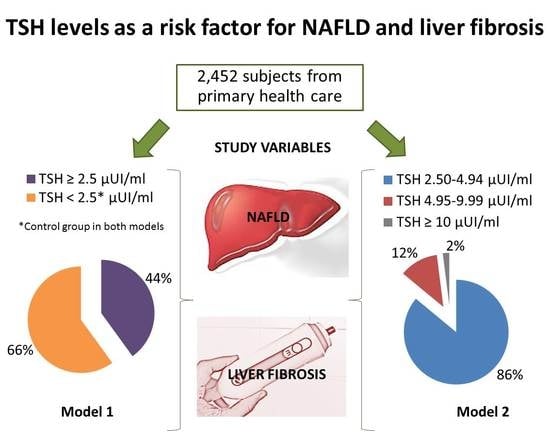
TSH Levels as an Independent Risk Factor for NAFLD and Liver Fibrosis in the General Population
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Clinical and Laboratory Parameters
2.3. Evaluation of MetS
2.4. Evaluation of NAFLD
2.5. Evaluation of Liver Fibrosis
2.6. Statistical Analysis
3. Results
3.1. Basal Characteristics
3.2. Relationship between TSH and NAFLD
3.3. Relationship between TSH and Hypertransaminasemia
3.4. Association between TSH and Liver Fibrosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bellentani, S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017, 37 (Suppl. S1), 81–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [Green Version]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Brunt, E.M.; Kleiner, D.E.; Carpenter, D.H.; Rinella, M.; Harrison, S.A.; Loomba, R.; Younossi, Z.; Neuschwander-Tetri, B.A.; Sanyal, A.J.; the American Association for the Study of Liver Diseases NASH Task Force. NAFLD: Reporting Histologic Findings in Clinical Practice. Hepatology 2021, 73, 2028–2038. [Google Scholar] [CrossRef] [PubMed]
- Diehl, A.M.; Day, C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2017, 377, 2063–2072. [Google Scholar] [CrossRef] [PubMed]
- Rosato, V.; Masarone, M.; Dallio, M.; Federico, A.; Aglitti, A.; Persico, M. NAFLD and Extra-Hepatic Comorbidities: Current Evidence on a Multi-Organ Metabolic Syndrome. Int. J. Environ. Res. Public Health 2019, 16, 3415. [Google Scholar] [CrossRef] [Green Version]
- Tanase, D.M.; Gosav, E.M.; Neculae, E.; Costea, C.F.; Ciocoiu, M.; Hurjui, L.L.; Tarniceriu, C.C.; Floria, M. Hypothyroidism-Induced Nonalcoholic Fatty Liver Disease (HIN): Mechanisms and Emerging Therapeutic Options. Int. J. Mol. Sci. 2020, 21, 5927. [Google Scholar] [CrossRef]
- Pagadala, M.R.; Zein, C.O.; Dasarathy, S.; Yerian, L.M.; Lopez, R.; McCullough, A.J. Prevalence of Hypothyroidism in Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2012, 57, 528–534. [Google Scholar] [CrossRef] [Green Version]
- Chung, G.E.; Kim, D.; Kim, W.; Yim, J.Y.; Park, M.J.; Kim, Y.J.; Yoon, J.-H.; Lee, H.-S. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J. Hepatol. 2012, 57, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wu, X.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Zhang, S.; Wang, Y.; Zhang, T.; Wang, X.; et al. High-Normal Thyroid Function Predicts Incident Nonalcoholic Fatty Liver Disease Among Middle-Aged and Older Euthyroid Subjects. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2021, glab037. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Dai, Y.; Qin, J. Serum Thyroid Hormones Levels are Significantly Associated with Nonalcoholic Fatty Liver Disease in Euthyroid Chinese Population. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef]
