Changes in Left Ventricular Ejection Fraction after Mitral Valve Repair for Primary Mitral Regurgitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Echocardiographic Data
2.3. Statistical Analysis
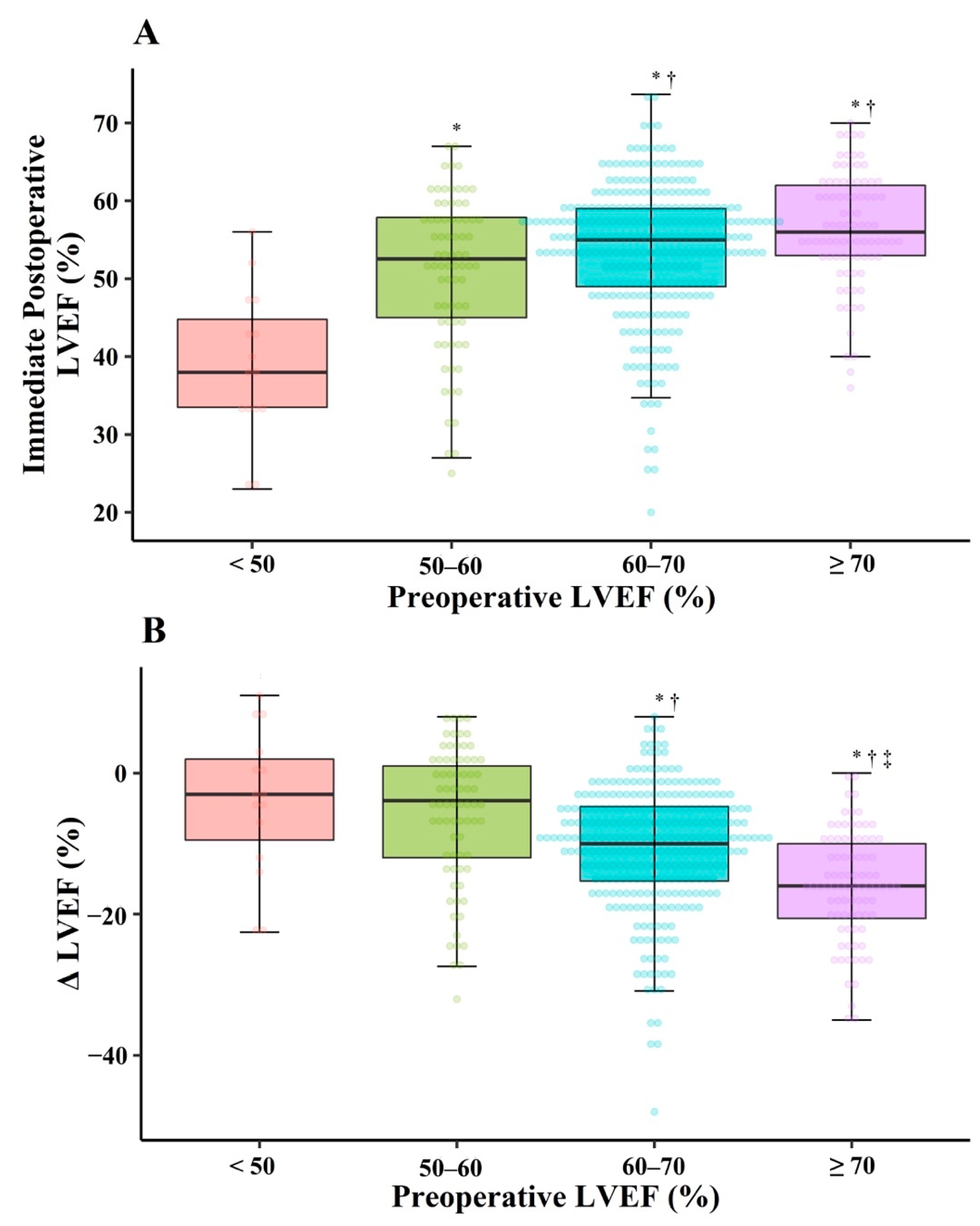
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e1159–e1195. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Sarano, M.; Tajik, A.; Schaff, H.; Orszulak, T.A.; McGoon, M.D.; Bailey, K.R.; Frye, R.L. Echocardiographic prediction of left ventricular function after correction of mitral regurgitation: Results and clinical implications. J. Am. Coll. Cardiol. 1994, 24, 1536–1543. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, T.; Ohtaki, E.; Tanaka, K.; Misu, K.; Tobaru, T.; Asano, R.; Nagayama, M.; Kitahara, K.; Umemura, J.; Sumiyoshi, T.; et al. Echocardiographic prediction ofleft ventricular dysfunction aftermitral valve repair for mitral regurgitation as anindicator to decide the optimal timing of repair. J. Am. Coll. Cardiol. 2003, 42, 458–463. [Google Scholar] [CrossRef] [Green Version]
- Suri, R.M.; Schaff, H.; Dearani, J.A.; Sundt, T.M.; Daly, R.C.; Mullany, C.J.; Enriquez-Sarano, M.; Orszulak, T.A. Recovery of left ventricular function after surgical correction of mitral regurgitation caused by leaflet prolapse. J. Thorac. Cardiovasc. Surg. 2009, 137, 1071–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tribouilloy, C.; Rusinaru, D.; Szymanski, C.; Mezghani, S.; Fournier, A.; Lévy, F.; Peltier, M.; Ben Ammar, A.; Carmi, D.; Remadi, J.-P.; et al. Predicting left ventricular dysfunction after valve repair for mitral regurgitation due to leaflet prolapse: Additive value of left ventricular end-systolic dimension to ejection fraction. Eur. J. Echocardiogr. 2011, 12, 702–710. [Google Scholar] [CrossRef] [Green Version]
- Witkowski, T.; Thomas, J.D.; Delgado, V.; van Rijnsoever, E.; Ng, A.C.; Hoke, U.; Ewe, S.H.; Auger, D.; Yiu, K.H.; Holman, E.R.; et al. Changes in Left Ventricular Function After Mitral Valve Repair for Severe Organic Mitral Regurgitation. Ann. Thorac. Surg. 2012, 93, 754–760. [Google Scholar] [CrossRef]
- Quintana, E.; Suri, R.M.; Thalji, N.M.; Daly, R.C.; Dearani, J.A.; Burkhart, H.M.; Li, Z.; Enriquez-Sarano, M.; Schaff, H. Left ventricular dysfunction after mitral valve repair—The fallacy of “normal” preoperative myocardial function. J. Thorac. Cardiovasc. Surg. 2014, 148, 2752–2762. [Google Scholar] [CrossRef] [Green Version]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Col-laboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef]
- De Simone, G.; Devereux, R.B.; Roman, M.J.; Ganau, A.; Saba, P.S.; Alderman, M.H.; Laragh, J.H. Assessment of left ventricular function by the midwall fractional shortening/end-systolic stress relation in human hypertension. J. Am. Coll. Cardiol. 1994, 23, 1444–1451. [Google Scholar] [CrossRef] [Green Version]
