardiac hemodynamic variables on retinal vessel density measurement on optical coherence tomography angiography in patients with myocardial infarction. J. Fr. Ophtalmol. 2020, 43, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhong, P.; Yuan, H.; Dong, X.; Peng, Q.; Huang, M.; Wu, Q.; Liu, B.; Xu, M.; Kuang, Y.; et al. Retinal microvasculature impairment in patients with congenital heart disease investigated by optical coherence tomography angiography. Clin. Exp. Ophthalmol. 2020, 48, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Arnould, L.; Guenancia, C.; Azemar, A.; Alan, G.; Pitois, S.; Bichat, F.; Zeller, M.; Gabrielle, P.-H.; Bron, A.M.; Creuzot-Garcher, C.; et al. The EYE-MI Pilot Study: A Prospective Acute Coronary Syndrome Cohort Evaluated with Retinal Optical Coherence Tomography Angiography. Investig. Opthalmol. Vis. Sci. 2018, 59, 4299–4306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida-Freitas, D.B.; Meira-Freitas, D.; Melo, L.A.S.D., Jr.; Paranhos, A., Jr.; Iared, W.; Ajzen, S. Color Doppler imaging of the ophthalmic artery in patients with chronic heart failure. Arq. Bras. Oftalmol. 2011, 74, 326–329. [Google Scholar] [CrossRef] [Green Version]
- Fouquet, S.; Vacca, O.; Sennlaub, F.; Paques, M. The 3D Retinal Capillary Circulation in Pigs Reveals a Predominant Serial Organization. Investig. Opthalmol. Vis. Sci. 2017, 58, 5754–5763. [Google Scholar] [CrossRef]
- Wong, T.Y.; Klein, R.; Klein, B.E.; Tielsch, J.M.; Hubbard, L.; Nieto, F. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Surv. Ophthalmol. 2001, 46, 59–80. [Google Scholar] [CrossRef]