- Eshraghian, A.; Dabbaghmanesh, M.H.; Eshraghian, H.; Fattahi, M.R.; Omrani, G.R. Nonalcoholic fatty liver disease in a cluster of Iranian population: Thyroid status and metabolic risk factors. Arch. Iran. Med. 2013, 16, 584–589. [Google Scholar]
- Lee, K.W.; Bang, K.B.; Rhee, E.J.; Kwon, H.J.; Lee, M.Y.; Cho, Y.K. Impact of hypothyroidism on the development of non-alcoholic fatty liver disease: A 4-year retrospective cohort study. Clin. Mol. Hepatol. 2015, 21, 372–378. [Google Scholar] [CrossRef]
- Caballería, L.; Pera, G.; Arteaga, I.; Rodríguez, L.; Alumà, A.; Morillas, R.M.; de la Ossa, N.; Díaz, A.; Expósito, C.; Miranda, D.; et al. High Prevalence of Liver Fibrosis Among European Adults With Unknown Liver Disease: A Population-Based Study. Clin. Gastroenterol. Hepatol. 2018, 16, 1138–1145.e5. [Google Scholar] [CrossRef] [Green Version]
- Ascaso, J.F.; Millán, J.; Hernández-Mijares, A.; Blasco, M.; Brea, Á.; Díaz, Á.; Pedro-Botet, J.; Pintó, X. Dislipidemia aterogénica 2019. Consensus document of the Atherogenic Dyslipidaemia Group of the Spanish Arteriosclerosis Society. Clín. Investig. Arterioscler. 2020, 32, 120–125. [Google Scholar] [CrossRef]
- Julián, M.T.; Pera, G.; Soldevila, B.; Caballería, L.; Julve, J.; Puig-Jové, C.; Morillas, R.; Torán, P.; Expósito, C.; Puig-Domingo, M.; et al. Atherogenic dyslipidemia, but not hyperglycemia, is an independent factor associated with liver fibrosis in subjects with type 2 diabetes and NAFLD: A population-based study. Eur. J. Endocrinol. 2021, 184, 587–596. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (NCEP); Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281.e4. [Google Scholar] [CrossRef] [Green Version]
- Berg, E.H.V.D.; van Tienhoven-Wind, L.J.; Amini, M.; Schreuder, T.C.; Faber, K.N.; Blokzijl, H.; Dullaart, R.P. Higher free triiodothyronine is associated with non-alcoholic fatty liver disease in euthyroid subjects: The Lifelines Cohort Study. Metabolism 2017, 67, 62–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carulli, L.; Ballestri, S.; Lonardo, A.; Lami, F.; Violi, E.; Losi, L.; Bonilauri, L.; Verrone, A.M.; Odoardi, M.R.; Scaglioni, F.; et al. Is nonalcoholic steatohepatitis associated with a high-though-normal thyroid stimulating hormone level and lower cholesterol levels? Intern. Emerg. Med. 2011, 8, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Feldt-Rasmussen, U.; Klose, M. Clinical Strategies in the Testing of Thyroid Function. Available online: https://www.ncbi.nlm.nih.gov/books/NBK285558/ (accessed on 20 November 2020).
