The Prevalence of Advanced Interatrial Block and Its Relationship to Left Atrial Function in Patients with Transthyretin Cardiac Amyloidosis
Abstract
:1. Introduction
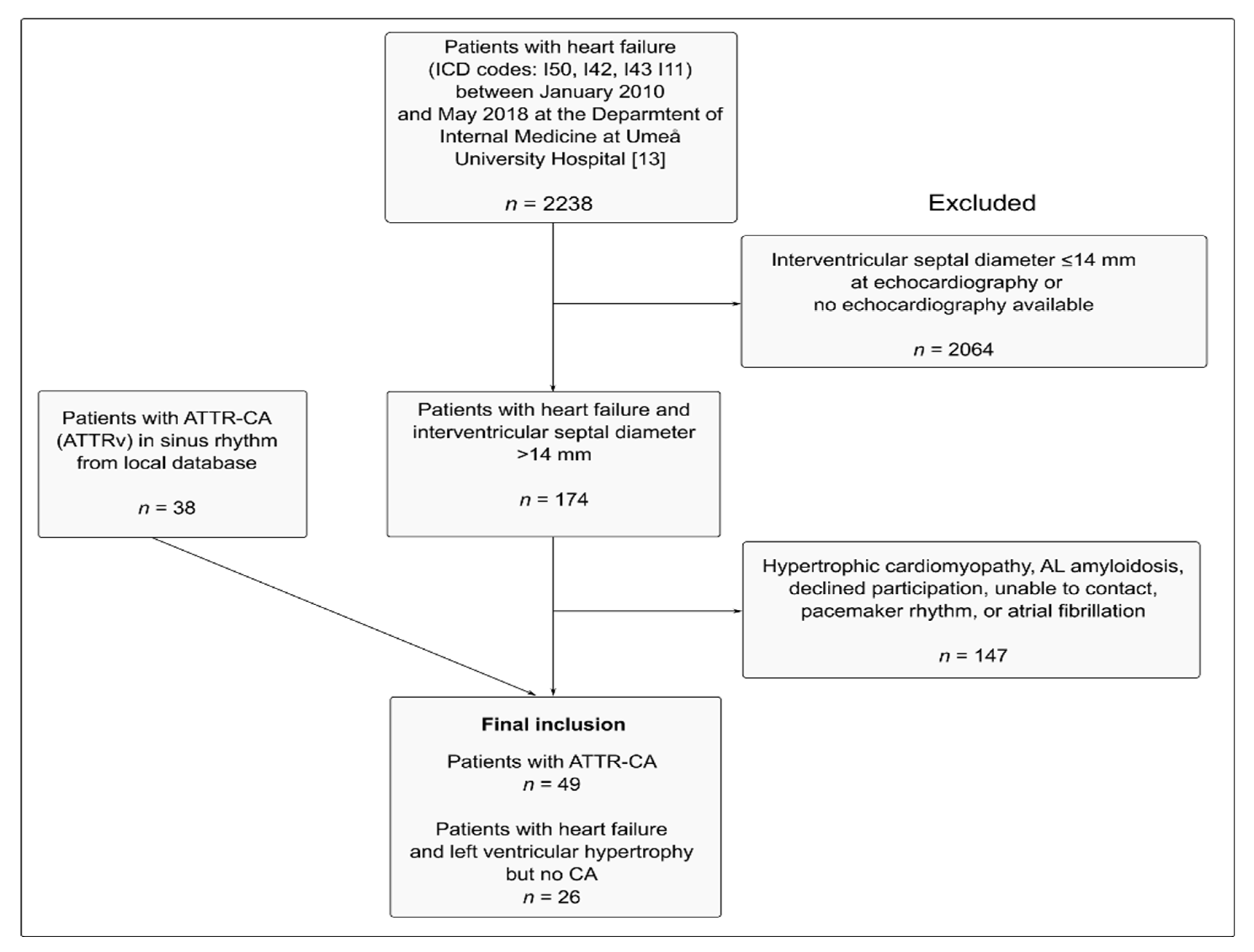
2. Material and Methods
2.1. Electrocardiography
2.2. Echocardiography
2.3. Statistical Analysis
3. Results
3.1. Prevalence of Advanced Interatrial Block in ATTR-CA
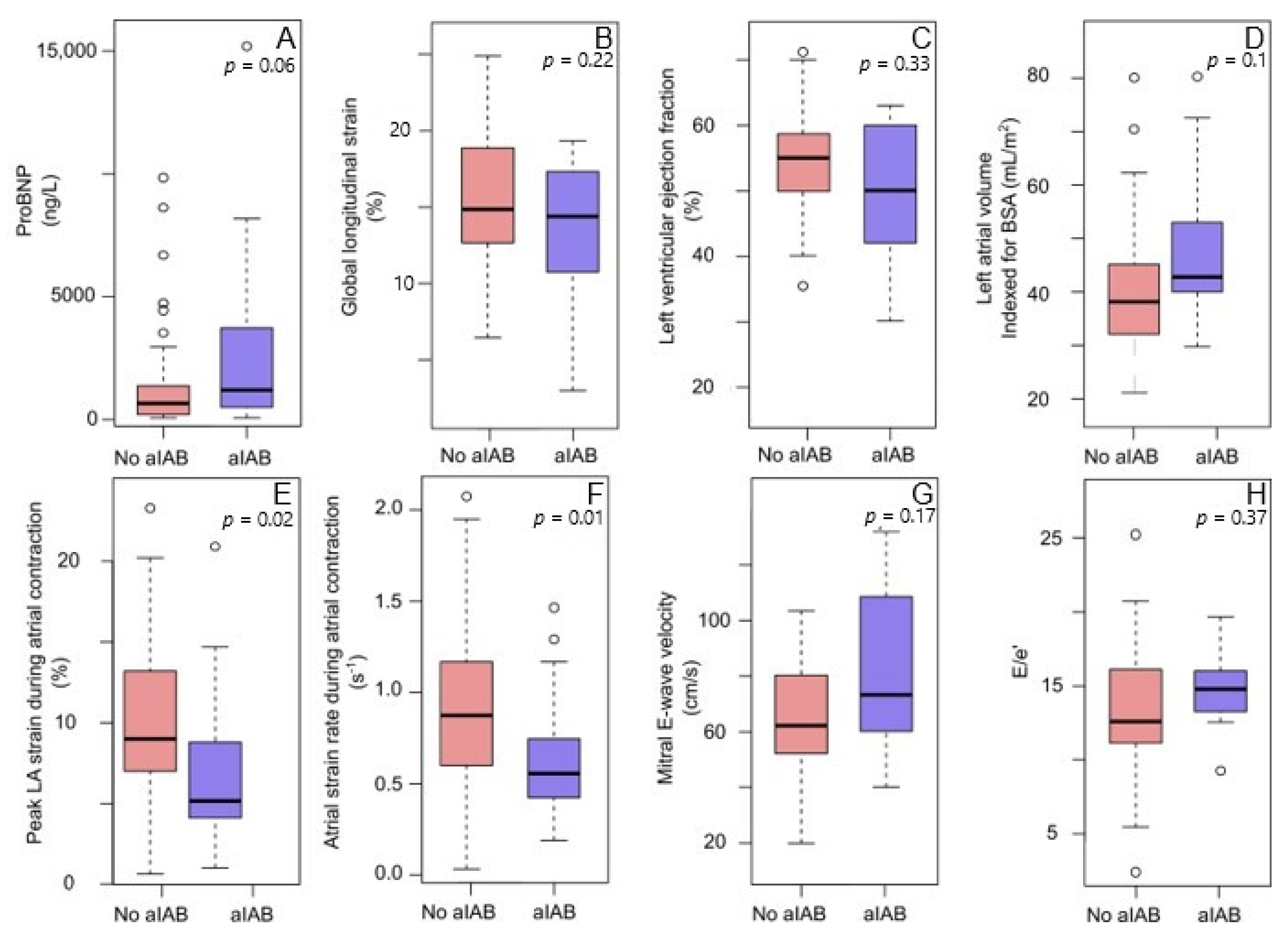
3.2. Advanced Interatrial Block and Left Atrial Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Ponti, R.; Ho, S.Y.; Salerno-Uriarte, J.A.; Tritto, M.; Spadacini, G. Electroanatomic analysis of sinus impulse propagation in normal human atria. J. Cardiovasc. Electrophysiol. 2002, 13, 1–10. [Google Scholar] [CrossRef]
- Roithinger, F.X.; Cheng, J.; SippensGroenewegen, A.; Lee, R.J.; Saxon, L.A.; Scheinman, M.M.; Lesh, M.D. Use of Electroanatomic Mapping to Delineate Transseptal Atrial Conduction in Humans. Circulation 1999, 100, 1791–1797. [Google Scholar] [CrossRef] [Green Version]
- Markides, V.; Schilling, R.J.; Ho, S.Y.; Chow, A.W.; Davies, D.W.; Peters, N.S. Characterization of Left Atrial Activation in the Intact Human Heart. Circulation 2003, 107, 733–739. [Google Scholar] [CrossRef]
- Mitrofanova, L.; Ivanov, V.; Platonov, P.G. Anatomy of the inferior interatrial route in humans. Europace 2005, 7, S49–S55. [Google Scholar] [CrossRef]
- Ariyarajah, V.; Spodick, D.H. The Bachmann Bundle and Interatrial Conduction. Cardiol. Rev. 2006, 14, 194–199. [Google Scholar] [CrossRef]
- de Luna, A.B.; Platonov, P.; Cosio, F.G.; Cygankiewicz, I.; Pastore, C.; Baranowski, R.; Spodick, D. Interatrial blocks. A separate entity from left atrial enlargement: A consensus report. J. Electrocardiol. 2012, 45, 445–451. [Google Scholar] [CrossRef] [PubMed]
- De Luna, A.B.; Martínez-Sellés, M.; Bayés-Genís, A.; Elosua, R.; Baranchuk, A. Surface ECG interatrial block-guided treatment for stroke prevention: Rationale for an attractive hypothesis. BMC Cardiovasc. Disord. 2017, 17, 211. [Google Scholar]
