Ability of Carotid Corrected Flow Time to Predict Fluid Responsiveness in Patients Mechanically Ventilated Using Low Tidal Volume after Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Postoperative Management
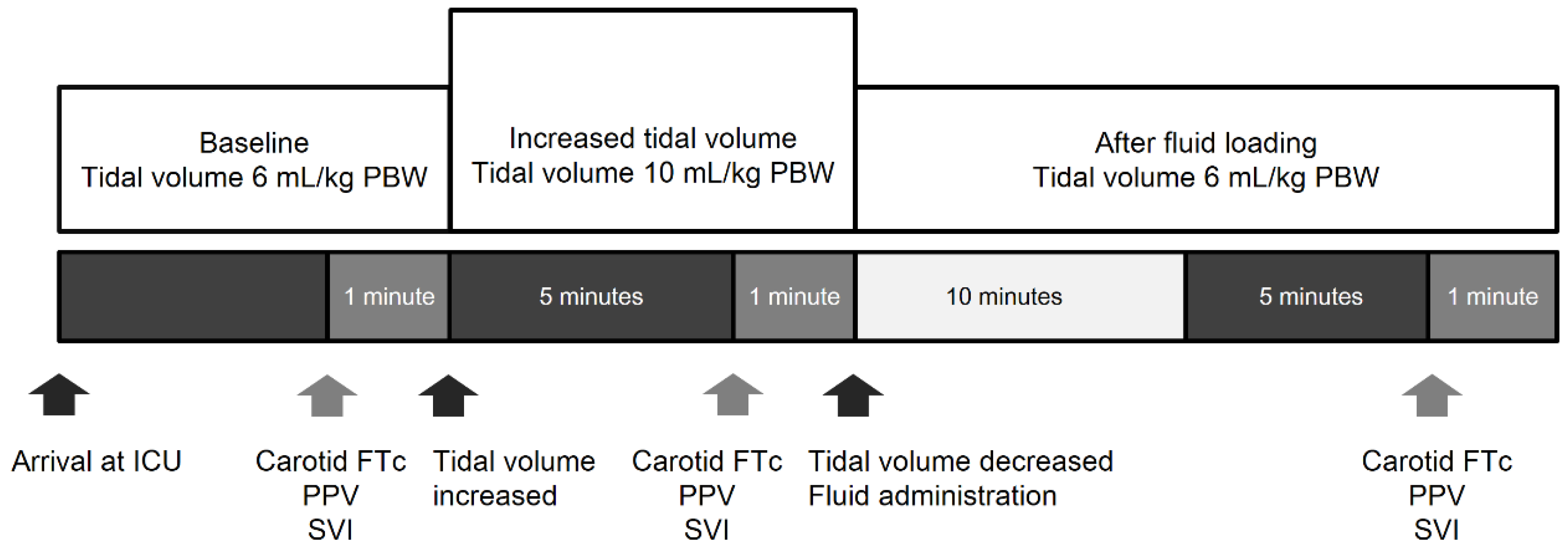
2.3. Study Protocol
2.4. Carotid Ultrasonography
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Hemodynamic Changes and Respiratory Variables
3.3. Prediction of Fluid Responsiveness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vincent, J.L.; De Backer, D. Circulatory shock. N. Engl. J. Med. 2013, 369, 1726–1734. [Google Scholar] [CrossRef] [Green Version]
- Messmer, A.S.; Zingg, C.; Müller, M.; Gerber, J.L.; Schefold, J.C.; Pfortmueller, C.A. Fluid overload and mortality in adult critical care patients—A systematic review and meta-analysis of observational studies. Crit. Care Med. 2020, 48, 1862–1870. [Google Scholar] [CrossRef]
- Malbrain, M.L.; Marik, P.E.; Witters, I.; Cordemans, C.; Kirkpatrick, A.W.; Roberts, D.J.; Van Regenmortel, N. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: A systematic review with suggestions for clinical practice. Anestezjol. Intensywna Ter. 2014, 46, 361–380. [Google Scholar] [CrossRef]
- Michard, F. Changes in arterial pressure during mechanical ventilation. Anesthesiology 2005, 103, 419–428. [Google Scholar] [CrossRef]
- De Backer, D.; Heenen, S.; Piagnerelli, M.; Koch, M.; Vincent, J.L. Pulse pressure variations to predict fluid responsiveness: Influence of tidal volume. Intensiv. Care Med. 2005, 31, 517–523. [Google Scholar] [CrossRef]
- Jozwiak, M.; Monnet, X.; Teboul, J.L. Prediction of fluid responsiveness in ventilated patients. Ann. Transl. Med. 2018, 6, 352. [Google Scholar] [CrossRef] [PubMed]
- Jalil, B.A.; Cavallazzi, R. Predicting fluid responsiveness: A review of literature and a guide for the clinician. Am. J. Emerg. Med. 2018, 36, 2093–2102. [Google Scholar] [CrossRef]
- Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.S.; Simonis, F.D.; Barbas, C.S.; Biehl, M.; Determann, R.M.; Elmer, J.; Friedman, G.; Gajic, O.; Goldstein, J.N.; Linko, R.; et al. Lung-protective ventilation with low tidal volumes and the occurrence of pulmonary complications in patients without acute respiratory distress syndrome: A systematic review and individual patient data analysis. Crit. Care Med. 2015, 43, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Chi, C.; Guo, L.; Guo, L.; Zhao, N.; Wang, W.; Pi, X.; Sun, B.; Lian, A.; et al. Lung ventilation strategies for acute respiratory distress syndrome: A systematic review and network meta-analysis. Sci. Rep. 2016, 6, 22855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shokoohi, H.; Berry, G.W.; Shahkolahi, M.; King, J.; King, J.; Salimian, M.; Poshtmashad, A.; Pourmand, A. The diagnostic utility of sonographic carotid flow time in determining volume responsiveness. J. Crit. Care 2017, 38, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Shin, S.; Kim, N.; Choi, T.; Choi, S.H.; Choi, Y.S. Carotid ultrasound measurements for assessing fluid responsiveness in spontaneously breathing patients: Corrected flow time and respirophasic variation in blood flow peak velocity. Br. J. Anaesth. 2018, 121, 541–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doctor, M.; Siadecki, S.D.; Cooper, D., Jr.; Rose, G.; Drake, A.B.; Ku, M.; Suprun, M.; Saul, T. Reliability, laterality and the effect of respiration on the measured corrected flow time of the carotid arteries. J. Emerg. Med. 2017, 53, 91–97. [Google Scholar] [CrossRef]
- Barjaktarevic, I.; Toppen, W.E.; Hu, S.; Aquije Montoya, E.; Ong, S.; Buhr, R.; David, I.J.; Wang, T.; Rezayat, T.; Chang, S.Y.; et al. Ultrasound assessment of the change in carotid corrected flow time in fluid responsiveness in undifferentiated shock. Crit. Care Med. 2018, 46, e1040–e1046. [Google Scholar] [CrossRef] [PubMed]
- Aboy, M.; McNames, J.; Thong, T.; Phillips, C.R.; Ellenby, M.S.; Goldstein, B. A novel algorithm to estimate the pulse pressure variation index deltapp. IEEE Trans. Bio-Med. Eng. 2004, 51, 2198–2203. [Google Scholar] [CrossRef]
- Blehar, D.J.; Glazier, S.; Gaspari, R.J. Correlation of corrected flow time in the carotid artery with changes in intravascular volume status. J. Crit. Care 2014, 29, 486–488. [Google Scholar] [CrossRef]
- Mohammadinejad, P.; Hossein-Nejad, H. Calculation of corrected flow time: Wodey’s formula vs. Bazett’s formula. J. Crit. Care 2018, 44, 154–155. [Google Scholar] [CrossRef]
