Pharmacodynamics and Outcomes of a De-Escalation Strategy with Half-Dose Prasugrel or Ticagrelor in East Asians Patients with Acute Coronary Syndrome: Results from HOPE-TAILOR Trial
Abstract
:1. Introduction
2. Materials and Methods
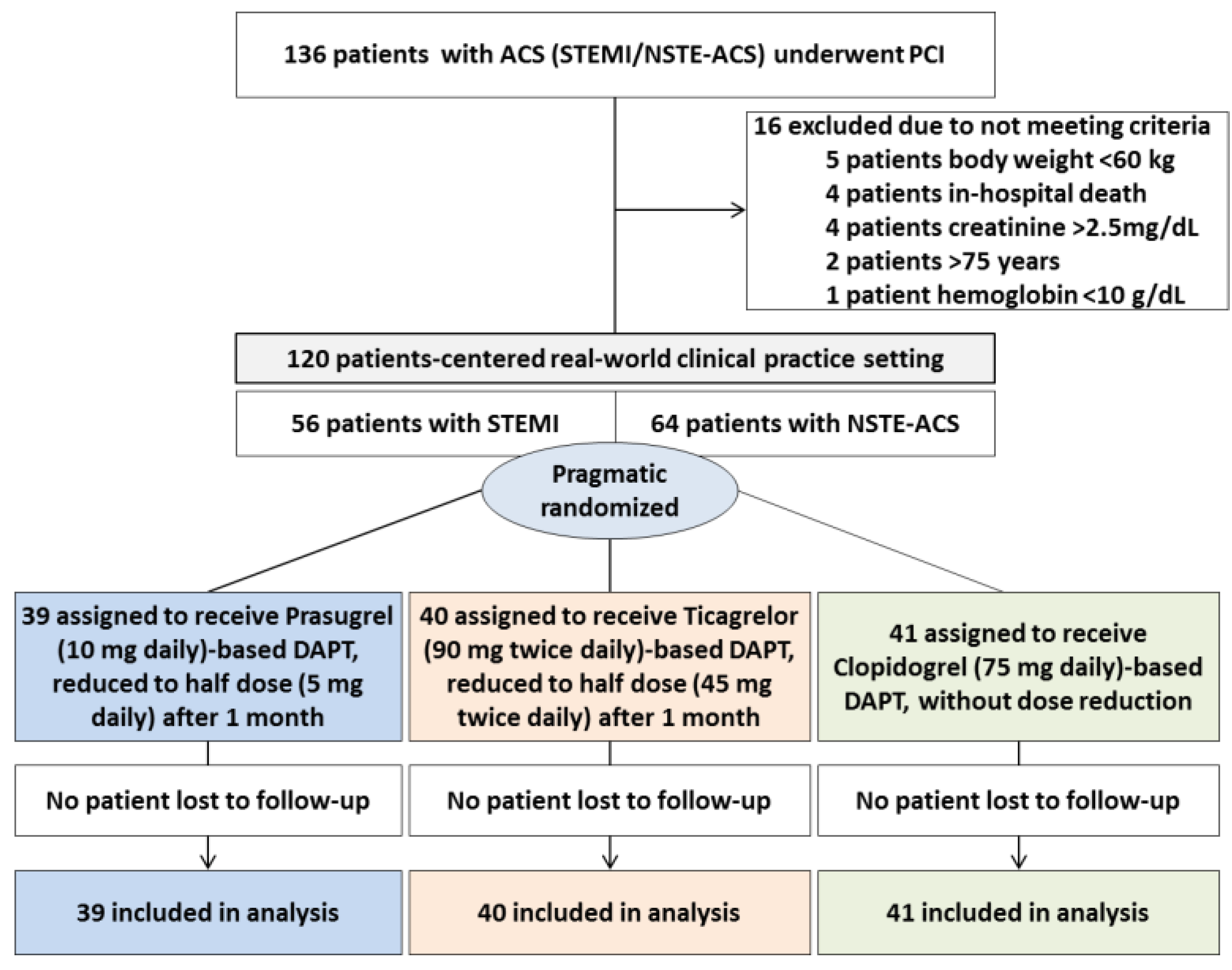
2.1. Study Design and Population
2.2. Platelet Function Test
2.3. Study Endpoint
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Pharmacodynamics Data for Oral P2Y12 Inhibitors
3.3. Clinical Outcomes
3.3.1. Safety Endpoints
3.3.2. Efficacy Endpoints
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [Green Version]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Capodanno, D.; Alfonso, F.; Levine, G.N.; Valgimigli, M.; Angiolillo, D.J. ACC/AHA Versus ESC Guidelines on Dual Antiplatelet Therapy: JACC Guideline Comparison. J. Am. Coll. Cardiol. 2018, 72 Pt A, 2915–2931. [Google Scholar] [CrossRef]
- Small, D.S.; Kothare, P.; Yuen, E.; Lachno, D.R.; Li, Y.G.; Winters, K.J.; Farid, N.A.; Ni, L.; Jakubowski, J.A.; Salazar, D.E.; et al. The pharmacokinetics and pharmacodynamics of prasugrel in healthy Chinese, Japanese, and Korean subjects compared with healthy Caucasian subjects. Eur. J. Clin. Pharmacol. 2010, 66, 127–135. [Google Scholar] [CrossRef]
- Teng, R. Pharmacokinetic, pharmacodynamic and pharmacogenetic profile of the oral antiplatelet agent ticagrelor. Clin. Pharmacokinet. 2012, 51, 305–318. [Google Scholar] [CrossRef]
- Saito, S.; Isshiki, T.; Kimura, T.; Ogawa, H.; Yokoi, H.; Nanto, S.; Takayama, M.; Kitagawa, K.; Nishikawa, M.; Miyazaki, S.; et al. Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: The PRASFIT-ACS study. Circ. J. 2014, 78, 1684–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, S.; Huang, C.H.; Park, S.J.; Emanuelsson, H.; Kimura, T. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome--randomized, double-blind, phase III PHILO study. Circ. J. 2015, 79, 2452–2460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, D.W.; Kwon, O.; Jang, J.S.; Yun, S.C.; Park, H.; Kang, D.Y.; Ahn, J.M.; Lee, P.H.; Lee, S.W.; Park, S.W.; et al. Clinically Significant Bleeding with Ticagrelor Versus Clopidogrel in Korean Patients with Acute Coronary Syndromes Intended for Invasive Management: A Randomized Clinical Trial. Circulation 2019, 140, 1865–1877. [Google Scholar] [CrossRef]