- E Rademakers, F.; Rogers, W.J.; Guier, W.H.; Hutchins, G.M.; O Siu, C.; Weisfeldt, M.L.; Weiss, J.L.; Shapiro, E.P. Relation of regional cross-fiber shortening to wall thickening in the intact heart. Three-dimensional strain analysis by NMR tagging. Circulation 1994, 89, 1174–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloner, R.A.; Jennings, R.B. Consequences of Brief Ischemia: Stunning, Preconditioning, and Their Clinical Implications. Circulation 2001, 104, 3158–3167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, C.-L.; Verma, A.; Uno, H.; Shin, S.-H.; Bourgoun, M.; Hassanein, A.H.; McMurray, J.J.; Velazquez, E.J.; Kober, L.; Pfeffer, M.A.; et al. Longitudinal and Circumferential Strain Rate, Left Ventricular Remodeling, and Prognosis After Myocardial Infarction. J. Am. Coll. Cardiol. 2010, 56, 1812–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zois, N.; Olsen, N.T.; Moesgaard, S.; Rasmussen, C.; Falk, T.; Häggström, J.; Pedersen, H.; Møller, J.; Olsen, L.H. Left Ventricular Twist and Circumferential Strain in Dogs with Myxomatous Mitral Valve Disease. J. Veter. Intern. Med. 2013, 27, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Zito, C.; Carerj, S.; Todaro, M.C.; Cusmà-Piccione, M.; Caprino, A.; Di Bella, G.; Oreto, L.; Oreto, G.; Khandheria, B.K. Myocardial Deformation and Rotational Profiles in Mitral Valve Prolapse. Am. J. Cardiol. 2013, 112, 984–990. [Google Scholar] [CrossRef]
- Enriquez-Sarano, M.; Tajik, A.J.; Schaff, H.; A Orszulak, T.; Bailey, K.R.; Frye, R.L. Echocardiographic prediction of survival after surgical correction of organic mitral regurgitation. Circulation 1994, 90, 830–837. [Google Scholar] [CrossRef] [Green Version]
- Suri, R.M.; Vanoverschelde, J.-L.; Grigioni, F.; Schaff, H.; Tribouilloy, C.; Avierinos, J.-F.; Barbieri, A.; Pasquet, A.; Huebner, M.; Rusinaru, D.; et al. Association Between Early Surgical Intervention vs Watchful Waiting and Outcomes for Mitral Regurgitation Due to Flail Mitral Valve Leaflets. JAMA 2013, 310, 609–616. [Google Scholar] [CrossRef]
- Rosenhek, R.; Rader, F.; Klaar, U.; Gabriel, H.; Krejc, M.; Kalbeck, D.; Schemper, M.; Maurer, G.; Baumgartner, H. Outcome of Watchful Waiting in Asymptomatic Severe Mitral Regurgitation. Circulation 2006, 113, 2238–2244. [Google Scholar] [CrossRef] [Green Version]
- Suri, R.M.; Schaff, H.; Dearani, J.A.; Sundt, T.M., III; Daly, R.C.; Mullany, C.J.; Sarano, M.E.; Orszulak, T.A. Determinants of early decline in ejection fraction after surgical correction of mitral regurgitation. J. Thorac. Cardiovasc. Surg. 2008, 136, 442–447. [Google Scholar] [CrossRef] [Green Version]