- Baloch, Z.; Carayon, P.; Conte-Devolx, B.; Demers, L.M.; Feldt-Rasmussen, U.; Henry, J.-F.; LiVosli, A.V.; Niccoli-Sire, P.; John, R.; Ruf, J.; et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 2003, 13, 3–126. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.; Phadke, A.; Sawant, P. Prevalence of hypothyroidism in nonalcoholic fatty liver disease in patients attending a tertiary hospital in western India. Indian J. Gastroenterol. 2015, 34, 169–173. [Google Scholar] [CrossRef]
- Loosen, S.H.; Demir, M.; Kostev, K.; Luedde, T.; Roderburg, C. Incidences of hypothyroidism and autoimmune thyroiditis are increased in patients with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2021. [Google Scholar] [CrossRef]
- Tahara, K.; Akahane, T.; Namisaki, T.; Moriya, K.; Kawaratani, H.; Kaji, K.; Takaya, H.; Sawada, Y.; Shimozato, N.; Sato, S.; et al. Thyroid-stimulating hormone is an independent risk factor of non-alcoholic fatty liver disease. JGH Open 2019, 4, 400–404. [Google Scholar] [CrossRef]
- Lee, J.; Ha, J.; Jo, K.; Lim, D.-J.; Lee, J.-M.; Chang, S.-A.; Kang, M.-I.; Cha, B.-Y.; Kim, M.-H. Male-specific association between subclinical hypothyroidism and the risk of non-alcoholic fatty liver disease estimated by hepatic steatosis index: Korea National Health and Nutrition Examination Survey 2013 to 2015. Sci. Rep. 2018, 8, 15145. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.; Gu, H.; Wu, J.; Sui, J. Thyroid function is associated with non-alcoholic fatty liver disease in euthyroid subjects. Endocr. Res. 2015, 40, 74–78. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, X.; Guan, L.; Jiang, Z.; Lin, H.; Jiang, Q.; Zhang, N.; Zhang, Y.; Zhang, X.; Yu, C.; et al. Free triiodothyronine levels are positively associated with non-alcoholic fatty liver disease in euthyroid middle-aged subjects. Endocr. Res. 2014, 40, 188–193. [Google Scholar] [CrossRef]
- Liu, L.; Li, P.; Mi, Y.; Liu, Y.; Liu, Y.; Zhang, P. Thyroid-stimulating hormone is associated with nonalcoholic steatohepatitis in patients with chronic hepatitis B. Medicine 2019, 98, e17945. [Google Scholar] [CrossRef]
- Grewal, H.; Joshi, S.; Sharma, R.; Mittal, P.; Goel, A. Non-alcoholic fatty liver disease in patients with hypothyroidism presenting at a rural tertiary care centre in north India. Trop. Doct. 2021, 51, 181–184. [Google Scholar] [CrossRef]