- Guo, L.Z.; Kim, M.H.; Shin, E.S.; Ann, S.H.; Jin, C.D.; Cho, Y.R.; Park, J.S.; Park, K.; Park, T.H.; Lee, M.S.; et al. Thienopyridine reloading in clopidogrel-loaded patients undergoing percutaneous coronary interventions: The PRAISE study. Int. J. Cardiol. 2016, 222, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.D.; Kim, M.H.; Guo, L.Z.; Jin, E.; Shin, E.S.; Ann, S.H.; Cho, Y.R.; Park, J.S.; Kim, S.J.; Lee, M.S. Pharmacodynamic study of prasugrel or clopidogrel in non-ST-elevation acute coronary syndrome with CYP2C19 genetic variants undergoing percutaneous coronary intervention (PRAISE-GENE trial). Int. J. Cardiol. 2020, 305, 11–17. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kim, M.H.; Cho, Y.R.; Sung Park, J.; Min Lee, K.; Park, T.H.; Yun, S.C. Performance of PRECISE-DAPT Score for Predicting Bleeding Complication During Dual Antiplatelet Therapy. Circ. Cardiovasc. Interv. 2018, 11, e006837. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Hong, S.J.; Cho, Y.H.; Yun, K.H.; Kim, Y.H.; Suh, Y.; Cho, J.Y.; Her, A.Y.; Cho, S.; Jeon, D.W.; et al. Effect of Ticagrelor Monotherapy vs Ticagrelor With Aspirin on Major Bleeding and Cardiovascular Events in Patients With Acute Coronary Syndrome: The TICO Randomized Clinical Trial. JAMA 2020, 323, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Baber, U.; Sharma, S.K.; Cohen, D.J.; Angiolillo, D.J.; Briguori, C.; Cha, J.Y.; Collier, T.; Dangas, G.; Dudek, D.; et al. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N. Engl. J. Med. 2019, 381, 2032–2042. [Google Scholar] [CrossRef]
- Jin, C.D.; Kim, M.H.; Bang, J.; Serebruany, V. A Prospective, Randomized, Open-Label, Blinded, Endpoint Study Exploring Platelet Response to Half-Dose Prasugrel and Ticagrelor in Patients with the Acute Coronary Syndrome: HOPE-TAILOR Study. Cardiology 2017, 138, 201–206. [Google Scholar] [CrossRef]
- van Werkum, J.W.; van der Stelt, C.A.; Seesing, T.H.; Hackeng, C.M.; ten Berg, J.M. A head-to-head comparison between the VerifyNow P2Y12 assay and light transmittance aggregometry for monitoring the individual platelet response to clopidogrel in patients undergoing elective percutaneous coronary intervention. J. Thromb. Haemost. 2006, 4, 2516–2518. [Google Scholar] [CrossRef]
- Tantry, U.S.; Bonello, L.; Aradi, D.; Price, M.J.; Jeong, Y.H.; Angiolillo, D.J.; Stone, G.W.; Curzen, N.; Geisler, T.; Ten Berg, J.; et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J. Am. Coll. Cardiol. 