- Tick, S.; Rossant, F.; Ghorbel, I.; Gaudric, A.; Sahel, J.-A.; Chaumet-Riffaud, P.; Paques, M. Foveal shape and structure in a normal population. Investig. Opthalmol. Vis. Sci. 2011, 52, 5105–5110. [Google Scholar] [CrossRef] [Green Version]
- Kuehlewein, L.; Tepelus, T.C.; An, L.; Durbin, M.K.; Srinivas, S.; Sadda, S.R. Noninvasive visualization and analysis of the human parafoveal capillary network using swept source OCT optical microangiography. Investig. Opthalmol. Vis. Sci. 2015, 56, 3984–3988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.S.; Lim, L.W.; Chow, V.S.; Chay, I.W.; Tan, S.; Cheong, K.X.; Tan, G.T.; Sadda, S.R. Optical Coherence Tomography Angiography Evaluation of the Parafoveal Vasculature and Its Relationship with Ocular Factors. Investig. Opthalmol. Vis. Sci. 2016, 57, OCT224–OCT234. [Google Scholar] [CrossRef] [PubMed]
- Samara, W.A.; Say, E.A.T.; Khoo, C.T.L.; Higgins, T.P.; Magrath, G.; Ferenczy, S.; Shields, C.L. Correlation of Foveal Avascular Zone size with Foveal Morphology in Normal Eyes Using Optical Coherence Tomography Angiography. Retina 2015, 35, 2188–2195. [Google Scholar] [CrossRef]
- Takase, N.; Nozaki, M.; Kato, A.; Ozeki, H.; Yoshida, M.; Ogura, Y. Enlargement of Foveal Avascular Zone in Diabetic Eyes Evaluated by en Face Optical Coherence Tomography Angiography. Retina 2015, 35, 2377–2383. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Park, J.-H.; Won, Y.; Lee, M.-W.; Shin, Y.-I.; Jo, Y.-J.; Kim, J.-Y. Retinal Microvascular Change in Hypertension as measured by Optical Coherence Tomography Angiography. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Sheikh, M.; Akil, H.; Pfau, M.; Sadda, S.R. Swept-Source OCT Angiography Imaging of the Foveal Avascular Zone and Macular Capillary Network Density in Diabetic Retinopathy. Investig. Opthalmol. Vis. Sci. 2016, 57, 3907–3913. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Jiang, C.; Wang, X.; Zhu, L.; Gu, R.; Xu, H.; Jia, Y.; Huang, D.; Sun, X. Macular Perfusion in Healthy Chinese: An Optical Coherence Tomography Angiogram Study. Investig. Opthalmol. Vis. Sci. 2015, 56, 3212–3217. [Google Scholar] [CrossRef] [PubMed]
- Lupidi, M.; Coscas, F.; Cagini, C.; Fiore, R.; Spaccini, E.; Fruttini, D.; Coscas, C. Automated quantitative analysis of retinal microvasculature in normal eyes on optical coherence tomography angiography. Am. J. Ophthal. 2016, 169, 9–23. [Google Scholar] [CrossRef]
- Remigio, M.C.D.A.; Brandt, C.T.; Santos, C.C.L.; Arantes, T.E.; Aguiar, M.I.R.D. Macular and peripapillary retinal nerve fibre layer thickness in patients with cyanotic congenital heart disease. Eye 2015, 29, 465–468. [Google Scholar] [CrossRef] [Green Version]
- Aydin, E.; Kazanci, L.; Yilmaz, M.B.; Akcay, F.A.; Bayata, S. Analysis of central macular thickness and choroidal thickness changes in patients with cardiovascular risk factors. Eye 2020, 34, 2068–2075. [Google Scholar] [CrossRef]