- Janovsky, C.C.P.S.; Cesena, F.H.; Valente, V.A.T.; Conceição, R.D.D.O.; Santos, R.D.; Bittencourt, M.S. Association between Thyroid-Stimulating Hormone Levels and Non-Alcoholic Fatty Liver Disease Is Not Independent from Metabolic Syndrome Criteria. Eur. Thyroid. J. 2018, 7, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Borges-Canha, M.; Neves, J.S.; Mendonça, F.; Silva, M.M.; Costa, C.; Cabral, P.M.; Guerreiro, V.; Lourenço, R.; Meira, P.; Salazar, D.; et al. Thyroid Function and the Risk of Non-Alcoholic Fatty Liver Disease in Morbid Obesity. Front. Endocrinol. 2020, 11, 572128. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Niu, Q.; Lv, H.; Li, Q.; Ma, Y.; Tan, J.; Liu, C. Elevated TPOAb is a Strong Predictor of Autoimmune Development in Patients of Type 2 Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease: A Case–Control Study. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 4369–4378. [Google Scholar] [CrossRef]
- Huang, B.; Yang, S.; Ye, S. Association between Thyroid Function and Nonalcoholic Fatty Liver Disease in Euthyroid Type 2 Diabetes Patients. J. Diabetes Res. 2020, 2020, 6538208. [Google Scholar] [CrossRef]
- Guo, Z.; Li, M.; Han, B.; Qi, X. Association of non-alcoholic fatty liver disease with thyroid function: A systematic review and meta-analysis. Dig. Liver Dis. 2018, 50, 1153–1162. [Google Scholar] [CrossRef]
- Mantovani, A.; Nascimbeni, F.; Lonardo, A.; Zoppini, G.; Bonora, E.; Mantzoros, C.S.; Targher, G. Association Between Primary Hypothyroidism and Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Thyroid 2018, 28, 1270–1284. [Google Scholar] [CrossRef] [PubMed]
- He, W.; An, X.; Li, L.; Shao, X.; Li, Q.; Yao, Q.; Zhang, J.-A. Relationship between Hypothyroidism and Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Front. Endocrinol. 2017, 8, 335. [Google Scholar] [CrossRef] [Green Version]
- Jaruvongvanich, V.; Sanguankeo, A.; Upala, S. Nonalcoholic Fatty Liver Disease Is Not Associated with Thyroid Hormone Levels and Hypothyroidism: A Systematic Review and Meta-Analysis. Eur. Thyroid. J. 2017, 6, 208–215. [Google Scholar] [CrossRef] [Green Version]
- Martínez Escudé, A.; Pera, G.; Arteaga, I.; Expósito, C.; Rodríguez, L.; Torán, P.; Caballeria, L. Relationship between hypothyroidism and non-alcoholic fatty liver disease in the Spanish population. Med. Clín. 2020, 154, 1–6. [Google Scholar] [CrossRef]
- Sinha, R.A.; You, S.-H.; Zhou, J.; Siddique, M.M.; Bay, B.-H.; Zhu, X.; Privalsky, M.L.; Cheng, S.-Y.; Stevens, R.D.; Summers, S.A.; et al. Thyroid hormone stimulates hepatic lipid catabolism via activation of autophagy. J. Clin. Investig. 2012, 122, 2428–2438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchs, C.; Claudel, T.; Trauner, M. Role of metabolic lipases and lipolytic metabolites in the pathogenesis of NAFLD. Trends Endocrinol. Metab. 2014, 25, 576–585. [Google Scholar] [CrossRef]