- Tse, G.; Wong, C.W.; Gong, M.; Wong, W.T.; Bazoukis, G.; Wong, S.H.; Li, G.; Wu, W.K.; Tse, L.A.; Lampropoulos, K.; et al. Predictive value of inter-atrial block for new onset or recurrent atrial fibrillation: A systematic review and meta-analysis. Int. J. Cardiol. 2018, 250, 152–156. [Google Scholar] [CrossRef]
- Chhabra, L.; Devadoss, R.; Chaubey, V.K.; Spodick, D.H. Interatrial Block in the Modern Era. Curr. Cardiol. Rev. 2014, 10, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Lindow, T.; Baranchuk, A. Interatrial block and ischemic stroke. J. Thorac. Dis. 2018, 10, 7052–7054. [Google Scholar] [CrossRef] [PubMed]
- Ruberg, F.L.; Grogan, M.; Hanna, M.; Kelly, J.W.; Maurer, M.S. Transthyretin Amyloid Cardiomyopathy. J. Am. Coll. Cardiol. 2019, 73, 2872–2891. [Google Scholar] [CrossRef]
- Nitsche, C.; Scully, P.R.; Patel, K.P.; Kammerlander, A.A.; Koschutnik, M.; Dona, C.; Wollenweber, T.; Ahmed, N.; Thornton, G.D.; Kelion, A.D.; et al. Prevalence and Outcomes of Concomitant Aortic Stenosis and Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2021, 77, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Lindmark, K.; Pilebro, B.; Sundström, T.; Lindqvist, P. Prevalence of wild type transtyrethin cardiac amyloidosis in a heart failure clinic. ESC Heart Fail. 2020, 8, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Bianco, M.; Parente, A.; Biolè, C.; Righetti, C.; Spirito, A.; Luciano, A.; Destefanis, P.; Nangeroni, G.; Angusti, T.; Anselmino, M.; et al. The prevalence of TTR cardiac amyloidosis among patients undergoing bone scintigraphy. J. Nucl. Cardiol. 2021, 1–6. [Google Scholar] [CrossRef]
- Röcken, C.; Peters, B.; Juenemann, G. Atrial amyloidosis. An arrhythmogenic substrate for persistent atrial fibrillation. ACC Curr. J. Rev. 2003, 12, 86–87. [Google Scholar] [CrossRef]
