The Addition of Transarterial Chemoembolization to Palliative Chemotherapy Extends Survival in Intrahepatic Cholangiocarcinoma
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Characteristics
3.2. Treatment
3.3. Survival
3.4. Locoregional Therapy
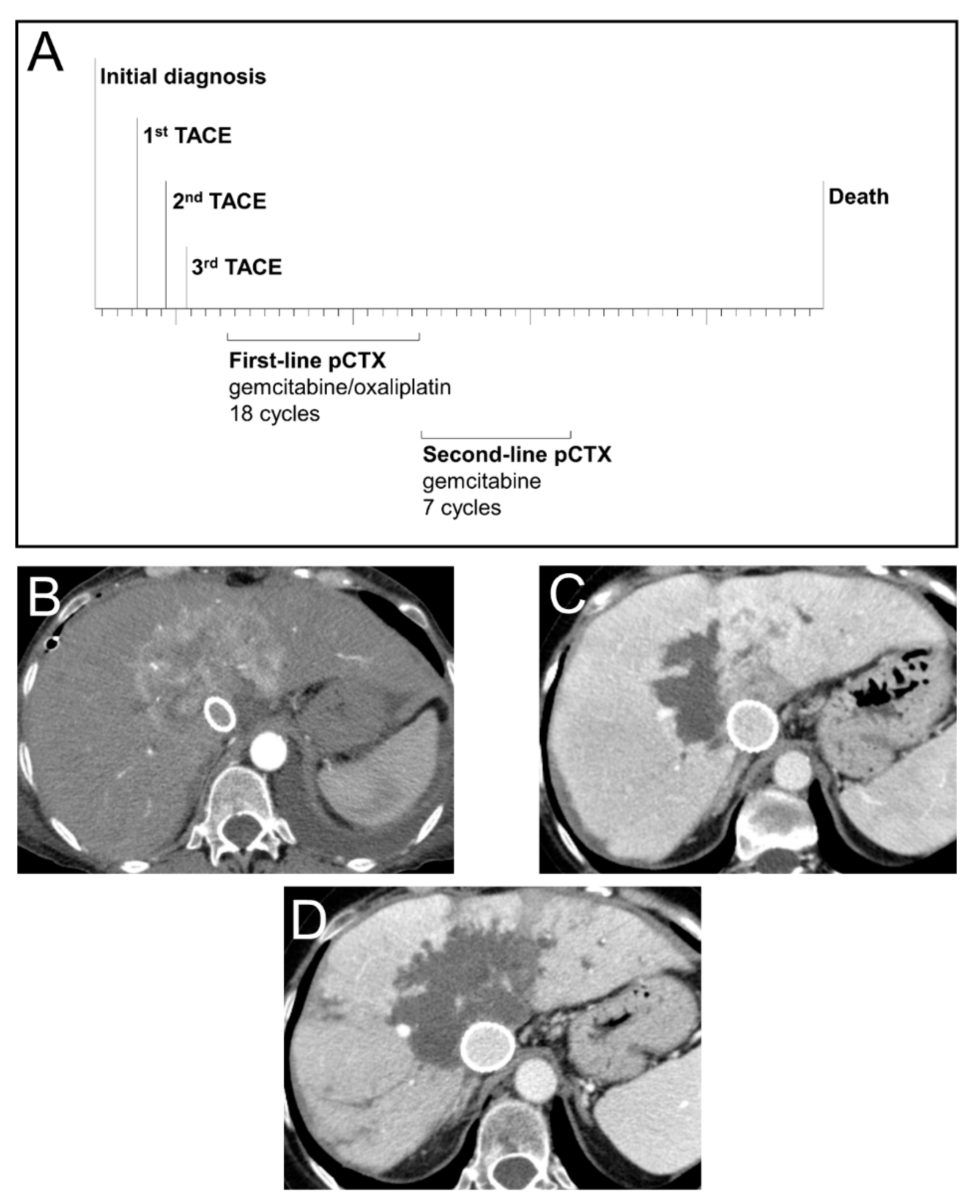
3.5. Case Study
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergquist, A.; Von Seth, E. Epidemiology of cholangiocarcinoma. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Endo, I.; Gonen, M.; Yopp, A.C.; Dalal, K.M.; Zhou, Q.; Klimstra, D.; D’Angelica, M.; DeMatteo, R.P.; Fong, Y.; Schwartz, L.; et al. Intrahepatic Cholangiocarcinoma: Rising frequency, improved survival, and determinants of outcome after resection. Ann. Surg. 2008, 248, 84–96. [Google Scholar] [CrossRef]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Okusaka, T.; Nakachi, K.; Fukutomi, A.; Mizuno, N.; Ohkawa, S.; Funakoshi, A.; Nagino, M.; Kondo, S.; Nagaoka, S.; Funai, J.; et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: A comparative multicentre study in Japan. Br. J. Cancer 2010, 103, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Borbath, I.; Khan, S.A.; Huguet, F.; Gruenberger, T.; Arnold, D. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v28–v37. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Furuse, J.; Jitlal, M.; Beare, S.; Mizuno, N.; Wasan, H.; Bridgewater, J.; Okusaka, T. Cisplatin and gemcitabine for advanced biliary tract cancer: A meta-analysis of two randomised trials. Ann. Oncol. 2014, 25, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. ABC-06|A randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin/5-FU chemotherapy (ASC + mFOLFOX) for patients (pts) with locally advanced/metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. J. Clin. Oncol. 2019, 37, 4003. [Google Scholar] [CrossRef]
- Shroff, R.T.; Javle, M.M.; Xiao, L.; Kaseb, A.O.; Varadhachary, G.R.; Wolff, R.A.; Raghav, K.P.S.; Iwasaki, M.; Masci, P.; Ramanathan, R.K.; et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Phelip, J.-M.; Edeline, J.; Blanc, J.-F.; Barbier, E.; Michel, P.; Bourgeois, V.; Neuzillet, C.; Malka, D.; Manfredi, S.; Desrame, J. Modified FOLFIRINOX versus CisGem first-line chemotherapy for locally advanced non resectable or metastatic biliary tract cancer (AMEBICA)-PRODIGE 38: Study protocol for a randomized controlled multicenter phase II/III study. Dig. Liver Dis. 2019, 51, 318–320. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Valle, J.W.; Van Cutsem, E.; Rimassa, L.; Furuse, J.; Ioka, T.; Melisi, D.; Macarulla, T.; Bridgewater, J.; Wasan, H.; et al. FIGHT-302: First-line pemigatinib vs gemcitabine plus cisplatin for advanced cholangiocarcinoma with FGFR2 rearrangements. Future Oncol. 2020, 16, 2385–2399. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Mazzaferro, V.; El-Rayes, B.F.; Droz Dit Busset, M.; Cotsoglou, C.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Personeni, N.; Braiteh, F.; et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer 2019, 120, 165–171. [Google Scholar] [CrossRef]
- Droz Dit Busset, M.; Braun, S.; El-Rayes, B.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Bhoori, S.; Niger, M.; Personeni, N.; et al. Efficacy of derazantinib (DZB) in patients (pts) with intrahepatic cholangiocarcinoma (iCCA) expressing FGFR2-fusion or FGFR2 mutations/amplifications. Ann. Oncol. 2019, 30, v276–v277. [Google Scholar] [CrossRef]
- Makawita, S.; Abou-Alfa, G.K.; Roychowdhury, S.; Sadeghi, S.; Borbath, I.; Goyal, L.; Cohn, A.; Lamarca, A.; Oh, D.-Y.; Macarulla, T.; et al. Infigratinib in patients with advanced cholangiocarcinoma with FGFR2 gene fusions/translocations: The PROOF 301 trial. Future Oncol. 2020, 16, 2375–2384. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Kelley, R.K.; Roychowdhury, S.; Weiss, K.H.; Abou-Alfa, G.K.; Macarulla, T.; Sadeghi, S.; Waldschmidt, D.; Zhu, A.X.; Goyal, L.; et al. AB051. P-19. A phase II study of infigratinib (BGJ398) in previously-treated advanced cholangiocarcinoma containing FGFR2 fusions. HepatoBiliary Surg. Nutr. 2019, 8, AB051. [Google Scholar] [CrossRef]
- Javle, M.; Lowery, M.; Shroff, R.T.; Weiss, K.H.; Springfeld, C.; Borad, M.J.; Ramanathan, R.K.; Goyal, L.; Sadeghi, S.; Macarulla, T.; et al. Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. J. Clin. Oncol. 2018, 36, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.H.; Hierro, C.; Heist, R.S.; Cleary, J.M.; Meric-Bernstam, F.; Tabernero, J.; Janku, F.; Gandhi, L.; Iafrate, A.J.; Borger, D.R.; et al. A Phase I, Open-Label, Multicenter, Dose-escalation Study of the Oral Selective FGFR Inhibitor Debio 1347 in Patients with Advanced Solid Tumors Harboring FGFR Gene Alterations. Clin. Cancer Res. 2019, 25, 2699–2707. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.C.H.; Goyal, L.; Bang, Y.-J.; Oh, D.-Y.; Chao, T.-Y.; Cleary, J.M.; Voss, M.H.; Meric-Bernstam, F.; Iyer, G.; Heist, R.S.; et al. AB065. P-36. Debio 1347 in patients with cholangiocarcinoma harboring an FGFR gene alteration: Preliminary results. HepatoBiliary Surg. Nutr. 2019, 8, AB065. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Arkenau, H.; Tran, B.; Bahleda, R.; Kelley, R.K.; Hierro, C.; Ahn, D.; Zhu, A.; Javle, M.; Winkler, R.; et al. Efficacy of TAS-120, an irreversible fibroblast growth factor receptor (FGFR) inhibitor, in cholangiocarcinoma patients with FGFR pathway alterations who were previously treated with chemotherapy and other FGFR inhibitors. Ann. Oncol. 2018, 29, v100. [Google Scholar] [CrossRef]
- Tran, B.; Meric-Bernstam, F.; Arkenau, H.-T.; Bahleda, R.; Kelley, R.; Hierro, C.; Ahn, D.; Zhu, A.; Javle, M.; Winkler, R.; et al. Efficacy of TAS-120, an irreversible fibroblast growth factor receptor inhibitor (FGFRi), in patients with cholangiocarcinoma and FGFR pathway alterations previously treated with chemotherapy and other FGFRi’s. Ann. Oncol. 2018, 29, ix49–ix50. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Kloeckner, R.; Galle, P.R.; Bruix, J. Local and Regional Therapies for Hepatocellular Carcinoma. Hepatology 2021, 73, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Kim, J.H.; Yoon, H.-J.; Lee, I.-S.; Yoon, H.-K.; Kim, K.-P. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin. Radiol. 2011, 66, 322–328. [Google Scholar] [CrossRef]
- Ray, C.E.; Edwards, A.; Smith, M.T.; Leong, S.; Kondo, K.; Gipson, M.; Rochon, P.J.; Gupta, R.; Messersmith, W.; Purcell, T.; et al. Metaanalysis of Survival, Complications, and Imaging Response following Chemotherapy-based Transarterial Therapy in Patients with Unresectable Intrahepatic Cholangiocarcinoma. J. Vasc. Interv. Radiol. 2013, 24, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, M.V.; Albert, M.; McNally, M.; Robertson, M.; Sun, W.; Fraker, D.; Olthoff, K.; Christians, K.; Pappas, S.; Rilling, W.; et al. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol: A 2-center study. Cancer 2011, 117, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Knüppel, M.; Kubicka, S.; Vogel, A.; Malek, N.P.; Schneider, M.; Papendorf, F.; Greten, T.; Wedemeyer, J.; Schneider, A. Combination of Conservative and Interventional Therapy Strategies for Intra- and Extrahepatic Cholangiocellular Carcinoma: A Retrospective Survival Analysis. Gastroenterol. Res. Prat. 2012, 2012, 19070. [Google Scholar] [CrossRef][Green Version]
- Schiffman, S.C.; Metzger, T.; Dubel, G.; Andrasina, T.; Kralj, I.; Tatum, C.; McMasters, K.M.; Scoggins, C.R.; Martin, R.C.G. Precision Hepatic Arterial Irinotecan Therapy in the Treatment of Unresectable Intrahepatic Cholangiocellular Carcinoma: Optimal Tolerance and Prolonged Overall Survival. Ann. Surg. Oncol. 2011, 18, 431–438. [Google Scholar] [CrossRef]