- Yang, S.Y.; Shim, J.K.; Song, Y.; Seo, S.J.; Kwak, Y.L. Validation of pulse pressure variation and corrected flow time as predictors of fluid responsiveness in patients in the prone position. Br. J. Anaesth. 2012, 110, 713–720. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Kwak, Y.L.; Song, J.W.; Kim, Y.J.; Shim, J.K. Respirophasic carotid artery peak velocity variation as a predictor of fluid responsiveness in mechanically ventilated patients with coronary artery disease. Br. J. Anaesth. 2014, 113, 61–66. [Google Scholar] [CrossRef] [Green Version]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Carpenter, J.; Bithell, J. Bootstrap confidence intervals: When, which, what? A practical guide for medical statisticians. Stat. Med. 2000, 19, 1141–1164. [Google Scholar] [CrossRef]
- Neto, A.S.; Schultz, M.J.; de Abreu, M.G. Intraoperative ventilation strategies to prevent postoperative pulmonary complications: Systematic review, meta-analysis, and trial sequential analysis. Best Pract. Res. Clin. Anaesthesiol. 2015, 29, 331–340. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, L.Q.; Li, G.Q.; Yu, X.; Li, G.F.; Li, Y.M. Pulse pressure variation adjusted by respiratory changes in pleural pressure, rather than by tidal volume, reliably predicts fluid responsiveness in patients with acute respiratory distress syndrome. Crit. Care Med. 2016, 44, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Myatra, S.N.; Prabu, N.R.; Divatia, J.V.; Monnet, X.; Kulkarni, A.P.; Teboul, J.L. The changes in pulse pressure variation or stroke volume variation after a "tidal volume challenge" reliably predict fluid responsiveness during low tidal volume ventilation. Crit. Care Med. 2017, 45, 415–421. [Google Scholar] [CrossRef]
- Valtier, B.; Cholley, B.P.; Belot, J.P.; de la Coussaye, J.E.; Mateo, J.; Payen, D.M. Noninvasive monitoring of cardiac output in critically ill patients using transesophageal doppler. Am. J. Respir. Crit. Care Med. 1998, 158, 77–83. [Google Scholar] [CrossRef]
- Dark, P.M.; Singer, M. The validity of trans-esophageal doppler ultrasonography as a measure of cardiac output in critically ill adults. Intensiv. Care Med. 2004, 30, 2060–2066. [Google Scholar] [CrossRef]
- Jalil, B.; Thompson, P.; Cavallazzi, R.; Marik, P.; Mann, J.; El-Kersh, K.; Guardiola, J.; Saad, M. Comparing changes in carotid flow time and stroke volume induced by passive leg raising. Am. J. Med. Sci. 2018, 355, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Kenny, J.-É.S.; Barjaktarevic, I.; Mackenzie, D.C.; Eibl, A.M.; Parrotta, M.; Long, B.F.; Eibl, J.K. Diagnostic characteristics of 11 formulae for calculating corrected flow time as measured by a wearable doppler patch. Intensiv. Care Med. Exp. 2020, 8, 1–11. [Google Scholar] [CrossRef]