2013, 62, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Sibbing, D.; Aradi, D.; Alexopoulos, D.; Ten Berg, J.; Bhatt, D.L.; Bonello, L.; Collet, J.P.; Cuisset, T.; Franchi, F.; Gross, L.; et al. Updated Expert Consensus Statement on Platelet Function and Genetic Testing for Guiding P2Y(12) Receptor Inhibitor Treatment in Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2019, 12, 1521–1537. [Google Scholar] [CrossRef]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Ahn, S.G.; Park, B.; Park, S.W.; Kang, Y.S.; Lee, J.W.; Youn, Y.J.; Ahn, M.S.; Kim, J.Y.; Yoo, B.S.; et al. A pharmacodynamic study of the optimal P2Y12 inhibitor regimen for East Asian patients with acute coronary syndrome. Korean J. Intern. Med. 2015, 30, 620–628. [Google Scholar] [CrossRef]
- Lee, Y.S.; Jin, C.D.; Kim, M.H.; Guo, L.Z.; Cho, Y.R.; Park, K.; Park, J.S.; Park, T.H.; Kim, Y.D. Comparison of Prasugrel and Ticagrelor Antiplatelet Effects in Korean Patients Presenting With ST-Segment Elevation Myocardial Infarction. Circ. J. 2015, 79, 1248–1254. [Google Scholar] [CrossRef] [Green Version]
- Yokoi, H.; Kimura, T.; Isshiki, T.; Ogawa, H.; Ikeda, Y. Pharmacodynamic assessment of a novel P2Y12 receptor antagonist in Japanese patients with coronary artery disease undergoing elective percutaneous coronary intervention. Thromb. Res. 2012, 129, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, D.; Xanthopoulou, I.; Gkizas, V.; Kassimis, G.; Theodoropoulos, K.C.; Makris, G.; Koutsogiannis, N.; Damelou, A.; Tsigkas, G.; Davlouros, P.; et al. Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with ST-segment-elevation myocardial infarction. Circ. Cardiovasc. Interv. 2012, 5, 797–804. [Google Scholar] [CrossRef] [Green Version]
- Park, D.W.; Lee, P.H.; Jang, S.; Lim, H.S.; Kang, D.Y.; Lee, C.H.; Ahn, J.M.; Yun, S.C.; Park, S.W.; Park, S.J. Effect of Low-Dose Versus Standard-Dose Ticagrelor and Clopidogrel on Platelet Inhibition in Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2018, 71, 1594–1595. [Google Scholar] [CrossRef]
- Schupke, S.; Neumann, F.J.; Menichelli, M.; Mayer, K.; Bernlochner, I.; Wohrle, J.; Richardt, G.; Liebetrau, C.; Witzenbichler, B.; Antoniucci, D.; et al. Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2019, 381, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Dewilde, W.J.M.; Oirbans, T.; Verheugt, F.W.A.; Kelder, J.C.; De Smet, B.J.G.L.; Herrman, J.-P.; Adriaenssens, T.; Vrolix, M.; Heestermans, A.A.C.M.; Vis, M.M.; et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet 2013, 381, 1107–1115. [Google Scholar] [CrossRef]
- Urban, P.; Mehran, R.; Colleran, R.; Angiolillo, D.J.; Byrne, R.A.; Capodanno, D.; Cuisset, T.; Cutlip, D.; Eerdmans, P.; Eikelboom, J.; et al. Defining High Bleeding Risk in Patients Undergoing Percutaneous Coronary Intervention. Circulation 2019, 140, 240–261. [Google Scholar] [CrossRef]