- Vogl, T.J.; Naguib, N.; Zangos, S.; Eichler, K.; Gruber, T. Repeated transarterial chemoperfusion and -embolization (TACE) in primary hepatic cholangiocarcinoma (CCC): Local tumor control and survival rate. J. Clin. Oncol. 2009, 27, e15595. [Google Scholar] [CrossRef]
- Vogl, T.J.; Naguib, N.N.N.; Nour-Eldin, N.-E.A.; Bechstein, W.O.; Zeuzem, S.; Trojan, J.; Gruber-Rouh, T. Transarterial chemoembolization in the treatment of patients with unresectable cholangiocarcinoma: Results and prognostic factors governing treatment success. Int. J. Cancer 2012, 131, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Baydoun, H.; Meirovich, H.; Maroun, G.; Coburn, N.; David, E. Locoregional options in the management of cholangiocarcinoma: Single center experience. Ann. Palliat. Med. 2021, 10, 1784–1791. [Google Scholar] [CrossRef]
- Bargellini, I.; Mosconi, C.; Pizzi, G.; Lorenzoni, G.; Vivaldi, C.; Cappelli, A.; Vallati, G.E.; Boni, G.; Cappelli, F.; Paladini, A.; et al. Yttrium-90 Radioembolization in Unresectable Intrahepatic Cholangiocarcinoma: Results of a Multicenter Retrospective Study. Cardiovasc. Interv. Radiol. 2020, 43, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Hyder, O.; Marsh, J.W.; Salem, R.; Petre, E.N.; Kalva, S.; Liapi, E.; Cosgrove, D.; Neal, D.; Kamel, I.; Zhu, A.X.; et al. Intra-arterial Therapy for Advanced Intrahepatic Cholangiocarcinoma: A Multi-institutional Analysis. Ann. Surg. Oncol. 2013, 20, 3779–3786. [Google Scholar] [CrossRef] [PubMed]
- Bridgewater, J.; Lopes, A.; Wasan, H.; Malka, D.; Jensen, L.; Okusaka, T.; Knox, J.; Wagner, D.; Cunningham, D.; Shannon, J.; et al. Prognostic factors for progression-free and overall survival in advanced biliary tract cancer. Ann. Oncol. 2016, 27, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Lowery, M.A.; Ptashkin, R.; Jordan, E.; Berger, M.F.; Zehir, A.; Capanu, M.; Kemeny, N.E.; O’Reilly, E.M.; El-Dika, I.; Jarnagin, W.R.; et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin. Cancer Res. 2018, 24, 4154–4161. [Google Scholar] [CrossRef]
- Zheng, Y.; Tu, X.; Zhao, P.; Jiang, W.; Liu, L.; Tong, Z.; Zhang, H.; Yan, C.; Fang, W.; Wang, W. A randomised phase II study of second-line XELIRI regimen versus irinotecan monotherapy in advanced biliary tract cancer patients progressed on gemcitabine and cisplatin. Br. J. Cancer 2018, 119, 291–295. [Google Scholar] [CrossRef]
- Taghizadeh, H.; Unseld, M.; Schmiderer, A.; Djanani, A.; Wilthoner, K.; Buchinger, D.; Prager, G.W. First evidence for the antitumor activity of nanoliposomal irinotecan with 5-fluorouracil and folinic acid in metastatic biliary tract cancer. Cancer Chemother. Pharmacol. 2020, 86, 109–115. [Google Scholar] [CrossRef]