- Mayer, J.; Boldt, J.; Poland, R.; Peterson, A.; Manecke, G.R., Jr. Continuous arterial pressure waveform-based cardiac output using the Flotrac/Vigileo: A review and meta-analysis. J. Cardiothorac. Vasc. Anesth. 2009, 23, 401–406. [Google Scholar] [CrossRef]
- Sangkum, L.; Liu, G.L.; Yu, L.; Yan, H.; Kaye, A.D.; Liu, H. Minimally invasive or noninvasive cardiac output measurement: An update. J. Anesth. 2016, 30, 461–480. [Google Scholar] [CrossRef]
- Lamia, B.; Kim, H.K.; Severyn, D.A.; Pinsky, M.R. Cross-comparisons of trending accuracies of continuous cardiac-output measurements: Pulse contour analysis, bioreactance, and pulmonary-artery catheter. J. Clin. Monit. 2018, 32, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Krige, A.; Bland, M.; Fanshawe, T. Fluid responsiveness prediction using Vigileo Flotrac measured cardiac output changes during passive leg raise test. J. Intensiv. Care 2016, 4, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamoto, S.; Tatara, T.; Okamoto, T.; Hirose, M. Complex effects of continuous vasopressor infusion on fluid responsiveness during liver resection: A randomised controlled trial. Eur. J. Anaesthesiol. 2019, 36, 667–675. [Google Scholar] [CrossRef]
- Kenny, J.S.; Barjaktarevic, I.; Eibl, A.M.; Parrotta, M.; Long, B.F.; Eibl, J.K.; Michard, F. A carotid doppler patch accurately tracks stroke volume changes during a preload-modifying maneuver in healthy volunteers. Crit. Care Explor. 2020, 2, e0072. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.; Sunkaria, R.K. Detection of onset, systolic peak and dicrotic notch in arterial blood pressure pulses. Meas. Control. 2017, 50, 170–176. [Google Scholar] [CrossRef] [Green Version]
| Overall (n = 36) | Responders (n = 16) | Non-Responders (n = 20) | p-Value | |
|---|---|---|---|---|
| Female, n (%) | 14 (38.9) | 5 (31.2) | 9 (45.0) | 0.619 |
| Age (year) | 55.0 ± 11.5 | 52.8 ± 13.0 | 56.9 ± 10.2 | 0.295 |
| Height (cm) | 165.6 ± 8.2 | 167.6 ± 6.6 | 164.0 ± 9.2 | 0.200 |
| Weight (kg) | 62.9 ± 12.0 | 65.7 ± 12.0 | 60.6 ± 11.9 | 0.210 |
| BMI (kg/m2) | 22.9 ± 2.9 | 23.3 ± 3.2 | 22.4 ± 3.0 | 0.411 |
| ASA physical status (I/II/III) | 13/18/5 | 5/10/1 | 8/8/4 | 0.343 |