- Di Bella, G.; Minutoli, F.; Madaffari, A.; Mazzeo, A.; Russo, M.; Donato, R.; Zito, C.; Aquaro, G.D.; Piccione, M.C.; Pedri, S.; et al. Left atrial function in cardiac amyloidosis. J. Cardiovasc. Med. 2016, 17, 113–121. [Google Scholar] [CrossRef]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE expert consensus on Atrial cardiomyopathies: Definition, characterisation, and clinical implication. J. Arrhythmia 2016, 32, 247–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, D.; Syed, I.S.; Martinez, M.; Oh, J.K.; Jaffe, A.S.; Grogan, M.; Edwards, W.D.; Gertz, M.A.; Klarich, K.W. Intracardiac Thrombosis and Anticoagulation Therapy in Cardiac Amyloidosis. Circulation 2009, 119, 2490–2497. [Google Scholar] [CrossRef] [Green Version]
- Maurer, M.S.; Bokhari, S.; Damy, T.; Dorbala, S.; Drachman, B.M.; Fontana, M.; Grogan, M.; Kristen, A.V.; Lousada, I.; Nativi-Nicolau, J.; et al. Expert Consensus Recommendations for the Suspicion and Diagnosis of Transthyretin Cardiac Amyloidosis. Circulation Hear. Fail. 2019, 12, e006075. [Google Scholar] [CrossRef]
- Maurer, M.S.; Hanna, M.; Grogan, M.; Dispenzieri, A.; Witteles, R.; Drachman, B.; Judge, D.P.; Lenihan, D.J.; Gottlieb, S.S.; Shah, J.S.; et al. Genotype and Phenotype of Transthyretin Cardiac Amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J. Am. Coll. Cardiol. 2016, 68, 161–172. [Google Scholar] [CrossRef]
- Bukhari, S.; Barakat, A.F.; Eisele, Y.S.; Nieves, R.; Jain, S.; Saba, S.; Follansbee, W.P.; Brownell, A.; Soman, P. Prevalence of Atrial Fibrillation and Thromboembolic Risk in Wild-Type Transthyretin Amyloid Cardiomyopathy. Circulation 2021, 143, 1335–1337. [Google Scholar] [CrossRef]
- Donnellan, E.; Wazni, O.M.; Hanna, M.; Elshazly, M.B.; Puri, R.; Saliba, W.; Kanj, M.; Vakamudi, S.; Patel, D.R.; Baranowski, B.; et al. Atrial Fibrillation in Transthyretin Cardiac Amyloidosis: Predictors, Prevalence, and Efficacy of Rhythm Control Strategies. JACC. Clin. Electrophysiol. 2020, 6, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016, 133, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Perugini, E.; Guidalotti, P.L.; Salvi, F.; Cooke, R.M.; Pettinato, C.; Riva, L.; Leone, O.; Farsad, M.; Ciliberti, P.; Reggiani, M.L.B.; et al. Noninvasive Etiologic Diagnosis of Cardiac Amyloidosis Using 99mTc-3,3-Diphosphono-1,2-Propanodicarboxylic Acid Scintigraphy. J. Am. Coll. Cardiol. 