- Wehner, G.J.; Jing, L.; Haggerty, C.M.; Suever, J.D.; Leader, J.B.; Hartzel, D.N.; Kirchner, H.L.; A Manus, J.N.; James, N.; Ayar, Z.; et al. Routinely reported ejection fraction and mortality in clinical practice: Where does the nadir of risk lie? Eur. Hear. J. 2020, 41, 1249–1257. [Google Scholar] [CrossRef] [Green Version]
- Kitkungvan, D.; Nabi, F.; Kim, R.J.; Bonow, R.O.; Khan, A.; Xu, J.; Little, S.H.; Quinones, M.A.; Lawrie, G.M.; Zoghbi, W.A.; et al. Myocardial Fibrosis in Patients With Primary Mitral Regurgitation With and Without Prolapse. J. Am. Coll. Cardiol. 2018, 72, 823–834. [Google Scholar] [CrossRef]
- Venkateshvaran, A.; Sarajlic, P.; Lund, L.H.; Fridén, C.; Nordgren, B.; Opava, C.H.; E Lundberg, I.; Larsson, S.; Manouras, A.; Bäck, M. Impaired left atrial dynamics and its improvement by guided physical activity reveal left atrial strain as a novel early indicator of reversible cardiac dysfunction in rheumatoid arthritis. Eur. J. Prev. Cardiol. 2018, 25, 1106–1108. [Google Scholar] [CrossRef] [PubMed]
- Sarajlic, P.; Fridén, C.; Lund, L.H.; Manouras, A.; Venkateshvaran, A.; Larsson, S.C.; Nordgren, B.; Opava, C.H.; Lundberg, I.E.; Bäck, M. Enhanced ventricular-arterial coupling during a 2-year physical activity programme in patients with rheumatoid arthritis: A prospective substudy of the physical activity in rheumatoid arthritis 2010 trial. J. Intern. Med. 2017, 284, 664–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flemming, M.A.; Oral, H.; Rothman, E.D.; Briesmiester, K.; Petrusha, J.A.; Starling, M.R. Echocardiographic markers for mitral valve surgery to preserve left ventricular performance in mitral regurgitation. Am. Hear. J. 2000, 140, 476–482. [Google Scholar] [CrossRef]
- Skudicky, D.; Essop, M.R.; Sareli, P. Time-Related Changes in Left Ventricular Function After Double Valve Replacement for Combined Aortic and Mitral Regurgitation in a Young Rheumatic Population. Circulation 1997, 95, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.H.; Souchek, J.; Oprian, C.A.; Miller, D.C.; Rahimtoola, S.; Giacomini, J.C.; Sethi, G.; Hammermeister, K.E. Determinants of survival and left ventricular performance after mitral valve replacement. Department of Veterans Affairs Cooperative Study on Valvular Heart Disease. Circulation 1990, 81, 1173–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Variables (n = 461) | |
|---|---|
| Clinical data | |
| Age (years) | 53.0 (42.0–62.5) |
| Male | 291 (63.1) |
| Body mass index (kg/m2) | 24.5 (22.4–26.5) |
| Diabetes mellitus | 34 (7.4) |
| Hypertension | 172 (37.3) |
| Atrial fibrillation | 81 (17.6) |
| Preoperative medication | |
| ACEI/ARB | 219 (47.5) |
| β blocker | 101 (21.9) |