- Lonardo, A.; Mantovani, A.; Lugari, S.; Targher, G. NAFLD in Some Common Endocrine Diseases: Prevalence, Pathophysiology, and Principles of Diagnosis and Management. Int. J. Mol. Sci. 2019, 20, 2841. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.K.; Sinha, R.A.; Yen, P.M. Novel Transcriptional Mechanisms for Regulating Metabolism by Thyroid Hormone. Int. J. Mol. Sci. 2018, 19, 3284. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.-S.; Zhu, S.-H.; Liu, W.-Y.; Pan, X.-Y.; Zhu, P.-W.; Li, Y.-Y.; Zheng, I.K.; Ma, H.-L.; You, J.; Targher, G.; et al. PNPLA3 polymorphism influences the association between high-normal TSH level and NASH in euthyroid adults with biopsy-proven NAFLD. Diabetes Metab. 2020, 46, 496–503. [Google Scholar] [CrossRef]
- Dullaart, R.P.; Berg, E.H.V.D.; Van Der Klauw, M.M.; Blokzijl, H. Low normal thyroid function attenuates serum alanine aminotransferase elevations in the context of metabolic syndrome and insulin resistance in white people. Clin. Biochem. 2014, 47, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Gionfra, F.; De Vito, P.; Pallottini, V.; Lin, H.-Y.; Davis, P.J.; Pedersen, J.Z.; Incerpi, S. The Role of Thyroid Hormones in Hepatocyte Proliferation and Liver Cancer. Front. Endocrinol. 2019, 10, 532. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Yoo, E.R.; Li, A.; Fernandes, C.; Tighe, S.; Cholankeril, G.; Hameed, B.; Ahmed, A. Low-Normal Thyroid Function Is Associated With Advanced Fibrosis Among Adults in the United States. Clin. Gastroenterol. Hepatol. 2019, 17, 2379–2381. [Google Scholar] [CrossRef]
- Bano, A.; Chaker, L.; Plompen, E.P.C.; Hofman, A.; Dehghan, A.; Franco, O.H.; Janssen, H.L.A.; Murad, S.D.; Peeters, R.P. Thyroid Function and the Risk of Nonalcoholic Fatty Liver Disease: The Rotterdam Study. J. Clin. Endocrinol. Metab. 2016, 101, 3204–3211. [Google Scholar] [CrossRef] [Green Version]
- D’Ambrosio, R.; Campi, I.; Maggioni, M.; Perbellini, R.; Giammona, E.; Stucchi, R.; Borghi, M.; Degasperi, E.; De Silvestri, A.; Persani, L.; et al. The relationship between liver histology and thyroid function tests in patients with non-alcoholic fatty liver disease (NAFLD). PLoS ONE 2021, 16, e0249614. [Google Scholar] [CrossRef]
- Martínez-Escudé, A.; Pera, G.; Rodríguez, L.; Arteaga, I.; Expósito-Martínez, C.; Torán-Monserrat, P.; Caballería, L. Risk of Liver Fibrosis According to TSH Levels in Euthyroid Subjects. J. Clin. Med. 2021, 10, 1350. [Google Scholar] [CrossRef]
- Bano, A.; Chaker, L.; Muka, T.; Mattace-Raso, F.U.S.; Bally, L.; Franco, O.H.; Peeters, R.P.; Razvi, S. Thyroid Function and the Risk of Fibrosis of the Liver, Heart, and Lung in Humans: A Systematic Review and Meta-Analysis. Thyroid 2020, 30, 806–820. [Google Scholar] [CrossRef]
- Liu, L.; Yu, Y.; Zhao, M.; Zheng, D.; Zhang, X.; Guan, Q.; Xu, C.; Gao, L.; Zhao, J.; Zhang, H. Benefits of Levothyroxine Replacement Therapy on Nonalcoholic Fatty Liver Disease in Subclinical Hypothyroidism Patients. Int. J. Endocrinol. 2017, 2017, 5753039. [Google Scholar] [CrossRef] [PubMed]