- Sorrentino, S.; Sartori, S.; Baber, U.; Claessen, B.E.; Giustino, G.; Chandrasekhar, J.; Chandiramani, R.; Cohen, D.J.; Henry, T.D.; Guedeney, P.; et al. Bleeding Risk, Dual Antiplatelet Therapy Cessation, and Adverse Events After Percutaneous Coronary Intervention: The PARIS Registry. Circ. Cardiovasc. Interv. 2020, 13, e008226. [Google Scholar] [CrossRef]
- Kim, M.H.; Cho, S.Y.; Serebruany, V. De-Escalation of Dual Antiplatelet Regimens in East Asian Patients Undergoing Coronary Intervention. Am. J. Ther. 2020, 27, e611–e612. [Google Scholar] [CrossRef]
- Aradi, D.; Kirtane, A.; Bonello, L.; Gurbel, P.A.; Tantry, U.S.; Huber, K.; Freynhofer, M.K.; ten Berg, J.; Janssen, P.; Angiolillo, D.J.; et al. Bleeding and stent thrombosis on P2Y12-inhibitors: Collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur. Heart J. 2015, 36, 1762–1771. [Google Scholar] [CrossRef] [Green Version]
- Huo, Y.; Jeong, Y.H.; Gong, Y.; Wang, D.; He, B.; Chen, J.; Fu, G.; Chen, Y.; Li, J.; Li, Y.; et al. 2018 update of expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Sci. Bull. 2019, 64, 166–179. [Google Scholar] [CrossRef] [Green Version]
- Cuisset, T.; Deharo, P.; Quilici, J.; Johnson, T.W.; Deffarges, S.; Bassez, C.; Bonnet, G.; Fourcade, L.; Mouret, J.P.; Lambert, M.; et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: The TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur. Heart J. 2017, 38, 3070–3078. [Google Scholar] [CrossRef] [Green Version]
- Sibbing, D.; Aradi, D.; Jacobshagen, C.; Gross, L.; Trenk, D.; Geisler, T.; Orban, M.; Hadamitzky, M.; Merkely, B.; Kiss, R.G.; et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): A randomised, open-label, multicentre trial. Lancet 2017, 390, 1747–1757. [Google Scholar] [CrossRef] [Green Version]
- Angiolillo, D.J.; Rollini, F.; Storey, R.F.; Bhatt, D.L.; James, S.; Schneider, D.J.; Sibbing, D.; So, D.Y.F.; Trenk, D.; Alexopoulos, D.; et al. International Expert Consensus on Switching Platelet P2Y12 Receptor-Inhibiting Therapies. Circulation 2017, 136, 1955–1975. [Google Scholar] [CrossRef] [PubMed]
- Kupka, D.; Sibbing, D. De-Escalation of P2Y12 Receptor Inhibitor Therapy after Acute Coronary Syndromes in Patients Undergoing Percutaneous Coronary Intervention. Korean Circ. J. 2018, 48, 863–872. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Kang, J.; Hwang, D.; Han, J.K.; Yang, H.M.; Kang, H.J.; Koo, B.K.; Rhew, J.Y.; Chun, K.J.; Lim, Y.H.; et al. Prasugrel-based de-escalation of dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (HOST-REDUCE-POLYTECH-ACS): An open-label, multicentre, non-inferiority randomised trial. Lancet 2020, 396, 1079–1089. [Google Scholar] [CrossRef]