- Chan, A. Normal Macular Thickness Measurements in Healthy Eyes Using Stratus Optical Coherence Tomography. Arch. Ophthalmol. 2006, 124, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Song, W.K.; Lee, S.C.; Lee, E.S.; Kim, C.Y.; Kim, S.S. Macular thickness variations with sex, age, and axial length in healthy subjects: A spectral domain–optical coherence tomography study. Investig. Opthalmol. Vis. Sci. 2010, 51, 3913–3918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, X.R.; Liang, Y.; Friedman, D.S.; Sun, L.P.; Wong, T.Y.; Tao, Q.S.; Bao, L.; Wang, N.L.; Wang, J.J. Normal macular thickness measurements using optical coherence tomography in healthy eyes of adult chinese persons: The handan eye study. Ophthalmology 2010, 117, 1585–1594. [Google Scholar] [CrossRef]
- Grover, S.; Murthy, R.K.; Brar, V.S.; Chalam, K.V. Normative data for macular thickness by high-definition spectral-domain optical coherence tomography (spectralis). Am. J. Ophthalmol. 2009, 148, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Saini, V.K.; Agarwal, P.; Gupta, S. Evaluation of Central Macular Thickness and Retinal Nerve Fiber Layer Thickness using Spectral Domain Optical Coherence Tomography in a Tertiary Care Hospital. J. Curr. Glaucoma Pract. DVD 2014, 8, 75–81. [Google Scholar] [CrossRef]
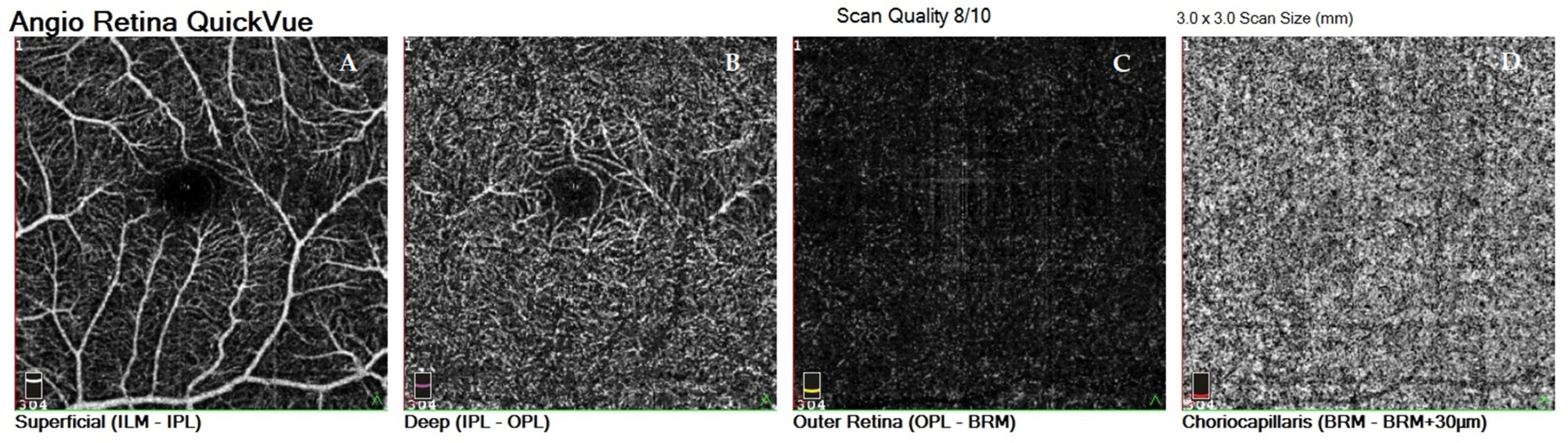

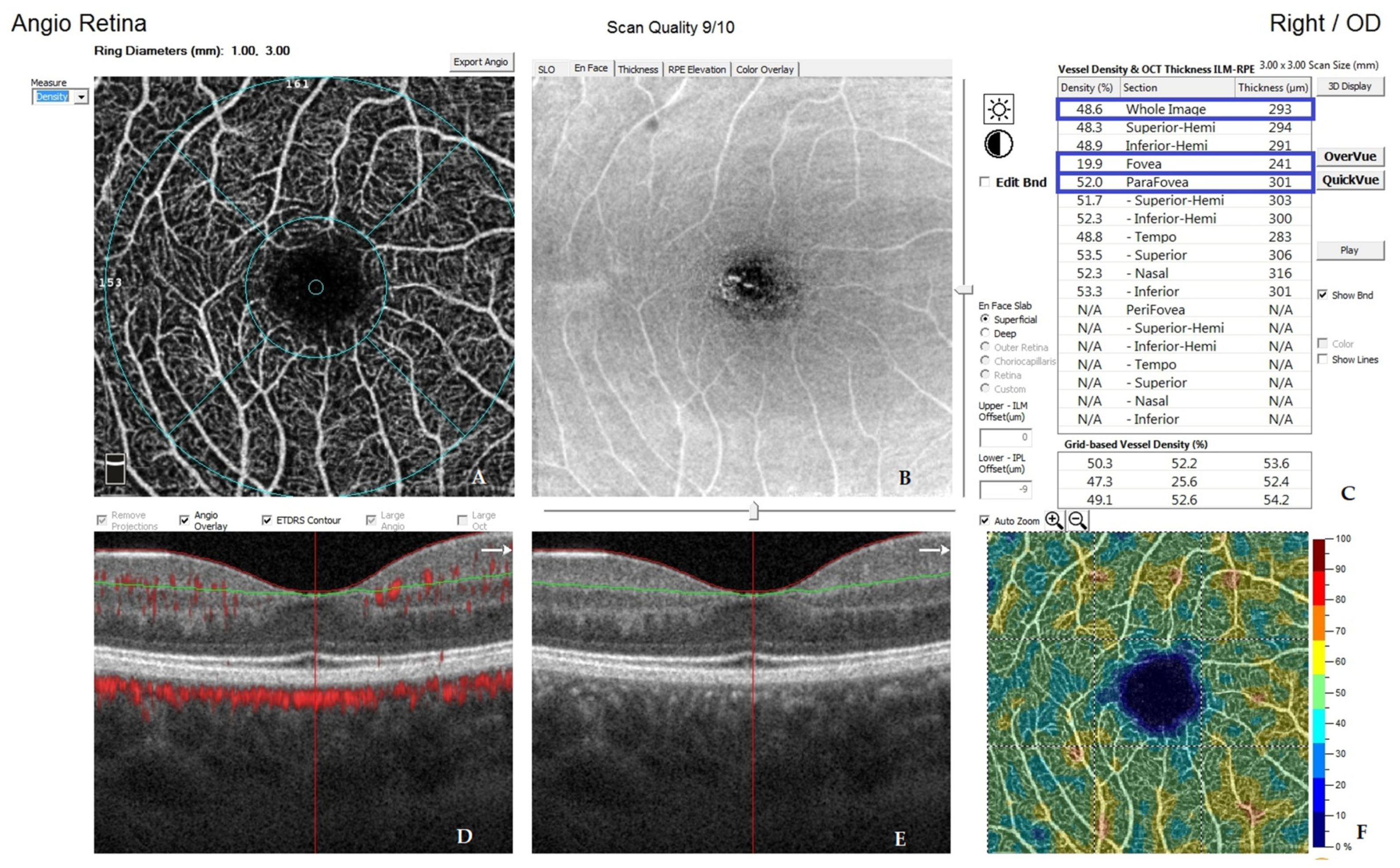
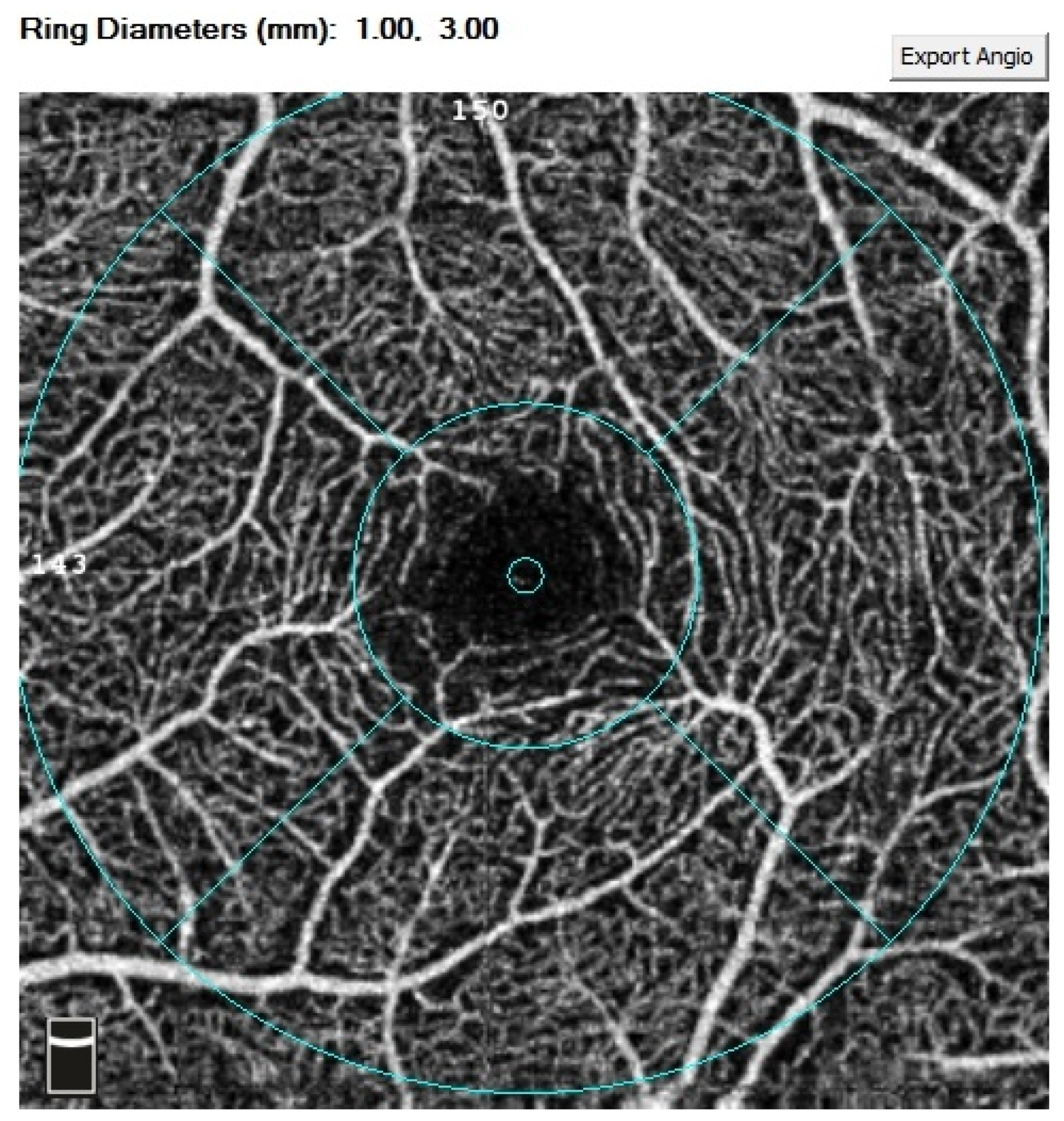
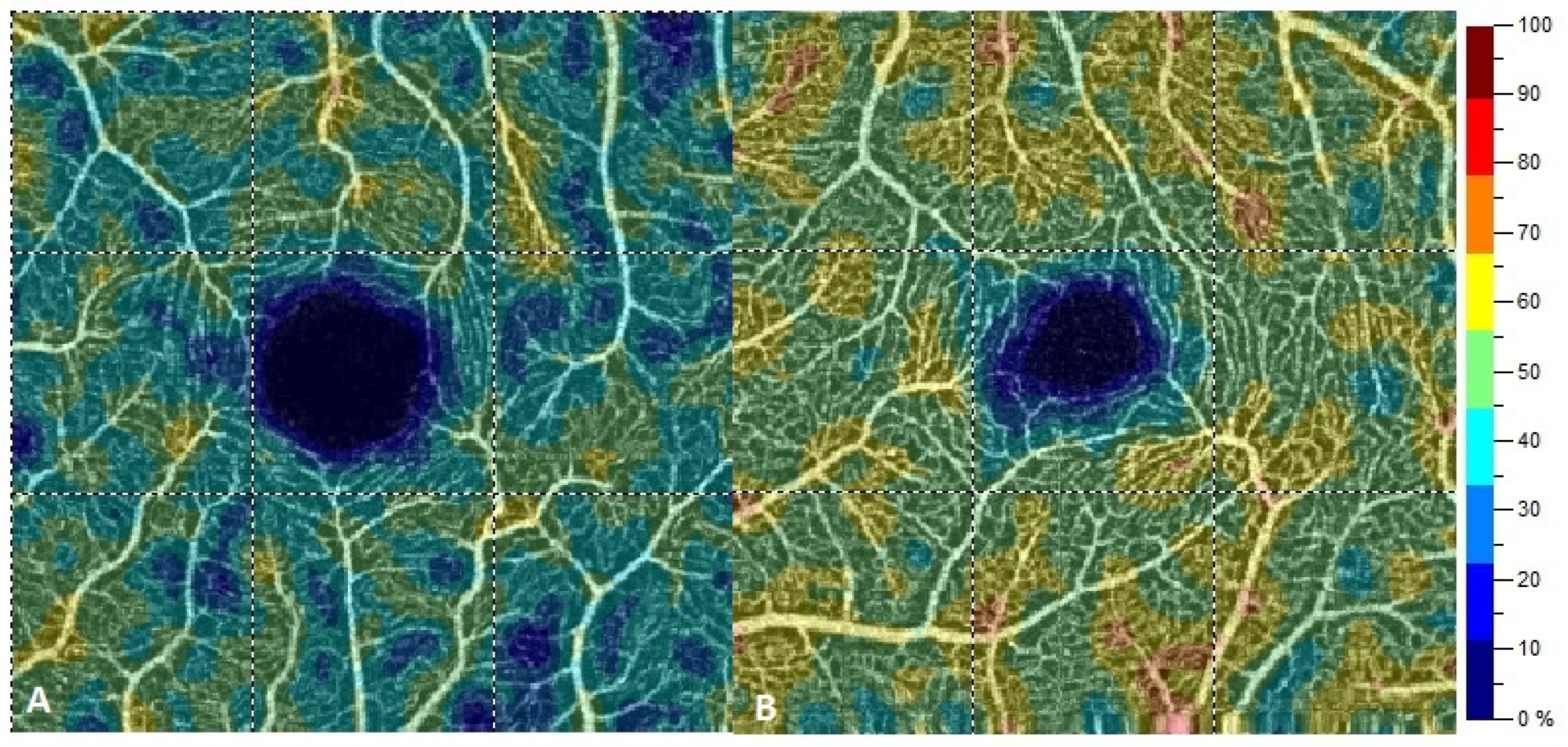


| Variable | Study Group | M | SD | 95% CI | Range | p |
|---|---|---|---|---|---|---|
| NT-proBNP (pg/mL) | DCM Group | 568.10 | 1045.27 | 177.79–958.41 | 15–3723 | - |
| LVEF (%) | DCM Group | 49.03 | 6.63 | 46.56–51.51 | 30–55 | - |
| Age (years) | Control Group | 11.27 | 3.33 | 10.02–12.51 | 4–17 | 0.11 |