2005, 46, 1076–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Luna, A.B.; Escobar-Robledo, L.A.; Aristizabal, D.; Restrepo, D.W.; Mendieta, G.; van Roessel, A.M.; Elosua, R.; Bayés-Genís, A.; Martínez-Sellés, M.; Baranchuk, A.; et al. Atypical advanced interatrial blocks: Definition and electrocardiographic recognition. J. Electrocardiol. 2018, 51, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef]
- Morris, D.A.; Takeuchi, M.; Krisper, M.; Köhncke, C.; Bekfani, T.; Carstensen, T.; Hassfeld, S.; Dorenkamp, M.; Otani, K.; Takigiku, K.; et al. Normal values and clinical relevance of left atrial myocardial function analysed by speckle-tracking echocardiography: Multicentre study. Europace Heart J. Cardiovasc. Imaging 2015, 16, 364–372. [Google Scholar] [CrossRef] [Green Version]
- Bayés-De-Luna, A.; Martínez-Sellés, M.; Elosua, R.; Bayés-Genís, A.; Mendieta, G.; Baranchuk, A.; Breithardt, G. Relation of Advanced Interatrial Block to Risk of Atrial Fibrillation and Stroke. Am. J. Cardiol. 2020, 125, 1745–1748. [Google Scholar] [CrossRef]
- Escobar-Robledo, L.A.; Bayés-De-Luna, A.; Lupón, J.; Baranchuk, A.; Moliner, P.; Martínez-Sellés, M.; Zamora, E.; de Antonio, M.; Domingo, M.; Cediel, G.; et al. Advanced interatrial block predicts new-onset atrial fibrillation and ischemic stroke in patients with heart failure: The “Bayes’ Syndrome-HF” study. Int. J. Cardiol. 2018, 271, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Brambatti, M.; Connolly, S.J.; Gold, M.R.; Morillo, C.; Capucci, A.; Muto, C.; Lau, C.P.; Van Gelder, I.C.; Hohnloser, S.H.; Carlson, M.; et al. Temporal Relationship Between Subclinical Atrial Fibrillation and Embolic Events. Circulation 2014, 129, 2094–2099. [Google Scholar] [CrossRef] [Green Version]
- Bayés de Luna, A.; Baranchuk, A.; Martínez-Sellés, M.; Platonov, P.G. Anticoagulation in patients at high risk of stroke without documented atrial fibrillation. Time for a paradigm shift? Ann. Noninvasive Electrocardiol. 2017, 22, e12417. [Google Scholar] [CrossRef] [PubMed]