| APACHE II score at ICU admission | 18.1 ± 4.9 | 17.4 ± 5.9 | 18.7 ± 4.0 | 0.469 |
| Diagnosis, n (%) | 0.550 | |||
| Head and neck cancer | 31 (86.1) | 13 (81.3) | 18 (90.0) | |
| Facial bone fracture | 1 (2.8) | 0 (0) | 1 (5.0) | |
| Intra-abdominal cancer | 3 (8.3) | 2 (12.5) | 1 (5.0) | |
| Cervical disc herniation | 1 (2.8) | 1 (6.3) | 0 (0) | |
| Comorbidities, n (%) | ||||
| Hypertension | 12 (33.3) | 7 (43.8) | 5 (25.0) | 0.406 |
| Diabetes mellitus | 5 (13.9) | 2 (12.5) | 3 (15.0) | >0.999 |
| Coronary artery disease | 1 (2.8) | 0 (0) | 1 (5.0) | >0.999 |
| Medications, n (%) | ||||
| Calcium channel blockers | 9 (25.0) | 5 (31.3) | 4 (20.0) | 0.699 |
| β-blockers | 2 (5.6) | 1 (6.3) | 1 (5.0) | >0.999 |
| Angiotensin receptor blockers | 8 (22.2) | 5 (31.3) | 3 (6.0) | 0.446 |
| Statin | 7 (19.4) | 2 (12.5) | 5 (25.0) | 0.605 |
| Fluid volume administered (mL/h) * | 282.7 [243.2−344.8] | 282.1 [222.4−376.3] | 282.7 [245.1−335.7] | 0.863 |
| Urine output (mL/h) † | 74.0 [60.9–136.2] | 74.0 [63.0–122.9] | 75.0 [59.6–154.8] | 0.814 |
| Blood loss (mL) | 110.0 [50.0−350.0] | 350.0 (75.0−650.0) | 100.0 (50.0−160.0) | 0.110 |
| Amount of pRBCs transfused (mL) | 0.0 [0.0−0.0] | 0.0 [0.0−0.0] | 0.0 [0.0−0.0] | 0.199 |
| Patients received vasopressors during surgery, n (%) | 17 (47.2) | 7 (43.8) | 10 (50.0) | 0.970 |
| Anesthesia time (min) | 455.0 [342.5−605.0] | 510.0 [415.0−905.0] | 390.0 [317.5−492.5] | 0.012 |
| Baseline | Increased VT Ventilation | After Fluid Loading | |
|---|---|---|---|
| VT, 6 mL/kg PBW | VT, 10 mL/kg PBW | VT, 6 mL/kg PBW | |
| Heart rate (beats/min) | |||
| Responders | 91.1 ± 16.0 | 91.4 ± 16.3 | 82.9 ± 13.8 ‡,§ |
| Non-responders | 81.4 ± 13.7 | 82.5 ± 15.6 | 78.7 ± 12.4 § |
| Mean arterial pressure (mmHg) | |||
| Responders | 77.3 ± 18.4 | 72.3 ± 18.2 *,† | 80.8 ± 15.8 § |
| Non-responders | 87.3 ± 12.6 | 83.8 ± 11.6† | 82.6 ± 10.3 ‡ |
| Driving pressure (cmH2O) | |||
| Responders | 7.0 [7.0−9.0] | 12.0 [10.5−13.0] † | 7.0 [7.0−8.5] § |
| Non-responders | 8.0 [6.0−8.0] | 13.0 [10.5−14.0] † | 8.0 [7.0−9.0] § |
| Respiratory system compliance (mL/cmH2O) | |||
| Responders | 57.0 [38.4−73.6] | 59.0 [51.6−79.0] | 59.2 [38.9−70.8] |
| Non-responders | 50.9 [44.7−71.4] | 54.5 [47.7−68.3] | 52.3 [43.3−72.3] |
| Stroke volume index (mL/m2) | |||
| Responders | 36.5 [33.0−38.5] * | 36.0 [31.0−39.0] | 47.5 [41.0−52.0] ‡,§ |