- Bruinstroop, E.; Dalan, R.; Cao, Y.; Bee, Y.M.; Chandran, K.; Cho, L.W.; Soh, S.B.; Teo, E.K.; Toh, S.-A.; Leow, M.K.S.; et al. Low-Dose Levothyroxine Reduces Intrahepatic Lipid Content in Patients With Type 2 Diabetes Mellitus and NAFLD. J. Clin. Endocrinol. Metab. 2018, 103, 2698–2706. [Google Scholar] [CrossRef] [PubMed]
- Ladenson, P.W.; Kristensen, J.D.; Ridgway, E.C.; Olsson, A.G.; Carlsson, B.; Klein, I.; Baxter, J.D.; Angelin, B. Use of the Thyroid Hormone Analogue Eprotirome in Statin-Treated Dyslipidemia. N. Engl. J. Med. 2010, 362, 906–916. [Google Scholar] [CrossRef] [Green Version]
- Harrison, A.S.; Bashir, M.R.; Guy, C.D.; Zhou, R.; Moylan, C.A.; Frias, J.P.; Alkhouri, N.; Bansal, M.B.; Baum, S.; Neuschwander-Tetri, A.B.; et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2019, 394, 2012–2024. [Google Scholar] [CrossRef]




| TSH < 2.5 (n = 1619) | TSH ≥ 2.5 (n = 833) | p-Value | TSH ≥ 2.5 | ||||
|---|---|---|---|---|---|---|---|
| TSH 2.50–4.94 (n = 718) | TSH 4.95–9.99 (n = 96) | TSH ≥ 10 (n = 19) | p-Value * | ||||
| Age (years) | 54 ± 12 | 55 ± 12 | 0.013 | 55 ± 12 | 56 ± 11 | 52 ± 9 | 0.042 |
| Female | 909 (56%) | 592 (71%) | <0.001 | 503 (70%) | 76 (79%) | 13 (68%) | <0.001 |
| Disease history | |||||||
| T2DM | 158 (9.8%) | 95 (11%) | 0.205 | 88 (12%) | 4 (4.2%) | 3 (16%) | 0.046 |
| HBP | 421 (26%) | 221 (27%) | 0.779 | 201 (28%) | 15 (16%) | 5 (26%) | 0.079 |
| Hypercholesterolemia | 599 (37%) | 344 (41%) | 0.038 | 299 (42%) | 36 (38%) | 9 (47%) | 0.157 |
| Hypertriglyceridemia | 163 (10%) | 101 (12%) | 0.120 | 88 (12%) | 12 (13%) | 1 (5.3%) | 0.337 |
| Atherogenic dyslipidemia | 139 (9.0%) | 107 (13%) | 0.001 | 90 (13%) | 14 (15%) | 3 (16%) | 0.009 |
| Global obesity (BMI ≥ 30) | 454 (28%) | 294 (35%) | <0.001 | 250 (35%) | 34 (35%) | 10 (53%) | 0.007 |
| Abdominal obesity | 705 (44%) | 436 (52%) | <0.001 | 370 (52%) | 53 (55%) | 13 (68%) | <0.001 |
| MetS | 405 (25%) | 249 (30%) | 0.010 | 216 (30%) | 27 (28%) | 6 (32%) | 0.076 |
| Physical examination | |||||||
| BMI | 28 ± 5 | 29 ± 5 | <0.001 | 29 ± 5 | 29 ± 5 | 30 ± 6 | 0.001 |
| WC-Male (cm) | 98 ± 11 | 100 ± 12 | 0.032 | 100 ± 12 | 100 ± 13 | 101 ± 8 | 0.201 |
| WC-Female (cm) | 90 ± 12 | 92 ± 13 | 0.001 | 91 ± 13 | 93 ± 12 | 96 ± 15 | 0.003 |
| SBP (mmHg) | 125 ± 17 | 125 ± 17 | 0.801 | 125 ± 17 | 126 ± 16 | 126 ± 19 | 0.903 |
| DBP (mmHg) | 80 ± 10 | 81 ± 10 | 0.013 | 81 ± 10 | 81 ± 11 | 83 ± 13 | 0.066 |
| Blood analysis | |||||||
| Platelets (109/L) | 244 ± 59 | 246 ± 60 | 0.471 | 245 ± 61 | 253 ± 60 | 251 ± 47 | 0.527 |
| ALT (U/L) | 23 ± 13 | 24 ± 16 | 0.047 | 24 ± 16 | 22 ± 14 | 29 ± 19 | 0.040 |
| AST (U/L) | 23 ± 8 | 24 ± 10 | 0.025 | 24 ± 10 | 23 ± 8 | 25 ± 8 | 0.058 |