| DCM Group | 9.9 | 3.57 | 8.57–11.23 | 5–17 | ||
| Biometry (mm) | Control Group | 22.75 | 0.69 | 22.49–23.01 | 21.35–24.69 | <0.05 |
| DCM Group | 22.17 | 0.88 | 21.85–22.5 | 20.615–24.08 | ||
| Spherical refractive error | Control Group | 0.12 | 0.84 | −0.19–0.44 | −1.75–1.75 | |
| DCM Group | 0.75 | 1.14 | 0.32–1.17 | −2.00–3.00 | ||
| Cylindrical refractive error | Control Group | 0.25 | 0.27 | 0.15–0.35 | 0.00–1.00 | 0.14 |
| DCM Group | 0.17 | 0.29 | 0.06–0.28 | 0.00–1.25 |
| Variable | Study Group | M | SD | 95% CI | Range | p |
|---|---|---|---|---|---|---|
| FAZ | Control Group | 0.24 | 0.09 | 0.21–0.28 | 0.087–0.445 | 0.2 |
| DCM Group | 0.27 | 0.07 | 0.24–0.3 | 0.138–0.4795 | ||
| wsVD | Control Group | 49.83 | 1.32 | 49.33–50.32 | 47.65–53.15 | <0.05 |
| DCM Group | 46.2 | 2.24 | 45.36–47.04 | 41.95–52.2 | ||
| fsVD | Control Group | 24.15 | 5.18 | 22.21–26.08 | 15.4–35.5 | |
| DCM Group | 18.07 | 5.09 | 16.16–19.97 | 9.35–27.3 | ||
| psVD | Control Group | 52.51 | 1.25 | 52.04–52.97 | 50.15–54.7 | |
| DCM Group | 49.24 | 2.5 | 48.31–50.18 | 43.7–55.75 | ||
| WT | Control Group | 323.55 | 13.16 | 318.64–328.46 | 294–342 | |
| DCM Group | 311.03 | 14.74 | 305.53–316.54 | 281.5–334 | ||
| FT | Control Group | 256.98 | 18.46 | 250.09–263.88 | 222.5–296.5 | |
| DCM Group | 244.57 | 15.56 | 238.76–250.38 | 221–285.5 | ||
| PFT | Control Group | 332.02 | 15.98 | 326.05–337.99 | 291–354.5 | |
| DCM Group | 320.63 | 15.19 | 314.96–326.31 | 292–344 | ||
| wdPD | Control Group | 50.04 | 3.71 | 48.65–51.42 | 41.05–56.1 | 0.55 |
| DCM Group | 49.7 | 2.98 | 48.58–50.81 | 44.8–54.4 | ||