- McNamara, M.G.; Lopes, A.; Wasan, H.; Malka, D.; Goldstein, D.; Shannon, J.; Okusaka, T.; Knox, J.J.; Wagner, A.D.; André, T.; et al. Landmark survival analysis and impact of anatomic site of origin in prospective clinical trials of biliary tract cancer. J. Hepatol. 2020, 73, 1109–1117. [Google Scholar] [CrossRef]
- Moehler, M.; Maderer, A.; Ehrlich, A.; Foerster, F.; Schad, A.; Nickolay, T.; Ruckes, C.; Weinmann, A.; Sivanathan, V.; Marquardt, J.U.; et al. Safety and efficacy of afatinib as add-on to standard therapy of gemcitabine/cisplatin in chemotherapy-naive patients with advanced biliary tract cancer: An open-label, phase I trial with an extensive biomarker program. BMC Cancer 2019, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Moehler, M.; Maderer, A.; Schimanski, C.; Kanzler, S.; Denzer, U.; Kolligs, F.T.; Ebert, M.P.; Distelrath, A.; Geissler, M.; Trojan, J.; et al. Gemcitabine plus sorafenib versus gemcitabine alone in advanced biliary tract cancer: A double-blind placebo-controlled multicentre phase II AIO study with biomarker and serum programme. Eur. J. Cancer 2014, 50, 3125–3135. [Google Scholar] [CrossRef] [PubMed]
- Piha-Paul, S.A.; Oh, D.-Y.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int. J. Cancer 2020, 147, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Arkenau, H.-T.; Martin-Liberal, J.; Calvo, E.; Penel, N.; Krebs, M.G.; Herbst, R.S.; Walgren, R.A.; Widau, R.C.; Mi, G.; Jin, J.; et al. Ramucirumab Plus Pembrolizumab in Patients with Previously Treated Advanced or Metastatic Biliary Tract Cancer: Nonrandomized, Open-Label, Phase I Trial (JVDF). Oncologist 2018, 23, 1407–e136. [Google Scholar] [CrossRef]


| pCTX + TACE (n = 14) | n † | pCTX (n = 59) | n † | p Value | |
|---|---|---|---|---|---|
| Age at initial diagnosis—years | |||||
| median (range) | 61.3 (36.7–79.3) | 14 | 66.8 (28.8–83.1) | 59 | 0.073 |
| Age at first-line pCTX—years | |||||
| median (range) | 61.7 (38.3–79.5) | 14 | 67.7 (29.3–83.4) | 59 | 0.076 |
| Gender—no. (%) | |||||
| female | 8 (57.1) | 14 | 29 (49.2) | 59 | 0.591 |
| male | 6 (42.9) | 30 (50.8) | |||
| BMI—kg/m2 | |||||
| median (range) | 26.4 (17.3–36.4) | 13 | 26.0 (15.8–35.3) | 53 | 0.332 |
| ECOG PS at first-line pCTX—no. (%) | |||||
| 0 | 13 (92.9) | 14 | 24 (49.0) | 49 | 0.028 |
| 1 | 1 (7.1) | 19 (38.8) | |||
| 2 | 0 (0.0) | 5 (8.5) | |||
| 3 | 0 (0.0) | 1 (2.0) | |||
| Initial resectability—no. (%) | |||||