| ALT and/or AST > 35 (U/L) | 189 (12%) | 122 (15%) | 0.036 | 107 (15%) | 10 (10%) | 5 (26%) | 0.040 |
| GGT (U/L) | 30 ± 26 | 31 ± 32 | 0.172 | 32 ± 32 | 27 ± 25 | 36 ± 40 | 0.233 |
| ALP (U/L) | 77 ± 24 | 79 ± 25 | 0.019 | 79 ± 25 | 83 ± 24 | 89 ± 15 | 0.008 |
| Ferritin (ng/mL) | 115 ± 114 | 105 ± 110 | 0.045 | 106 ± 110 | 104 ± 114 | 82 ± 63 | 0.181 |
| Glycemia (mg/dL) | 100 ± 24 | 101 ± 28 | 0.181 | 101 ± 28 | 100 ± 32 | 96 ± 21 | 0.418 |
| HbA1c (%) | 5.7 ± 0.7 | 5.7 ± 0.7 | 0.199 | 5.7 ± 0.7 | 5.7 ± 0.8 | 5.6 ± 0.3 | 0.479 |
| Total cholesterol (mg/dL) | 210 ± 39 | 216 ± 38 | <0.001 | 214 ± 39 | 225 ± 35 | 243 ± 37 | <0.001 |
| HDL (mg/dL) | 55 ± 13 | 56 ± 13 | 0.018 | 56 ± 13 | 56 ± 12 | 53 ± 7 | 0.069 |
| LDL (mg/dL) | 133 ± 34 | 135 ± 34 | 0.176 | 133 ± 33 | 144 ± 32 | 161 ± 39 | <0.001 |
| TG (mg/dL) | 115 ± 68 | 130 ± 78 | <0.001 | 129 ± 77 | 133 ± 82 | 154 ± 58 | <0.001 |
| FLI < 60 (n = 1589) | FLI ≥ 60 (n = 863) | p-Value | |
|---|---|---|---|
| TSH level (μIU/mL) | 2.3 ± 2.4 | 2.9 ± 6.2 | <0.001 |
| Groups TSH (n, %) | |||
| TSH < 2.5 (μIU/mL) | 1089 (69%) | 530 (61%) | <0.001 |
| TSH ≥ 2.5 (μIU/mL) | 500 (31%) | 333 (39%) | |
| TSH 2.50–4.94 (μIU/mL) | 434 (27%) | 284 (33%) | <0.001 * |
| TSH 4.95–9.99 (μIU/mL) | 61 (3.8%) | 35 (4.1%) | |
| TSH ≥ 10 (μIU/mL) | 5 (0.3%) | 14 (1.6%) |
| FLI ≥ 60 | |
|---|---|
| OR (CI 95%) p-value | |
| Univariate | |
| TSH 2.50–4.94 (μIU/mL) | 1.34 (1.12–1.61) 0.001 |
| TSH 4.95–9.99 (μIU/mL) | 1.18 (0.77–1.81) 0.451 |
| TSH ≥ 10 (μIU/mL) | 5.75 (2.06–16.06) < 0.001 |
| Multivariate * | |
| Adjusted to global obesity | |
| TSH 2.50–4.94 (μIU/mL) | 1.46 (1.13–1.89) 0.004 |
| TSH 4.95–9.99 (μIU/mL) | 1.28 (0.71–2.31) 0.410 |
| TSH ≥ 10 (μIU/mL) | 8.71 (2.51–30.25) < 0.001 |
| Adjusted to total cholesterol | |
| TSH 2.50–4.94 (μIU/mL) | 1.49 (1.23–1.81) < 0.001 |
| TSH 4.95–9.99 (μIU/mL) | 1.29 (0.83–2.02) 0.262 |
| TSH ≥ 10 (μIU/mL) | 6.75 (2.38–19.13) < 0.001 |
| Adjusted to MetS | |
| TSH 2.50–4.94 (μIU/mL) | 1.45 (1.16–1.80) < 0.001 |
| TSH 4.95–9.99 (μIU/mL) | 1.34 (0.82–2.19) 0.247 |
| TSH ≥ 10 (μIU/mL) | 9.33 (3.11–27.97) < 0.001 |
| Adjusted to MetS parameters ** | |
| TSH 2.50–4.94 (μIU/mL) | 1.31 (1.00–1.71) 0.046 |
| TSH 4.95–9.99 (μIU/mL) | 1.14 (0.63–2.06) 0.655 |
| TSH ≥ 10 (μIU/mL) | 8.22 (1.77–38.04) 0.007 |
| ALT and/or AST > 35 U/L | |
|---|---|
| OR (CI 95%) p-value | |
| Univariate | |
| TSH 2.50–4.94 (μIU/mL) | 1.33 (1.03–1.71) 0.031 |
| TSH 4.95–9.99 (μIU/mL) | 0.88 (0.45–1.72) 0.709 |
| TSH ≥ 10 (μIU/mL) | 2.70 (0.96–7.59) 0.059 |
| Multivariate * | |
| TSH 2.50–4.94 (μIU/mL) | 1.34 (1.02–1.75) 0.035 |
| TSH 4.95–9.99 (μIU/mL) | 0.98 (0.49–1.97) 0.962 |
| TSH ≥ 10 (μIU/mL) | 2.24 (0.76–6.61) 0.145 |