- Sadiq Ali, F.; Enriquez, A.; Conde, D.; Redfearn, D.; Michael, K.; Simpson, C.; Abdollah, H.; de Luna, A.B.; Hopman, W.; Baranchuk, A. Advanced Interatrial Block Predicts New Onset Atrial Fibrillation in Patients with Severe Heart Failure and Cardiac Resynchronization Therapy. Ann. Noninvasive Electrocardiol. 2015, 20, 586–591. [Google Scholar] [CrossRef]
- Vicent, L.; Fernández-Cordón, C.; Nombela-Franco, L.; Escobar-Robledo, L.A.; Ayesta, A.; Solé, A.A.; Gómez-Doblas, J.J.; Bernal, E.; Tirado-Conte, G.; Cobiella, J.; et al. Baseline ECG and Prognosis After Transcatheter Aortic Valve Implantation: The Role of Interatrial Block. J. Am. Hear. Assoc. 2020, 9, e017624. [Google Scholar] [CrossRef] [PubMed]
- Bruña, V.; Velásquez-Rodríguez, J.; Valero-Masa, M.J.; Pérez-Guillem, B.; Vicent, L.; Díez-Delhoyo, F.; Devesa, C.; Sousa-Casasnovas, I.; Juárez, M.; De Luna, A.B.; et al. Prognostic of Interatrial Block after an Acute ST-Segment Elevation Myocardial Infarction. Cardiology 2019, 142, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Martín-Demiguel, I.; Núñez-Gil, I.J.; Pérez-Castellanos, A.; Vedia, O.; Uribarri, A.; Durán-Cambra, A.; Martín-García, A.; Corbí-Pascual, M.; Marzo, M.G.; Martínez-Sellés, M. Prevalence and Significance of Interatrial Block in Takotsubo Syndrome (from the RETAKO Registry). Am. J. Cardiol. 2019, 123, 2039–2043. [Google Scholar] [CrossRef]
- Fanjul, F.; Campins, A.; Asensio, J.; Sampériz, G.; Yañez, A.; Romaguera, D.; Fiol, M.; Riera, M. Interatrial blocks prevalence and risk factors for human immunodeficiency virus-infected persons. PLoS ONE 2019, 14, e0223777. [Google Scholar] [CrossRef]
- Lacalzada-Almeida, J.; Izquierdo-Gómez, M.M.; García-Niebla, J.; Elosua, R.; Jiménez-Sosa, A.; Baranchuk, A.; Bayes de Luna, A. Advanced interatrial block is a surrogate for left atrial strain reduction which predicts atrial fibrillation and stroke. Ann. Noninvasive Electrocardiol. 2019, 24, e12632. [Google Scholar] [CrossRef] [Green Version]