| Variable | Overall (n = 120) | Prasugrel (n = 39) | Ticagrelor (n = 40) | Clopidogrel (n = 41) | p-Value |
|---|---|---|---|---|---|
| Age, year | 60 ± 10 | 57 ± 10 a | 61 ± 9 | 63 ± 10 b | 0.050 |
| Male gender | 109 (90.8) | 37 (94.9) | 34 (85.0) | 38 (92.7) | 0.323 |
| Body weight, kg | 70.5 ± 9.6 | 72.1 ± 10.8 | 69.9 ± 9.2 | 69.7 ± 9.0 | 0.543 |
| BMI, kg/m2 | 24.8 ± 2.3 | 25.0 ± 2.4 | 24.6 ± 2.3 | 24.9 ± 2.3 | 0.802 |
| Diabetes mellitus | 29 (24.2) | 6 (15.4) | 9 (22.5) | 14 (34.1) | 0.149 |
| Hypertension | 52 (43.3) | 13 (33.3) | 21 (52.5) | 18 (43.9) | 0.230 |
| Dyslipidemia | 22 (18.3) | 7 (17.9) | 10 (25.0) | 5 (12.2) | 0.336 |
| Current smoking | 27 (22.5) | 8 (20.5) | 9 (22.5) | 10 (24.4) | 0.962 |
| Previous MI | 14 (11.7) | 4 (10.3) | 4 (10.0) | 6 (14.6) | 0.823 |
| Previous PCI | 22 (18.3) | 5 (12.8) | 8 (20.0) | 9 (22.0) | 0.581 |
| Clinical presentation | 0.001 | ||||
| UA | 32 (26.7) | 6 (15.4)a | 3 (7.5)a | 23 (56.1) b | |
| NSTEMI | 32 (26.7) | 10 (25.6) | 10 (25.0) | 12 (29.3) | |
| STEMI | 56 (46.7) | 23 (59.0) a | 27 (67.5) a | 6 (14.6) b | |
| Hemoglobin, g/dL | 13.3 ± 1.9 | 14.0 ± 2.0 | 13.5 ± 1.8 | 13.4 ± 1.8 | 0.320 |
| Platelet, 103/mm3 | 222.5 ± 53.6 | 219.6 ± 45.9 | 233.3 ± 65.3 | 216.4 ± 46.4 | 0.501 |
| Troponin I, peak, pg/mL | 10.83 (1.10–62.11) | 39.74 (4.43–88.31) a | 39.27 (6.94–77.19) a | 1.13 (0.15–8.07) b | 0.001 |
| GFR, mL/min/1.73 m2 | 83.4 ± 24.0 | 84.2 ± 23.6 | 84.0 ± 23.4 | 82.1 ± 25.8 | 0.945 |
| LDL-C, mg/dL | 109.3 ± 38.9 | 112.0 ± 31.1 | 119.7 ± 46.1 a | 96.1 ± 34.7 b | 0.023 |
| Procedural characteristics | |||||
| Radial approach | 95 (79.2) | 34 (87.2) | 29 (72.5) | 32 (78.0) | 0.469 |
| Multivessel disease | 47 (39.2) | 14 (35.9) | 16 (40.0) | 17 (41.5) | 0.892 |
| Multivessel PCI | 33 (27.5) | 11 (28.2) | 9 (22.5) | 13 (31.7) | 0.666 |
| PCI | 0.844 | ||||
| Stenting | 116 (96.7) | 38 (97.4) | 38 (95.0) | 40 (97.6) | |
| No. > 2 | 33 (27.5) | 9 (23.1) | 13 (32.5) | 11 (26.8) | 0.644 |
| Length (mm) | 22 (16–34) | 20 (17–27) | 26 (18–35) | 23 (14–40) | 0.289 |
| DCB | 2 (1.7) | 0 (0) | 1 (2.5) | 1 (2.4) | |
| POBA | 2 (1.7) | 1 (2.6) | 1 (2.5) | 0 (0) | |
| Discharge medication | |||||
| Aspirin | 120 (100.0) | 38 (100.0) | 40 (100.0) | 41 (100.0) | >0.999 |
| ACEi/ARB | 30 (25.0) | 9 (23.1) | 9 (22.5) | 12 (29.3) | 0.776 |
| Beta-blocker | 89 (74.2) | 30 (76.9) | 30 (75.0) | 28 (68.3) | 0.661 |
| Statin | 113 (94.2) | 37 (94.9) | 38 (95.0) | 38 (92.7) | 0.999 |