| Non-responders | 41.5 [38.0−51.5] | 40.0 [35.0−50.0] † | 45.0 [38.0−53.0] ‡,§ |
| FTcB (ms) | |||
| Responders | 329.3 ± 19.0* | 328.9 ± 18.8 * | 362.3 ± 20.9 ‡,§ |
| Non-responders | 363.5 ± 21.1 | 362.8 ± 20.0 | 366.7 ± 25.3 |
| FTcW (ms) | |||
| Responders | 311.8 ± 18.3 * | 310.1 ± 18.6 * | 341.8 ± 25.9 ‡,§ |
| Non-responders | 344.3 ± 22.7 | 342.8 ± 21.5 | 348.3 ± 27.8 |
| PPV (%) | |||
| Responders | 9.0 [7.0−13.0] * | 16.0 [14.0−22.5] *,† | 6.0 [5.0−9.5] *,‡,§ |
| Non-responders | 6.5 [5.0−9.0] | 9.5 [7.0−13.0] † | 5.0 [4.0−6.5] ‡,§ |
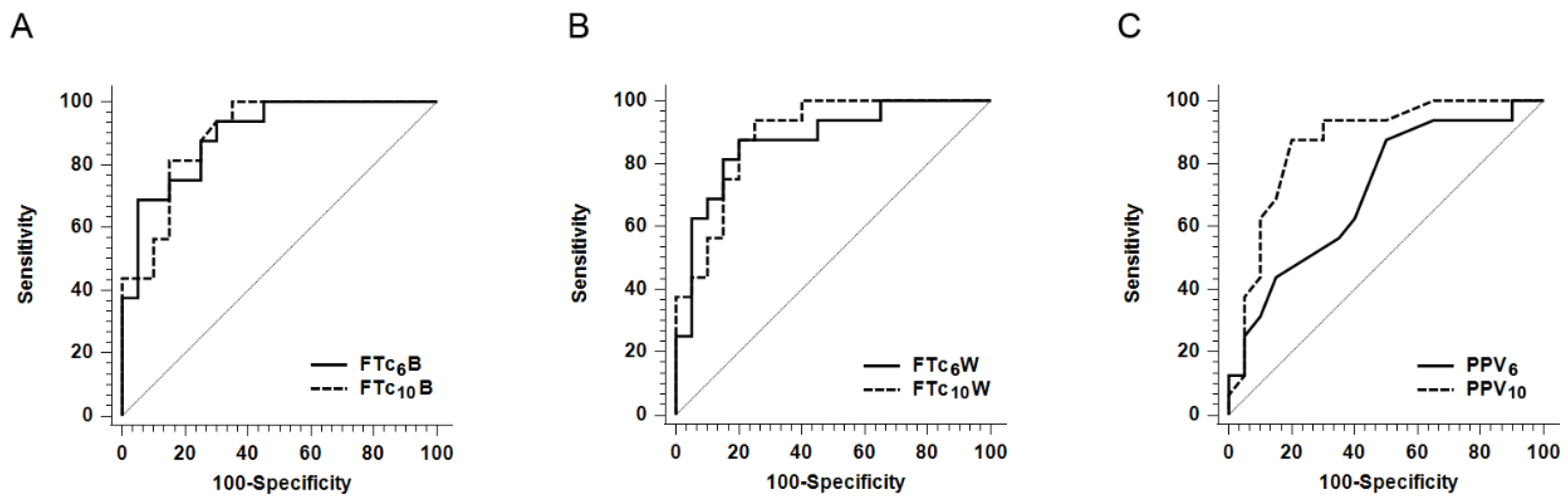
| AUROC (95% CI) | p-Value | Cut-Off Value * | Grey Zone † | Patients in Grey Zone (%) | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | |
|---|---|---|---|---|---|---|---|
| FTc6B | 0.897 (0.750–0.973) | <0.0001 | 338.5 ms | 317.5−350.7 ms | 13 (36) | 68.8 (41.3–89.0) | 95.0 (75.1–99.9) |
| FTc10B | 0.895 (0.748–0.972) | <0.0001 | 345.1 ms | 316.6−355.6 ms | 16 (44) | 81.3 (54.4–96.0) | 85.0 (62.1–96.8) |
| FTc6W | 0.875 (0.722–0.961) | <0.0001 | 325.8 ms | 322.9−349.4 ms | 12 (33) | 87.5 (61.7–98.4) | 80.0 (56.3–94.3) |
| FTc10W | 0.891 (0.744–0.970) | <0.0001 | 335.8 ms | 328.7−339.4 ms | 8 (22) | 93.8 (69.8–99.8) | 75.0 (50.9–91.3) |
| PPV6 | 0.714 (0.539–0.852) | 0.0139 | 6% | 5−9% | 23 (64) | 87.5 (61.7–98.4) | 50.0 (27.2–72.8) |
| PPV10 | 0.867 (0.712–0.957) | <0.0001 | 13% | 12−17% | 14 (39) | 87.5 (51.7–98.4) | 80.0 (56.3–94.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.; Kim, J.; Na, S.; Nam, W.S.; Kim, D.-H. Ability of Carotid Corrected Flow Time to Predict Fluid Responsiveness in Patients Mechanically Ventilated Using Low Tidal Volume after Surgery. J. Clin. Med. 2021, 10, 2676. https://doi.org/10.3390/jcm10122676
Jung S, Kim J, Na S, Nam WS, Kim D-H. Ability of Carotid Corrected Flow Time to Predict Fluid Responsiveness in Patients Mechanically Ventilated Using Low Tidal Volume after Surgery. Journal of Clinical Medicine. 2021; 10(12):2676. https://doi.org/10.3390/jcm10122676
Chicago/Turabian StyleJung, Seungho, Jeongmin Kim, Sungwon Na, Won Seok Nam, and Do-Hyeong Kim. 2021. "Ability of Carotid Corrected Flow Time to Predict Fluid Responsiveness in Patients Mechanically Ventilated Using Low Tidal Volume after Surgery" Journal of Clinical Medicine 10, no. 12: 2676. https://doi.org/10.3390/jcm10122676






