Obesity Strongly Predicts COVID-19-Related Major Clinical Adverse Events in Coptic Clergy
Abstract
:1. Introduction
2. Methods
2.1. Clinical Events
2.2. Cardiovascular Risk Factors Assessment
2.3. Statistical Analysis
3. Results
3.1. Demographic Indices of the Participating Clergy
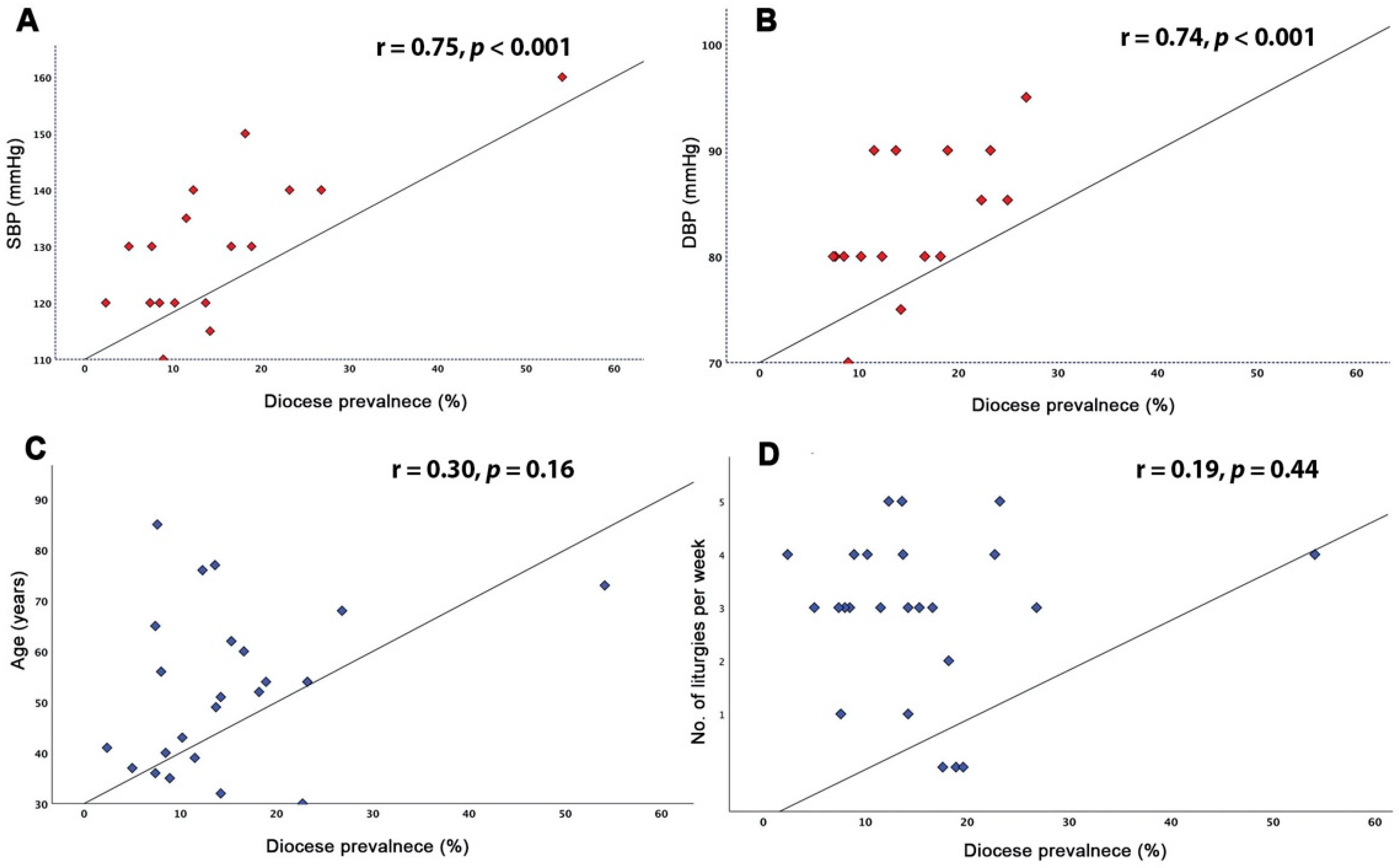
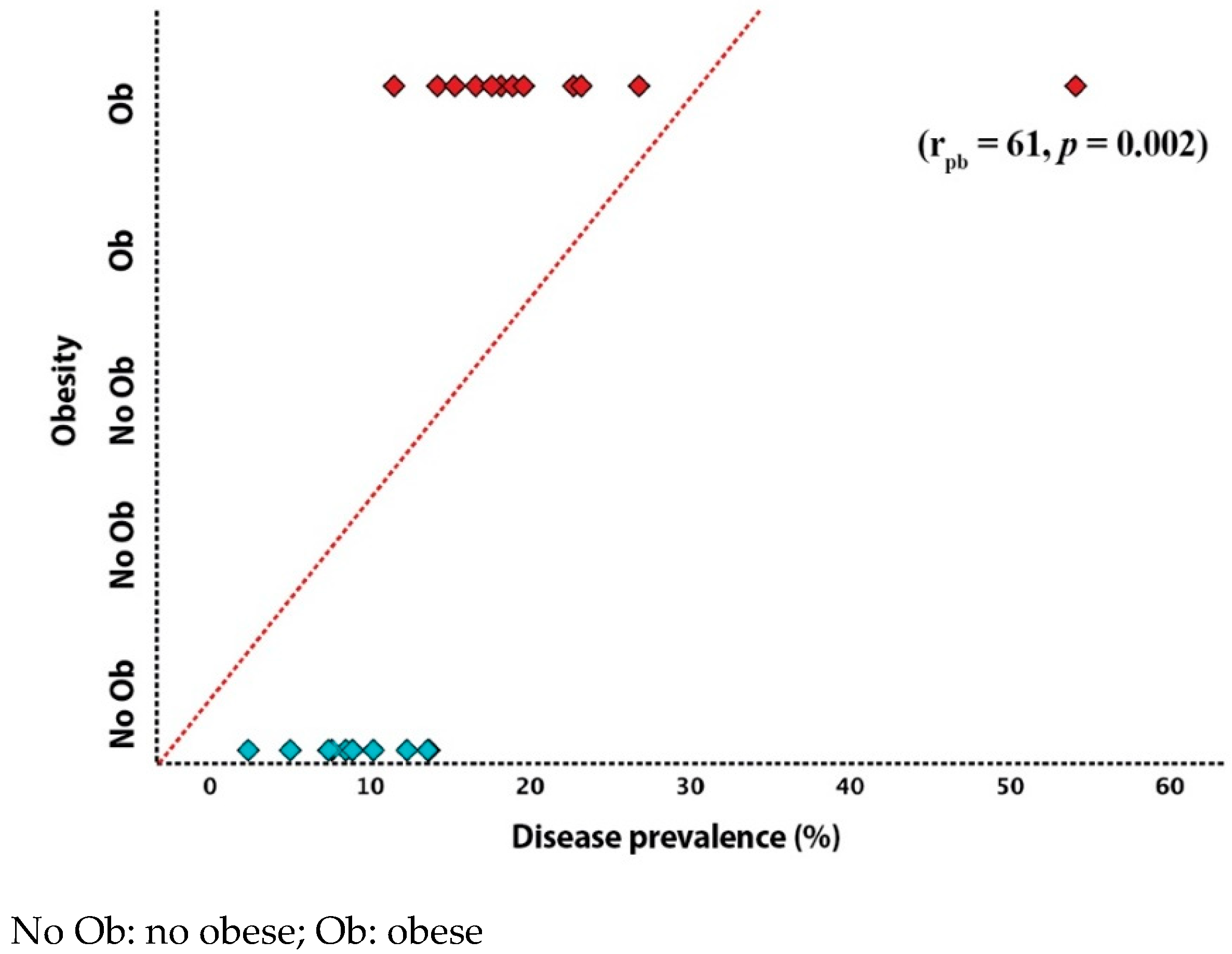
3.2. Impact of Cardiovascular Risk Factors on Disease Prevalence
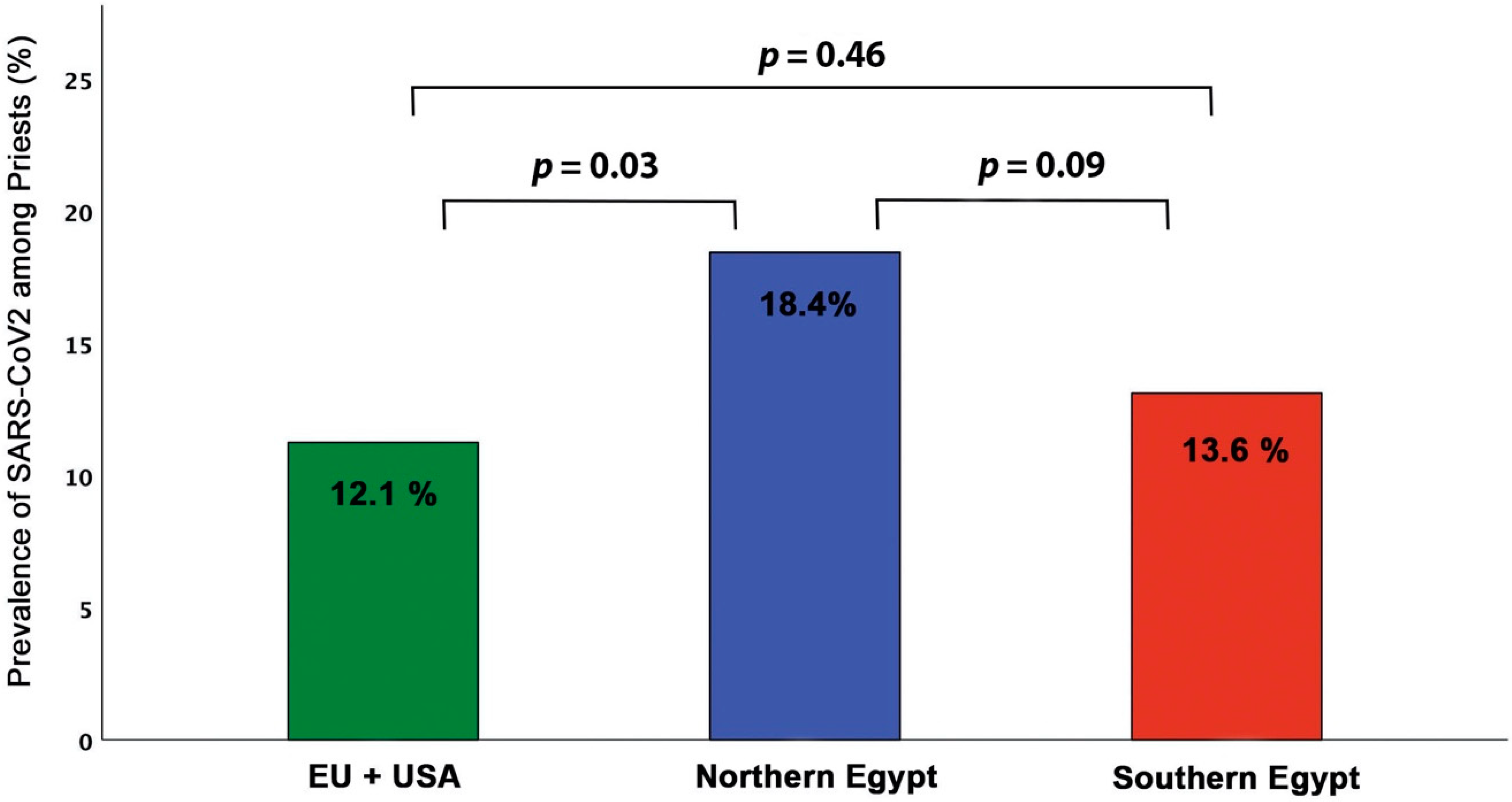
3.3. Geographical Impact on Disease Prevalence
3.4. Predictors of Clinical Events
3.5. Comparison with Data from other Communities
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef] [Green Version]
- Peters, A.; Vetter, P.; Guitart, C.; Lotfinejad, N.; Pittet, D. Understanding the emerging coronavirus: What it means for health security and infection prevention. J. Hosp. Infect. 2020, 104, 440–448. [Google Scholar] [CrossRef]
- Tartari, E.; Muthukumaran, P.; Peters, A.; Allegranzi, B.; Pittet, D. Monitoring your institution: The WHO hand hygiene self-assessment framework—Is it worth it? Clin. Microbiol. Infect. 2019, 25, 925–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Infection Prevention and Control during Health Care when Novel Coronavirus (Ncov) Infection Is Suspected. Interim Guidance, 2020. Available online: https://apps.who.int/iris/rest/bitstreams/1266296/retrieve (accessed on 25 January 2020).
- World Health Organization. Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 9 January 2021).