- Nochioka, K.; Quarta, C.C.; Claggett, B.; Roca, G.Q.; Rapezzi, C.; Falk, R.H.; Solomon, S.D. Left atrial structure and function in cardiac amyloidosis. Europace Hear. J. Cardiovasc. Imaging 2017, 18, 1128–1137. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Fareh, S.; Leung, T.K.; Nattel, S. Promotion of atrial fibrillation by heart failure in dogs: Atrial remodeling of a different sort. Circulation 1999, 100, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Henein, M.Y.; Suhr, O.B.; Arvidsson, S.; Pilebro, B.; Westermark, P.; Hörnsten, R.; Lindqvist, P. Reduced left atrial myocardial deformation irrespective of cavity size: A potential cause for atrial arrhythmia in hereditary transthyretin amyloidosis. Amyloid 2018, 25, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rausch, K.; Shiino, K.; Putrino, A.; Lam, A.K.-Y.; Scalia, G.M.; Chan, J. Reproducibility of global left atrial strain and strain rate between novice and expert using multi-vendor analysis software. Int. J. Cardiovasc. Imaging 2018, 35, 419–426. [Google Scholar] [CrossRef] [PubMed]
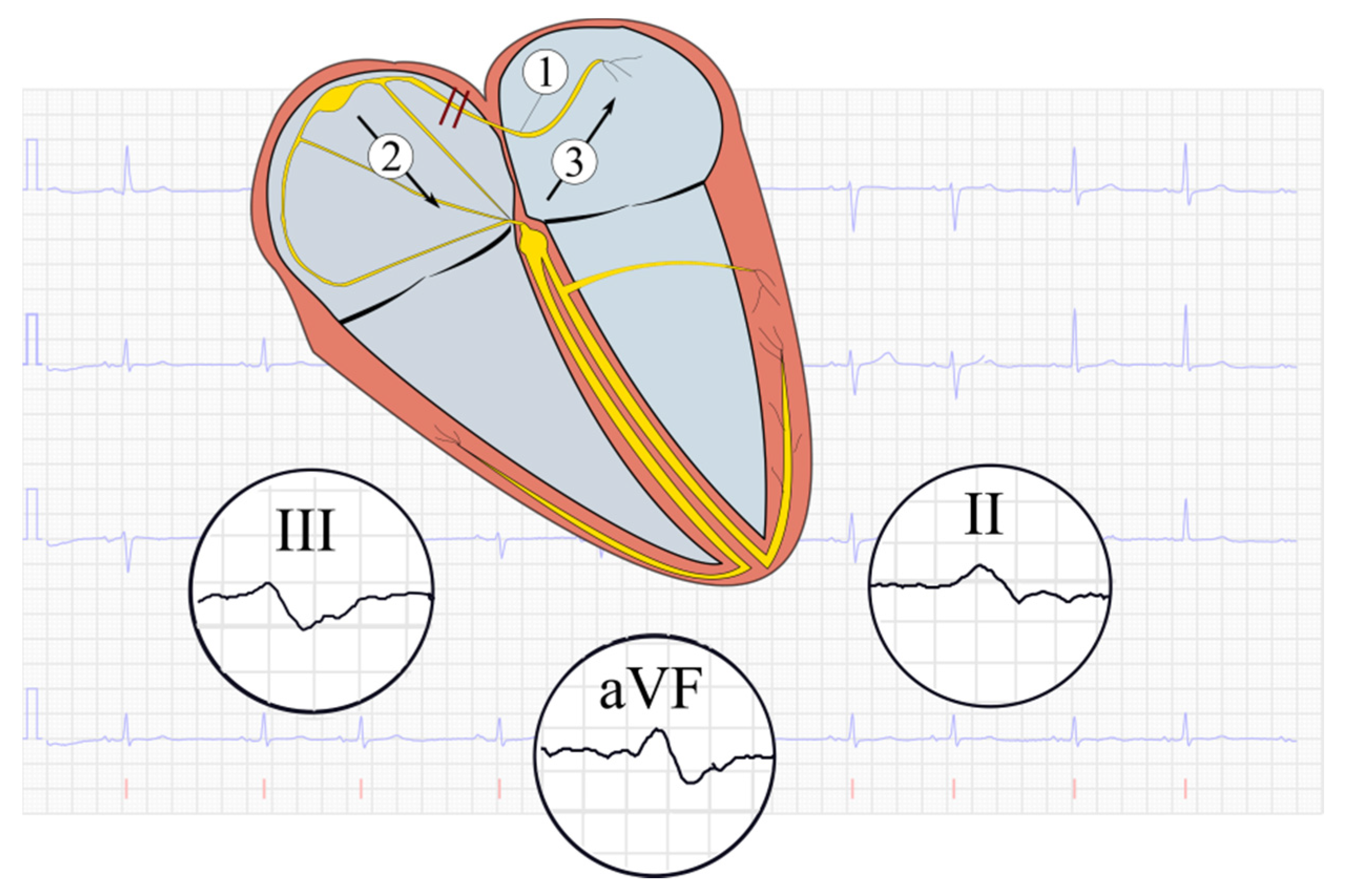
| All Patients | HF No ATTR-CA | ATTR-CA | p-Value | |
|---|---|---|---|---|
| n | 75 | 26 | 49 | |
| Age, years (mean (SD)) | 75.4 (8.0) | 75.8 (7.5) | 75.1 (8.5) | 0.73 |
| Body mass index (mean (SD)) | 26.0 (4.8) | 29.2 (5.5) | 24.9 (4.1) | |
| Perugini score (%) | <0.001 | |||
| 0 | 22 (31.9) | 22 (100.0) | 0 (0.0) | |
| 1 | 3 (4.3) | 0 (0.0) | 3 (6.4) | |
| 2 | 18 (26.1) | 0 (0.0) | 18 (38.3) | |
| 3 | 26 (37.7) | 0 (0.0) | 26 (55.3) | |
| NT-ProBNP, ng/L (median (IQR)) | 782 (231, 1616) | 408 (1216, 5440) | 844 (241, 1640) | 0.42 |
| Troponin T, ng/L (median (IQR)) | 25 (16, 40) | 22 (13, 34) | 30 (19, 45) | 0.27 |
| Systolic BP, mm Hg (mean (SD)) | 137.6 (20.0) | 145.4(23.2) | 133.3 (16.9) | 0.01 |
| Diastolic BP, mm Hg (mean (SD)) | 81.3 (10.2) | 85.6 (11.4) | 78.9 (87) | 0.006 |
| Heart failure medications (n, %) | 52 (69.3) | 25 (96.2) | 27 (55.1) | 0.001 |
| LVDD, mm (mean (SD)) | 44.6 (6.4) | 49.2 (5.8) | 42.1 (5.5) | <0.001 |
| IVSD, mm (median (IQR)) | 16 (15, 20) | 16(15, 17) | 18 (15, 21) | 0.067 |
| PWT, mm (median (IQR)) | 13 (10, 14) | 11 (9, 12) | 13 (12, 15) | <0.001 |