| fdVD | Control Group | 36.62 | 5.36 | 34.61–38.62 | 24.9–45.6 | 0.08 |
| DCM Group | 33.94 | 5.37 | 31.93–35.95 | 18.95–41.35 | ||
| pdVD | Control Group | 51.85 | 3.79 | 50.44–53.27 | 42.1–58.55 | 0.79 |
| DCM Group | 52.13 | 3.11 | 50.97–53.29 | 46.15–56.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakusiewicz, K.; Kanigowska, K.; Hautz, W.; Ziółkowska, L. The Impact of Chronic Heart Failure on Retinal Vessel Density Assessed by Optical Coherence Tomography Angiography in Children with Dilated Cardiomyopathy. J. Clin. Med. 2021, 10, 2659. https://doi.org/10.3390/jcm10122659
Rakusiewicz K, Kanigowska K, Hautz W, Ziółkowska L. The Impact of Chronic Heart Failure on Retinal Vessel Density Assessed by Optical Coherence Tomography Angiography in Children with Dilated Cardiomyopathy. Journal of Clinical Medicine. 2021; 10(12):2659. https://doi.org/10.3390/jcm10122659
Chicago/Turabian StyleRakusiewicz, Klaudia, Krystyna Kanigowska, Wojciech Hautz, and Lidia Ziółkowska. 2021. "The Impact of Chronic Heart Failure on Retinal Vessel Density Assessed by Optical Coherence Tomography Angiography in Children with Dilated Cardiomyopathy" Journal of Clinical Medicine 10, no. 12: 2659. https://doi.org/10.3390/jcm10122659
APA StyleRakusiewicz, K., Kanigowska, K., Hautz, W., & Ziółkowska, L. (2021). The Impact of Chronic Heart Failure on Retinal Vessel Density Assessed by Optical Coherence Tomography Angiography in Children with Dilated Cardiomyopathy. Journal of Clinical Medicine, 10(12), 2659. https://doi.org/10.3390/jcm10122659