- Ratzinger, J.C. The Spirit of the Liturgy by Joseph Cardinal Ratzinger; Amazon: Seattle, WA, USA, 2000; p. 174. [Google Scholar]
- Aherfi, S.; Gautret, P.; Chaudet, H.; Raoult, D.; La Scola, B. Clusters of COVID-19 associated with Purim celebration in the Jewish community in Marseille, France, March 2020. Int. J. Infect. Dis. 2020, 100, 88–94. [Google Scholar] [CrossRef]
- Budaev, S. Safety and Reverence: How Roman Catholic Liturgy Can Respond to the COVID-19 Pandemic. J. Relig. Health 2021, 1–22. [Google Scholar] [CrossRef]
- Biagioni, M.C. 400 Priests Have Died from Covid-19: “Stories of Heroes That Helped Everyone Keep Hope Alive. Available online: https://www.agensir.it/europa/2020/09/29/400-priests-have-died-from-covid-19-stories-of-heroes-that-helped-everyone-keep-hope-alive/ (accessed on 29 September 2020).
- Rosenberg, D. The Government Can’t Save Ultra-Orthodox Jews from COVID-19. Religious Leaders Can. Available online: https://foreignpolicy.com/2020/10/12/the-government-cant-save-ultra-orthodox-jews-from-covid-19-religious-leaders-can/ (accessed on 20 April 2021).
- Burgess, K. 64% of London’s Ultra-Orthodox Jews Infected with Covid. The Times. 3 February 2021. Available online: https://www.thetimes.co.uk/article/64-of-londons-ultra-orthodox-jews-infected-with-covid-5dhkw3lz0 (accessed on 20 April 2021).
- Bramstedt, K.A. COVID-19 as a Cause of Death for Catholic Priests in Italy: An Ethical and Occupational Health Crisis. Health Soc. Care Chaplain. 2020, 8, 180–190. [Google Scholar] [CrossRef]
- Monteiro, A.C.; Suri, R.; Emeruwa, I.O.; Stretch, R.J.; Cortes-Lopez, R.Y.; Sherman, A.; Lindsay, C.C.; Fulcher, J.A.; Goodman-Meza, D.; Sapru, A.; et al. Obesity and smoking as risk factors for invasive mechanical ventilation in COVID-19: A retrospective, observational cohort study. PLoS ONE 2020, 15, e0238552. [Google Scholar] [CrossRef]
- King, C.S.; Sahjwani, D.; Brown, A.W.; Feroz, S.; Cameron, P.; Osborn, E.; Desai, M.; Djurkovic, S.; Kasarabada, A.; Hinerman, R.; et al. Outcomes of mechanically ventilated patients with COVID-19 associated respiratory failure. PLoS ONE 2020, 15, e0242651. [Google Scholar] [CrossRef]
- Stefan, N.; Birkenfeld, A.L.; Schulze, M.B.; Ludwig, D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat. Rev. Endocrinol. 2020, 16, 341–342. [Google Scholar] [CrossRef] [Green Version]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M.; et al. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity 2020, 28, 1195–1199. [Google Scholar] [CrossRef]
- Belanger, M.J.; Hill, M.A.; Angelidi, A.M.; Dalamaga, M.; Sowers, J.R.; Mantzoros, C.S. Covid-19 and Disparities in Nutrition and Obesity. N. Engl. J. Med. 2020, 383, e69. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.A.; Ausiello, D.; Salzman, J.; Devlin, T.; Langer, R.; Beddingfield, B.J.; Fears, A.C.; Doyle-Meyers, L.A.; Redmann, R.K.; Killeen, S.Z.; et al. Exhaled aerosol increases with COVID-19 infection, age, and obesity. Proc. Natl. Acad. Sci. USA 2021, 118, 2021830118. [Google Scholar] [CrossRef]