| LVEF, % (median (IQR)) | 55 (46, 58) | 55 (46, 60) | 50 (46, 55) | 0.20 |
| E, cm/s (mean (SD)) | 68.26 (21.41) | 58.68 (20.25) | 73.36 (20.43) | 0.005 |
| e’, cm/s (median (IQR)) | 4.0 (3.8, 6.0) | 4.0 (3.0, 5.0) | 5.0 (4.0, 6.0) | 0.07 |
| E/e’ (median (IQR)) | 14.9 (12.8, 16.7) | 14.0 (11.3, 16.2) | 15.2 (13.3 18.3) | 0.31 |
| GLS, % (mean (SD)) | 14.1 (4.7) | 13.2 (4.6) | 14.6 (4.7) | 0.21 |
| Relative wall thickness (median (IQR)) | 0.46 (0.38, 0.55) | 0.41 (0.38, 0.48) | 0.60 (0.52, 0.75) | 0.01 |
| LAVi, mL/m2 (mean (SD)) | 40.54 (16.61) | 41.00 (18.80) | 39.38 (10.13) | 0.82 |
| Tricuspid regurgitant velocity, cm/s (mean (SD)) | 26.9 (8.3) | 28.1 (9.5) | 26.4 (7.9) | 0.54 |
| Peak atrial longitudinal strain, % (median (IQR)) | 12.20 (7.57, 16.05) | 10.30 (6.45, 14.95) | 13.80 (8.55, 18.50) | 0.17 |
| Peak atrial longitudinal strain (atrial contraction), % (median (IQR)) | 8.32 (5.15, 12.15) | 8.16 (6.10, 10.75) | 8.32 (5.00, 14.00) | 0.74 |
| Atrial systolic strain rate, s−1 (median (IQR)) | 0.80 (0.57, 1.20) | 0.74 (0.65, 0.96) | 0.80 (0.55, 1.94) | 0.38 |
| Atrial strain rate (atrial contraction), s−1 (median (IQR)) | 0.81 (0.52, 1.20) | 0.88 (0.57, 1.20) | 0.81 (0.46, 1.10) | 0.64 |
| HF No ATTR-CA | ATTR-CA | p-Value | |
|---|---|---|---|
| n | 26 | 49 | |
| Heart rate, min−1 (mean (SD)) | 64.7 (12.5) | 74.8 (11.6) | 0.001 |
| P wave duration, ms (mean (SD)) | 103.1 (20.2) | 112.0 (22.0) | 0.09 |
| P wave duration ≥120 ms (n, %) | 10 (38.5) | 25 (51.0) | 0.43 |
| Advanced IAB (n, %) | 5 (21.4) | 13 (26.5) | 0.67 |
| Typical aIAB | 4 (15.4) | 8 (16.3) | |
| Atypical aIAB | 1 (3.8) | 5 (10.2) | |
| PR interval, ms (mean (SD)) | 199.8 (37.7) | 211.8 (38.4) | 0.20 |
| AV-block I, ms (n, %) | 9 (34.6) | 21 (42.9) | 0.66 |
| QRS duration, ms (median [IQR]) | 106 (101, 121) | 106 (92, 128) | 0.24 |
| Left bundle branch block | 3 (11.5) | 6 (12.2) | 1.0 |
| Left anterior hemiblock | 0 (0) | 12 (24.5) | 0.006 |
| Right bundle branch block | 0 (0) | 2 (4.1) | 0.54 |
| Right bundle branch block and left anterior hemiblock | 0 (0) | 3 (12.7) | 0.04 |