| yes | 4 (28.6) | 14 | 24 (40.7) | 59 | 0.402 |
| Recurrence resectability—no. (%) | |||||
| yes | 0 (0.0) | 4 | 2 (8.3) | 24 | 1.000 |
| UICC stage—no. (%) | |||||
| 1 | 0 (0.0) | 14 | 7 (13.7) | 51 | 0.003 |
| 2 | 10 (71.4) | 10 (19.6) | |||
| 3 | 0 (0.0) | 2 (3.9) | |||
| 4 | 4 (28.6) | 32 (62.7) | |||
| Grading—no. (%) | |||||
| 1 | 0 (0.0) | 7 | 1 (2.8) | 36 | 0.740 |
| 2 | 5 (71.4) | 21 (58.3) | |||
| 3 | 2 (28.6) | 14 (38.9) | |||
| CA19-9 (U/mL) | |||||
| median (range) | 33 (4–4271) | 14 | 66 (2–696664) | 44 | 0.502 |
| CEA (ng/mL) | |||||
| median (range) | 1.1 (0.2–39.0) | 13 | 1.7 (0.5–4328.1) | 34 | 0.517 |
| Albumin (g/L) | |||||
| median (range) | 37.5 (28.0–42.0) | 14 | 31.0 (19.0–42.0) | 34 | <0.001 |
| Bilirubin (mg/dL) | |||||
| median (range) | 0.7 (0.2–1.3) | 14 | 0.9 (0.3–7.7) | 49 | 0.007 |
| pCTX + TACE (n = 14) | n † | pCTX (n = 59) | n † | p Value | |
|---|---|---|---|---|---|
| Vital status—no. (%) | |||||
| alive | 3 (21.4) | 14 | 7 (11.9) | 59 | 0.392 |
| dead | 11 (78.6) | 52 (88.1) | |||
| alive-receiving pCTX treatment | 1 (7.1) | 14 | 3 (5.1) | 59 | 1.000 |
| Adjuvant CTX—no. (%) | |||||
| yes | 0 (0.0) | 14 | 8 (13.6) | 59 | 0.340 |
| pCTX lines—no. (%) | |||||
| 1 | 4 (28.6) | 14 | 23 (39.0) | 59 | 0.380 |
| 2 | 4 (28.6) | 24 (40.7) | |||
| 3 | 4 (28.6) | 7 (11.9) | |||
| 4 | 2 (14.3) | 4 (6.8) | |||
| 5 | 0 (0.0) | 1 (1.7) | |||
| median (range) | 2 (1–4) | 2 (1–5) | |||
| First-line pCTX—no. (%) | |||||
| Gemcitabine | 1 (7.1) | 14 | 13 (22.0) | 59 | 0.672 |
| GemCis/GemOx | 9 (64.3) | 25 (42.4) | |||
| FOLFOX/CAPOX | 1 (7.1) | 7 (11.9) | |||
| FOLFIRINOX | 1 (7.1) | 5 (8.5) | |||
| Other | 2 (14.3) | 9 (15.3) | |||
| Cycles (median (range)) | 6 (2–18) | 14 | 4 (1–27) | 59 | 0.200 |
| Second-line pCTX—no. (%) | |||||
| Gemcitabine | 2 (20.0) | 10 | 7 (19.4) | 36 | 0.563 |
| GemCis/GemOx | 2 (20.0) | 8 (22.2) | |||
| FOLFOX/CAPOX | 1 (10.0) | 11 (30.6) | |||
| FOLFIRINOX | 0 (0.0) | 1 (2.8) | |||
| Other | 5 (50.0) | 9 (25.0) | |||
| Cycles (median (range)) | 5 (1–11) | 10 | 4 (1–16) | 36 | 0.293 |
| Time surgery to recurrence—months | |||||
| median (range) | 8.3 (1.9–13.3) | 4 | 8.6 (0.2–42.5) | 24 | 0.550 |
| Time recurrence to last follow-up/death—months | |||||
| median (range) | 22.5 (18.0–44.4) | 4 | 14.5 (1.6–79.3) | 24 | 0.341 |
| Survival-Months | pCTX + TACE | pCTX | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Median | Q1 | Q3 | n | Median | Q1 | Q3 | ||
| since initial diagnosis | 14 | 30.1 | 16.7 | 46.8 | 59 | 17.4 | 6.3 | 25.1 | 0.019 |