- Schiffrin, E.L.; Flack, J.M.; Ito, S.; Muntner, P.; Webb, R.C. Hypertension and COVID-19. Am. J. Hypertens. 2020, 33, 373–374. [Google Scholar] [CrossRef]
- Kamyshnyi, A.; Krynytska, I.; Matskevych, V.; Marushchak, M.; Lushchak, O. Arterial Hypertension as a Risk Comorbidity Associated with COVID-19 Pathology. Int. J. Hypertens. 2020, 2020, 8019360. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Diabetes, obesity, metabolism, and SARS-CoV-2 infection: The end of the beginning. Cell Metab. 2021, 33, 479–498. [Google Scholar] [CrossRef]
- Gregory, J.M.; Slaughter, J.C.; Duffus, S.H.; Smith, T.J.; LeStourgeon, L.M.; Jaser, S.S.; McCoy, A.B.; Luther, J.M.; Giovannetti, E.R.; Boeder, S.; et al. COVID-19 Severity Is Tripled in the Diabetes Community: A Prospective Analysis of the Pandemic’s Impact in Type 1 and Type 2 Diabetes. Diabetes Care 2021, 44, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Carey, I.M.; Critchley, J.; Dewilde, S.; Harris, T.; Hosking, F.J.; Cook, D.G. Risk of Infection in Type 1 and Type 2 Diabetes Compared with the General Population: A Matched Cohort Study. Diabetes Care 2018, 41, 513–521. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Rahman, K.M.; Sun, Y.; Qureshi, M.O.; Abdi, I.; Chughtai, A.A.; Seale, H. Current knowledge of COVID-19 and infection prevention and control strategies in healthcare settings: A global analysis. Infect. Control. Hosp. Epidemiol. 2020, 41, 1196–1206. [Google Scholar] [CrossRef]
- Zhao, L.; Stamler, J.; Yan, L.L.; Zhou, B.; Wu, Y.; Liu, K.; Daviglus, M.L.; Dennis, B.H.; Elliott, P.; Ueshima, H.; et al. Blood pressure differences between northern and southern Chinese: Role of dietary factors: The International Study on Macronutrients and Blood Pressure. Hypertension 2004, 43, 1332–1337. [Google Scholar] [CrossRef]
- Elliott, P.; Kesteloot, H.; Appel, L.J.; Dyer, A.R.; Ueshima, H.; Chan, Q.; Brown, I.J.; Zhao, L.; Stamler, J. Dietary phosphorus and blood pressure: International study of macro- and micro-nutrients and blood pressure. Hypertension 2008, 51, 669–675. [Google Scholar] [CrossRef] [Green Version]
- Mecenas, P.; Bastos, R.T.D.R.M.; Vallinoto, A.C.R.; Normando, D. Effects of temperature and humidity on the spread of COVID-19: A systematic review. PLoS ONE 2020, 15, e0238339. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.H.; Peiris, J.S.M.; Lam, S.Y.; Poon, L.L.M.; Yuen, K.Y.; Seto, W.H. The Effects of Temperature and Relative Humidity on the Viability of the SARS Coronavirus. Adv. Virol. 2011, 2011, 734690. [Google Scholar] [CrossRef] [PubMed]

| Variable | Priests (n = 255) |
|---|---|
| Demographic and clinical data | |
| Age (years) | 49.5 ± 12 |
| BMI (m/kg2) | 32 ± 6.2 |
| SBP (mmHg) | 127 ± 13 |
| DBP (mmHg) | 83 ± 9.5 |
| Underweight (n, %) | 0 (0) |
| Normal weight (n, %) | 21 (8.4) |
| Overweight (n, %) | 101 (39.8) |
| Obese (n, %) | 133 (52.2) |
| AH (n, %) | 71 (27.9) |
| DM (n, %) | 60 (23.5) |
| DM type 1 (n, %) | 6 (2.65%) |
| Dyslipidemia | 67 (26.5) |
| CHD (n, %) | 24 (9.6) |