| Pathological Q waves (n, %) | 2 (7.7) | 7 (14.3) | 0.48 |
| ST-segment changes (n, %) | 10 (38.5) | 14 (28.6) | 0.44 |
| Reduced Atrial Strain Rate during Atrial Contraction | ||||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariable Analysis | |||||
| Odds Ratio (95% CI) | AUC (95% CI) | p | AUC (95% CI) | p | ||
| ATTR-CA | 0.98 (0.37–2.56) | 0.50 (0.37–0.64) | 0.96 | - | ||
| P wave duration | 1.02 (0.98–1.04) | 0.62 (0.49–0.74) | 0.10 | - | ||
| Advanced interatrial block | 5.17 (1.35–19.9) | 0.63 (0.52–0.75) | 0.02 | 4.40 (1.00–19.19) | 0.76 (0.66–0.89) | 0.049 |
| Age | 1.08 (1.01–1.16) | 0.65 (0.53–0.78) | 0.02 | 1.08 (1.01–1.16) | 0.03 | |
| LAVI | 1.06 (1.01–1.11) | 0.70 (0.58–0.82) | <0.01 | 1.07 (1.01–1.12) | 0.01 | |
| Sex | 0.98 (0.39–2.47) | 0.50 (0.37–0.64) | 0.97 | - | ||
| LVEF | 1.0 (0.95–1.06) | 0.50 (0.37–0.63) | 0.99 | - | ||
| Global longitudinal strain | 0.92 (0.83–1.02) | 0.63 (0.49–0.76) | 0.10 | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindow, T.; Lindqvist, P. The Prevalence of Advanced Interatrial Block and Its Relationship to Left Atrial Function in Patients with Transthyretin Cardiac Amyloidosis. J. Clin. Med. 2021, 10, 2764. https://doi.org/10.3390/jcm10132764
Lindow T, Lindqvist P. The Prevalence of Advanced Interatrial Block and Its Relationship to Left Atrial Function in Patients with Transthyretin Cardiac Amyloidosis. Journal of Clinical Medicine. 2021; 10(13):2764. https://doi.org/10.3390/jcm10132764
Chicago/Turabian StyleLindow, Thomas, and Per Lindqvist. 2021. "The Prevalence of Advanced Interatrial Block and Its Relationship to Left Atrial Function in Patients with Transthyretin Cardiac Amyloidosis" Journal of Clinical Medicine 10, no. 13: 2764. https://doi.org/10.3390/jcm10132764
APA StyleLindow, T., & Lindqvist, P. (2021). The Prevalence of Advanced Interatrial Block and Its Relationship to Left Atrial Function in Patients with Transthyretin Cardiac Amyloidosis. Journal of Clinical Medicine, 10(13), 2764. https://doi.org/10.3390/jcm10132764