| since unresectability | 14 | 26.2 | 16.7 | 42.8 | 59 | 13.1 | 6.1 | 19.0 | 0.008 |
| Survival of Propensity Score Matched Patients | |||||||||
| since initial diagnosis | 10 | 26.8 | 13.6 | 46.8 | 10 | 9.4 | 4.2 | 21.2 | 0.051 |
| since unresectability | 10 | 22.5 | 13.6 | 43.4 | 10 | 8.5 | 3.4 | 13.5 | 0.020 |
| TACE Treatments | |
|---|---|
| median (range) | 3.5 (1–13) |
| TACE method—no. (%) | |
| cTACE (mitomycin C) | 2 (14.3) |
| DEB-TACE (doxorubicin) | 9 (64.3) |
| sequential combination | 3 (21.4) |
| Time unresectability to first TACE treatment—days | |
| median (range) | 84.5 (13–828) |
| TACE/CTX relation—no. (%) | |
| TACE before first CTX administration | 7 (50.0) |
| TACE after first CTX administration | 7 (50.0) |
| Time of hospitalization per TACE treatment—days | |
| median (range) | 2 (1–26) |
| Adverse events—no. (%) | |
| no adverse events | 36 (56.3) |
| postembolization syndrome | 24 (37.5) |
| severe adverse events | 3 (4.7) |
| other | 1 (1.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gairing, S.J.; Thol, F.; Müller, L.; Hahn, F.; Thomaidis, T.; Czauderna, C.; Bartsch, F.; Pitton, M.B.; Marquardt, J.U.; Wörns, M.-A.; et al. The Addition of Transarterial Chemoembolization to Palliative Chemotherapy Extends Survival in Intrahepatic Cholangiocarcinoma. J. Clin. Med. 2021, 10, 2732. https://doi.org/10.3390/jcm10122732
Gairing SJ, Thol F, Müller L, Hahn F, Thomaidis T, Czauderna C, Bartsch F, Pitton MB, Marquardt JU, Wörns M-A, et al. The Addition of Transarterial Chemoembolization to Palliative Chemotherapy Extends Survival in Intrahepatic Cholangiocarcinoma. Journal of Clinical Medicine. 2021; 10(12):2732. https://doi.org/10.3390/jcm10122732
Chicago/Turabian StyleGairing, Simon Johannes, Felix Thol, Lukas Müller, Felix Hahn, Thomas Thomaidis, Carolin Czauderna, Fabian Bartsch, Michael Bernhard Pitton, Jens Uwe Marquardt, Marcus-Alexander Wörns, and et al. 2021. "The Addition of Transarterial Chemoembolization to Palliative Chemotherapy Extends Survival in Intrahepatic Cholangiocarcinoma" Journal of Clinical Medicine 10, no. 12: 2732. https://doi.org/10.3390/jcm10122732
APA StyleGairing, S. J., Thol, F., Müller, L., Hahn, F., Thomaidis, T., Czauderna, C., Bartsch, F., Pitton, M. B., Marquardt, J. U., Wörns, M.-A., Galle, P. R., Moehler, M., Weinmann, A., Kloeckner, R., & Foerster, F. (2021). The Addition of Transarterial Chemoembolization to Palliative Chemotherapy Extends Survival in Intrahepatic Cholangiocarcinoma. Journal of Clinical Medicine, 10(12), 2732. https://doi.org/10.3390/jcm10122732