| Family history for CHD (n, %) | 22 (8.9) |
| Family history for stroke (n, %) | 14 (5.8) |
| Liturgies per week | 3.44 ± 1.0 |
| Source of infection | |
| Home (n, %) | 32 (12.8) |
| Church (n, %) | 76 (30.1) |
| Personal (n, %) | 41 (16.3) |
| Unknown (n, %) | 102 (40.1) |
| Outcome data | |
| Home treatment (n, %) | 210 (82.7) |
| Hospital treatment (n, %) | 44 (17.3) |
| Intensive care (n, %) | 21 (8.4) |
| Home treatment (days) | 17.9 ± 10.3 |
| Hospital treatment (days) | 10.2 ± 9.4 |
| Intensive care (days) | 7.4 ± 3.4 |
| Mechanical ventilator (n, %) | 16 (6.72) |
| Death (n, %) | 10 (3.92) |
| MAE (n, %) | 26 (10.2) |
| Variable | r | p Value |
|---|---|---|
| Age | 0.30 | 0.16 |
| BMI | −0.12 | 0.57 |
| CHD | −0.38 | 0.09 |
| DM | 0.10 | 0.68 |
| SBP | 0.75 | <0.001 |
| DBP | 0.74 | <0.001 |
| Obesity | 0.61 | 0.002 |
| Liturgies per week | 0.19 | 0.44 |
| Variable | EU+ USA (n = 31) | Northern Egypt (n = 175) | Southern Egypt (n = 49) | p-Value |
|---|---|---|---|---|
| Demographic and clinical data | ||||
| Age (years) | 52.7 ± 11 | 49.6 ± 12 | 47.4± 11 | NS |
| BMI (m/kg2) | 31 ± 9.1 | 32 ± 5.7 | 33 ± 5.3 | NS |
| SBP (mmHg) | 126 ± 10 | 127 ± 14 | 125 ± 11 | NS |
| DBP (mmHg) | 82 ± 6.1 | 83 ± 9.6 | 84 ± 11 | NS |
| Underweight (n, %) | 0 (0) | 0 (0) | 0 (0) | NS |
| Normal weight (n, %) | 5 (15.4) | 10 (6.10) a | 5 (11.5) | 0.03 |
| Overweight (n, %) | 16 (51.6) | 72 (41.2) a | 12 (25.5) b,c | 0.04 |
| Obese (n, %) | 10 (30.7) | 92 (52.6) a | 29 (59.5) b | 0.02 |
| AH (n, %) | 11 (36.4) | 66 (38) | 12 (24.3) b,c | 0.001 |
| DM (n, %) | 9 (28.6) | 46 (26.6) | 14 (29.7) | NS |
| Dyslipidemia | 9 (31.8) | 60 (34.6) a | 10 (22.2) c | 0.03 |
| CHD (n, %) | 3 (9.5) | 20 (11.36) | 1 (2.85) b,c | 0.004 |
| Family history of CHD (n, %) | 7 (23.8) | 19 (10.8) | 0 (0) b,c | 0.01 |
| Family history of stroke (n, %) | 3 (10.7) | 10 (5.92) | 9 (4.4) b | 0.04 |
| Liturgies per week (n, %) | 3.6 ± 1.2 | 3.4 ± 1.0 a | 3.5 ± 0.8 | NS |
| Source of infection | ||||
| Home (n, %) | 3 (9.7) | 20 (11.7) | 13 (28.2) b,c | 0.02 |
| Church (n, %) | 11 (35.5) | 54 (30.9) | 15 (32.6) | NS |
| Personal (n, %) | 5 (16.1) | 32 (18.4) | 7 (15.2) | NS |
| Unknown (n, %) | 12 (40.1) | 68 (38.9) | 11 (23.9) b,c | 0.001 |
| Outcome data | ||||
| Home treatment (n, %) | 27 (88) | 129 (74.2) | 46 (97.7) c | 0.04 |
| Hospital treatment (n, %) | 10 (31.8) | 26 (15.1) a | 8 (18.4) | 0.03 |
| Intensive care (n, %) | 3 (8.7) | 19 (10.8) | 5 (10.2) | NS |
| Home treatment (days) | 17.2 ± 11 | 18.1 ± 11 | 17.7 ± 6.9 | NS |
| Hospital treatment (days) | 12.5 ± 12 | 9.7 ± 6.4 a | 8.6 ± 4.5 | 0.001 |
| Intensive care (days) | 4.2 ± 3.9 | 6.9 ± 3.2 | 13 ± 8.4 a | 0.01 |
| Mechanical ventilator (n, %) | 2 (4.8) | 10 (5.71) | 4 (8.2) | NS |
| Death (n, %) | 0 (0) | 7 (4.1) a | 3 (6.4) b | <0.001 |
| MAE (n, %) | 2 (6.4) | 17 (10.2) a | 7 (14.9) b,c | <0.001 |
| Variable | Univariate Predictors OR (95% CI) | p-Value | Multivariate Predictors OR (95% CI) | p-Value |
|---|---|---|---|---|
| Age | 1.085 (1.029 to 1.153) | 0.003 | 1.001 (0.918 to 1.092) | 0.98 |