| TE ≥ 8.0 kPa | TE ≥ 9.2 kPa | |
|---|---|---|
| OR (CI 95%) p–value | OR (CI 95%) p–value | |
| Univariate | ||
| TSH 2.50–4.94 (μIU/mL) | 1.72 (1.18–2.49) 0.004 | 2.24 (1.37–3.68) 0.001 |
| TSH 4.95–9.99 (μIU/mL) | 1.50 (0.63–3.54) 0.358 | 1.55 (0.47–5.15) 0.474 |
| TSH ≥ 10 (μIU/mL) | 2.64 (0.60–11.67) 0.200 | 2.67 (0.35–20.6) 0.346 |
| Multivariate * | ||
| Adjusted to global obesity | ||
| TSH 2.50–4.94 (μIU/mL) | 1.74 (1.17–2.58) 0.006 | 2.30 (1.37–3.87) 0.002 |
| TSH 4.95–9.99 (μIU/mL) | 1.72 (0.69–4.25) 0.243 | 1.82 (0.52–6.36) 0.346 |
| TSH ≥ 10 (μIU/mL) | 2.39 (0.50–11.37) 0.272 | 2.44 (0.29–20.27) 0.410 |
| Adjusted to total cholesterol | ||
| TSH 2.50–4.94 (μIU/mL) | 1.98 (1.34–2.90) < 0.001 | 2.64 (1.59–4.39) < 0.001 |
| TSH 4.95–9.99 (μIU/mL) | 2.00 (0.83–4.86) 0.124 | 2.15 (0.63–7.32) 0.222 |
| TSH ≥ 10 (μIU/mL) | 5.01 (1.10–22.85) 0.037 | 5.44 (0.68–43.54) 0.111 |
| Adjusted to MetS | ||
| TSH 2.50–4.94 (μIU/mL) | 1.76 (1.19–2.61) 0.005 | 2.32 (1.39–3.88) 0.001 |
| TSH 4.95–9.99 (μIU/mL) | 1.94 (0.79–4.75) 0.147 | 2.17 (0.63–7.51) 0.220 |
| TSH ≥ 10 (μIU/mL) | 3.34 (0.71–15.78) 0.128 | 3.54 (0.43–29.37) 0.241 |
| Adjusted to MetS parameters ** | ||
| TSH 2.50–4.94 (μIU/mL) | 1.64 (1.10–2.46) 0.016 | 2.17 (1.28–3.68) 0.004 |
| TSH 4.95–9.99 (μIU/mL) | 1.78 (0.72–4.44) 0.213 | 2.00 (0.57–7.02) 0.277 |
| TSH ≥ 10 (μIU/mL) | 2.46 (0.50–12.09) 0.269 | 2.66 (0.31–22.98) 0.375 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Escudé, A.; Pera, G.; Costa-Garrido, A.; Rodríguez, L.; Arteaga, I.; Expósito-Martínez, C.; Torán-Monserrat, P.; Caballería, L. TSH Levels as an Independent Risk Factor for NAFLD and Liver Fibrosis in the General Population. J. Clin. Med. 2021, 10, 2907. https://doi.org/10.3390/jcm10132907
Martínez-Escudé A, Pera G, Costa-Garrido A, Rodríguez L, Arteaga I, Expósito-Martínez C, Torán-Monserrat P, Caballería L. TSH Levels as an Independent Risk Factor for NAFLD and Liver Fibrosis in the General Population. Journal of Clinical Medicine. 2021; 10(13):2907. https://doi.org/10.3390/jcm10132907
Chicago/Turabian StyleMartínez-Escudé, Alba, Guillem Pera, Anna Costa-Garrido, Lluís Rodríguez, Ingrid Arteaga, Carmen Expósito-Martínez, Pere Torán-Monserrat, and Llorenç Caballería. 2021. "TSH Levels as an Independent Risk Factor for NAFLD and Liver Fibrosis in the General Population" Journal of Clinical Medicine 10, no. 13: 2907. https://doi.org/10.3390/jcm10132907
APA StyleMartínez-Escudé, A., Pera, G., Costa-Garrido, A., Rodríguez, L., Arteaga, I., Expósito-Martínez, C., Torán-Monserrat, P., & Caballería, L. (2021). TSH Levels as an Independent Risk Factor for NAFLD and Liver Fibrosis in the General Population. Journal of Clinical Medicine, 10(13), 2907. https://doi.org/10.3390/jcm10132907