| Calcium channel blocker | 29 (24.4) | 9 (23.1) | 9 (23.1) | 11 (26.8) | 0.895 |
| Proton pump inhibitor | 25 (20.8) | 8 (20.5) | 10 (25.0) | 7 (17.1) | 0.692 |
| Time | P2Y12 Reaction Unit (PRU) | Overall Effect | Pairwise Comparisons (95% CI of diff.) | ||||
|---|---|---|---|---|---|---|---|
| 1 month | Prasugrel | Ticagrelor | Clopidogrel | p < 0.001 | P vs. T | P vs. C | T vs. C |
| Mean ± SD | 40.9 ± 41.4 | 20.0 ± 25.3 * | 159.0 ± 70.1 | −20.9 (−36.2 to −5.6) | 118.3 (92.5 to 144.1) | 139.2 (113.8 to 162.7) | |
| Median (IQR) | 11 (5–72) | 7 (3–30) ** | 167 (97–212) | −4 (−38 to 0) | 156 (92 to 152) | 160 (122 to 172) | |
| 3months | Prasugrel | Ticagrelor | Clopidogrel | p < 0.001 | P vs. T | P vs. C | T vs. C |
| Mean ± SD | 93.2 ± 57.1 | 31.0 ± 34.5 | 153.1 ± 69.4 | −62.3 (−41.2 to −83.3) | 59.9 (31.5 to 88.2) | 122.2 (97.8 to 146.5) | |
| Median (IQR) | 84 (47–145) | 15 (6–45) | 169 (107–199) | −69 (−81 to −39) | 85 (33 to 95) | 154 (101 to 156) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, C.-D.; Kim, M.-H.; Song, K.; Jin, X.; Lee, K.-M.; Park, J.-S.; Cho, Y.-R.; Yun, S.-C.; Lee, M.S. Pharmacodynamics and Outcomes of a De-Escalation Strategy with Half-Dose Prasugrel or Ticagrelor in East Asians Patients with Acute Coronary Syndrome: Results from HOPE-TAILOR Trial. J. Clin. Med. 2021, 10, 2699. https://doi.org/10.3390/jcm10122699
Jin C-D, Kim M-H, Song K, Jin X, Lee K-M, Park J-S, Cho Y-R, Yun S-C, Lee MS. Pharmacodynamics and Outcomes of a De-Escalation Strategy with Half-Dose Prasugrel or Ticagrelor in East Asians Patients with Acute Coronary Syndrome: Results from HOPE-TAILOR Trial. Journal of Clinical Medicine. 2021; 10(12):2699. https://doi.org/10.3390/jcm10122699
Chicago/Turabian StyleJin, Cai-De, Moo-Hyun Kim, Kai Song, Xuan Jin, Kwang-Min Lee, Jong-Sung Park, Young-Rak Cho, Sung-Cheol Yun, and Michael S. Lee. 2021. "Pharmacodynamics and Outcomes of a De-Escalation Strategy with Half-Dose Prasugrel or Ticagrelor in East Asians Patients with Acute Coronary Syndrome: Results from HOPE-TAILOR Trial" Journal of Clinical Medicine 10, no. 12: 2699. https://doi.org/10.3390/jcm10122699
APA StyleJin, C. -D., Kim, M. -H., Song, K., Jin, X., Lee, K. -M., Park, J. -S., Cho, Y. -R., Yun, S. -C., & Lee, M. S. (2021). Pharmacodynamics and Outcomes of a De-Escalation Strategy with Half-Dose Prasugrel or Ticagrelor in East Asians Patients with Acute Coronary Syndrome: Results from HOPE-TAILOR Trial. Journal of Clinical Medicine, 10(12), 2699. https://doi.org/10.3390/jcm10122699