| BMI | 1.022 (0.925 to 1.130) | 0.66 | ||
| Diabetes | 0.745 (0.045 to 3.706) | 0.71 | ||
| Obesity | 5.461 (1.015 to 16.94) | 0.06 | ||
| AH | 0.511 (0.103 to 2.530) | 0.41 | ||
| Dyslipidemia | 1.070 (0.257 to 4.014) | 0.81 | ||
| CHD | 1.429 (1.271 to 3.144) | 0.002 | 3.007 (0.282 to 6.059) | 0.36 |
| No. of liturgies | 0.800 (0.415 to 1.541) | 0.51 | ||
| Home treatment | 1.910 (0.232 to 15.74) | 0.54 | ||
| Hospital treatment | 4.615 (3.836 to 5.958) | 0.001 | 3.116 (2.586 to 4.796) | 0.007 |
| Mean home days | 0.784 (0.671 to 0.915) | 0.002 | 0.922 (0.803 to 1.059) | 0.25 |
| Mean hospital days | 1. 010 (0.928 to 1.100) | 0.21 |
| Variable | Univariate Predictors OR (95% CI) | p-Value | Multivariate Predictors OR (95% CI) | p-Value |
|---|---|---|---|---|
| Age | 1.031 (0.991 to 1.008) | 0.04 | 1.055 (0.024 to 1.141) | 0.01 |
| AH | 1.938 (1.172 to 2.001) | 0.01 | 1.931 (1.169 to 2.004) | 0.007 |
| Diabetes | 0.702 (0.222 to 2.170) | 0.52 | ||
| BMI | 1.011 (0.901 to 1.209) | 0.26 | ||
| Obesity | 3.366 (1.055 to 9.785) | 0.02 | 4.180 (2.479 to 12.15) | 0.01 |
| Dyslipidemia | 0.710 (0.312 to 2.231) | 0.55 | ||
| CHD | 4.122 (1.202 to 15.01) | 0.02 | 3.625 (0.802 to 17.89) | 0.09 |
| No. of liturgies | 0.608 (0.451 to 1.342) | 0.31 | ||
| Home days | 0.997 (0.806 to 1.011) | 0.04 | 1.480 (0.209 to 7.032) | 0.62 |
| Hospital days | 0.990 (0.801 to 1.068) | 0.33 | ||
| Northern Egypt | ||||
| Age | 1.081 (1.033 to 1.166) | 0.003 | 1.077 (0.980 to 1.613) | 0.21 |
| AH | 1.520 (1.111 to 2.509) | 0.04 | 1.542 (1.042 to 2.931) | 0.03 |
| CHD | 1.429 (1.271 to 3.144) | 0.002 | 3.001 (0.200 to 6.012) | 0.24 |
| Southern Egypt | ||||
| Age | 1.011 (0.909 to 1.380) | 0.04 | 2.110 (0.991 to 3.101) | 0.31 |
| AH | 0.902 (0.400 to 1.970) | 0.03 | 0.809 (0.106 to 2.121) | 0.08 |
| Obesity | 1.901 (1.001 to 3.122) | 0.01 | 2.990 (1.202 to 3.015) | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henein, M.Y.; Bytyçi, I.; Nicoll, R.; Shenouda, R.; Ayad, S.; Vancheri, F.; Cameli, M. Obesity Strongly Predicts COVID-19-Related Major Clinical Adverse Events in Coptic Clergy. J. Clin. Med. 2021, 10, 2752. https://doi.org/10.3390/jcm10132752
Henein MY, Bytyçi I, Nicoll R, Shenouda R, Ayad S, Vancheri F, Cameli M. Obesity Strongly Predicts COVID-19-Related Major Clinical Adverse Events in Coptic Clergy. Journal of Clinical Medicine. 2021; 10(13):2752. https://doi.org/10.3390/jcm10132752
Chicago/Turabian StyleHenein, Michael Y., Ibadete Bytyçi, Rachel Nicoll, Rafik Shenouda, Sherif Ayad, Federico Vancheri, and Matteo Cameli. 2021. "Obesity Strongly Predicts COVID-19-Related Major Clinical Adverse Events in Coptic Clergy" Journal of Clinical Medicine 10, no. 13: 2752. https://doi.org/10.3390/jcm10132752
APA StyleHenein, M. Y., Bytyçi, I., Nicoll, R., Shenouda, R., Ayad, S., Vancheri, F., & Cameli, M. (2021). Obesity Strongly Predicts COVID-19-Related Major Clinical Adverse Events in Coptic Clergy. Journal of Clinical Medicine, 10(13), 2752. https://doi.org/10.3390/jcm10132752