| Calcium channel blocker | 146 (31.7) |
| Digoxin | 71 (15.4) |
| Diuretics | 229 (49.7) |
| Preoperative echocardiography | |
| LV ejection fraction (%) | 64.6 (60.7–68.3) |
| LV end-diastolic volume index (mL/m2) | 92.9 (76.3–113.6) |
| LV end-systolic volume index (mL/m2) | 32.3 (26.0–40.8) |
| LV end-diastolic diameter (mm) | 60.0 (56.0–64.0) |
| LV end-systolic diameter (mm) | 38.0 (34.0–42.0) |
| Mitral regurgitation | |
| moderate/moderate to severe/severe | 19 (4.1)/17 (3.7)/425 (92.2) |
| prolapse/Flail/both | 302 (65.5)/152 (33.0)/7 (1.5) |
| Gr<50 (n = 15) | Gr50–60 (n = 74) | Gr60–70 (n = 281) | Gr≥70 (n = 85) | p-Value a | p-Value b | ||
|---|---|---|---|---|---|---|---|
| LV volume (mL/m2) | |||||||
| EDVI | Pre | 116.7 ± 26.8 | 99.4 ± 28.4 | 93.0 ± 26.1 * | 100.0 ± 29.8 | 0.002 | 0.188 |
| (preload) | Post | 98.2 ± 23.9 | 74.9 ± 20.4 * | 66.3 ± 19.0 *,† | 65.6 ± 20.9 *,† | <0.001 | <0.001 |
| Diff | −18.5 ± 25.5 | −24.5 ± 22.8 | −26.7 ± 19.2 | −34.4 ± 22.6 *,†,‡ | 0.003 | <0.001 | |
| ESVI | Pre | 58.2 (52.8–81.7) | 39.0 (34.1–51.0) § | 32.0 (26.1–39.7) §,∥ | 26.4 (21.3–31.8) §,∥¶ | <0.001 | <0.001 |
| Post | 61.4 (52.0–77.1) | 34.7 (27.5–42.9) § | 29.0 (22.1–37.1) §,∥ | 26.6 (20.1–35.1) §,∥ | <0.001 | <0.001 | |
| Diff | −11.9 (−19.2–9.3) | −5.2 (−12.8–−0.4) | −1.8 (−7.9–2.4) ∥ | 0.6 (−5.2–7.2) ∥¶ | <0.001 | <0.001 | |
| LV afterload (kdyne/cm2) | |||||||
| cESS | Pre | 177.1 ± 38.4 | 155.8 ± 43.2 | 140.1 ± 32.8 * | 129.0 ± 35.0 *,† | <0.001 | <0.001 |
| Post | 196.5 ± 59.9 | 158.9 ± 57.0 | 135.6 ± 37.0 * | 116.7 ± 27.5 *,†,‡ | <0.001 | <0.001 | |
| LV systolic function (%) | |||||||
| mFS | Pre | 14.1 ± 2.3 | 18.6 ± 2.8 * | 20.1 ± 2.8 *,† | 20.6 ±2.5 *,† | <0.001 | <0.001 |
| Post | 11.1 ± 2.3 | 14.8 ± 2.8 * | 15.0 ± 2.9 * | 15.4 ± 2.8 * | <0.001 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joung, K.-W.; Kim, S.-O.; Nam, J.-S.; Moon, Y.-J.; Bae, H.-J.; Chin, J.-H.; Jung, S.-H.; Choi, I.-C. Changes in Left Ventricular Ejection Fraction after Mitral Valve Repair for Primary Mitral Regurgitation. J. Clin. Med. 2021, 10, 2830. https://doi.org/10.3390/jcm10132830
Joung K-W, Kim S-O, Nam J-S, Moon Y-J, Bae H-J, Chin J-H, Jung S-H, Choi I-C. Changes in Left Ventricular Ejection Fraction after Mitral Valve Repair for Primary Mitral Regurgitation. Journal of Clinical Medicine. 2021; 10(13):2830. https://doi.org/10.3390/jcm10132830
Chicago/Turabian StyleJoung, Kyoung-Woon, Seon-Ok Kim, Jae-Sik Nam, Young-Jin Moon, Hyeun-Joon Bae, Ji-Hyun Chin, Sung-Ho Jung, and In-Cheol Choi. 2021. "Changes in Left Ventricular Ejection Fraction after Mitral Valve Repair for Primary Mitral Regurgitation" Journal of Clinical Medicine 10, no. 13: 2830. https://doi.org/10.3390/jcm10132830






